Abstract
The search for sustainable development has increased interest in the improvement of technologies that use renewable energy sources. One of the alternatives in the production of renewable energy comes from the use of waste including urban solids, animal excrement from livestock, and biomass residues from agro-industrial plants. These materials may be used in the production of biogas, making its production highly sustainable and environmentally friendly. The present study aimed to evaluate the cultivated and uncultivated microbial community from a substrate (starter) used as an adapter for biogas production in anaerobic digestion processes. 16S rDNA metabarcoding revealed the domain of bacteria belonging to the phyla Firmicutes, Bacteroidota, Chloroflexi and Synergistota. The methanogenic group was represented by the phyla Halobacterota and Euryarchaeota. Through 16S rRNA sequencing of isolates recovered from the starter culture, the genera Rhodococcus (Actinobacteria phylum), Vagococcus, Lysinibacillus, Niallia, Priestia, Robertmurraya, Proteiniclasticum (Firmicutes phylum), and Luteimonas (Proteobacteria phylum) were identified, genera that were not observed in the metabarcoding data. The volatile solids, volatile organic acids, and total inorganic carbon reached 659.10 g kg−1, 717.70 g kg−1, 70,005.0 g kg−1, respectively. The cultured groups are involved in the metabolism of sugars and other compounds derived from lignocellulosic material, as well as in anaerobic methane production processes. The results demonstrate that culture-dependent approaches, such as isolation and sequencing, and culture-independent studies, such as the Metabarcoding approach, are complementary methodologies that, when integrated provide robust and comprehensive information about the microbial communities involved in processes of the production of biogas in anaerobic digestion processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Population growth has increased the demand for limited supplies of food and fuel by nations around the world. However, petroleum-derived fuels, which, in addition to being composed of highly polluting substances to the environment, are originated from finite sources of energy, factors that indicate the need to replace these fuels in the medium term with those produced from renewable sources. (Gielen et al. 2019). Innovative technologies are being developed to efficiently convert biomass into valuable products such as biogas (Vale et al. 2019). Biogas derived from animal manure and plant residues (lignocellulosic biomass) has been considered an alternative and renewable biofuel with ample energy capacity and can be a sustainable option over the use of fossil fuels (Anthony et al. 2019; Gulhane et al. 2018). One of the major limitations that biogas production still has is the lack of a higher yield in the anaerobic digestion (AD) process so that the technology can be transferred to large-scale biodigesters. This deficiency can be overcome with greater knowledge of the microbial communities involved in anaerobic digestion (Muturi et al. 2021). Biogas production from renewable and sustainable resources is becoming a prominent alternative in most developed and developing countries (Murunga et al. 2016), which has increased its use, a more viable option for modern society.
AD is a process derived from microbial metabolism that produces biogas (methane) from the conversion of organic matter (Orhorhoro et al. 2018). This process, which occurs in the absence of oxygen, involves different microbial groups, each one being responsible for the degradation of a category of organic compounds present in the system. In AD, methane (CH4) production occurs over 4 stages, which are hydrolysis, acidogenesis, acetogenesis and methanogenesis (Vrieze and Verstraete 2016). In hydrolysis, carbohydrates, proteins and fats from animal manure and food residues are broken down into soluble compounds, such as monosaccharides, amino acids, and fatty acids, by the action of enzymes produced by hydrolytic bacteria of genera such as Bacillus, Bacteroides and Eubacterium (Soares et al. 2017). The products of hydrolysis are transformed into volatile fatty acids and alcohols by acidogenic bacterial genera (acidogenesis), including Clostridium and Bacteroides (Valijanian et al. 2018; Yan et al. 2020; Xu et al. 2021). The products from previous steps are transformed into acetic acid, hydrogen, and CO2 by acetogenic bacteria, including Desulfococcus and Desulfotomaculum. Finally, methane formation (methanogenesis) occurs due to the presence of methanogenic archaea including Methanosarcina and Methanobacterium (Covey and Megonigal 2019; Knoblauch et al. 2018; Valijanian et al. 2018; Xu et al. 2021).
Therefore, the implementation of AD processes in biodigesters improves the production of biogas, since in these systems the conditions are controlled, and the processes can be standardized according to the market needs for this biofuel. In addition, the characterization of microbial communities in environmental samples using culture-dependent and culture-independent methods are widely used technologies (Arguita-Maeso et al. 2020; Wei et al. 2021), and essential for the optimization of the process. In this sense, this work aimed to analyze an inoculum used by the company CIBiogás—International Center for Renewable Energies, to optimize the anaerobic digestion tests developed by the corporation, using culture-dependent and independent methods for the taxonomic characterization of the communities present in the inoculum, as well as to identify the main groups involved in AD processes. Such information will be used to optimize and develop strategies for a better understanding of biomethane production.
Materials and methods
Sampling and isolation
An inoculum sample (starter) produced in a biodigester was provided by the company CIBiogás located in the city of Foz do Iguaçu, Brazil and was used in the present work to assess the functional and taxonomic diversity of associated microorganisms. Sampling was carried out on 1/20/2020 in the laboratory of the company CIBiogás. Forty mL of the contents of the biodigester were collected, properly placed in 50 mL flasks under sterile conditions. The sample was homogenized in an automatic shaker and serial dilution (10–1, 10–2 and 10–3) was performed. Aliquots of 50 µL of each dilution were inoculated to the respective culture media, in triplicate, as described as follows:
Hydrolytic culture media (HM), composed by the inorganic salts (SI) KH2PO4 10 g.L−1; MgCl2.6H2O 6.6 g.L−1; NaCl 8 g.L−1; Na2SO4 0.28 g.L−1; NH4Cl 8 g.L−1 and CaCl2.2H2O 1 g.L−1 plus the trace elements ZnSO4.7H2O 0.1 g.L−1; MnCl2.4H2O 0.03 g.L−1; H3BO3 0.3 g.L−1; CoCl2.6H2O 0.2 g.L−1; CaCl2.2H2O 0.01 g.L−1; NiCl2.6H2O 0.02 g.L−1 and agar 20 g.L−1. For each 1000 mL of the hydrolytic culture media, the following reagents were added, separately for each analysis: (i) for isolation of cellulase-producing bacteria (CMC): CMC (carboxymethyl cellulose) 0.2%; (ii) for isolation of amylase-producing bacteria (AM): soluble starch 0.2%; (iii) for isolation of ligninase-producing bacteria (GUA and RBBR): guaiacol (99%) and 425 μL and RBBR (Remazol Briliant Blue R) 1000 mg.L−1, separately; (iv) for isolation of lipase-producing bacteria (OL): olive oil 1%; (v) for isolation of protease-producing bacteria (LE): skimmed milk 10%; (vi) for isolation of distinct bacteria (NA-nutrient agar): meat extract 3 g.L−1; peptone 5 g.L−1, pH 6.8. The different culture media containing the sample inoculum were incubated at 37 °C for 5 to 7 days. Morphologically distinct colonies were purified and preserved at − 80 °C in 20% glycerol.
Acidogenic culture medium (ACD)—basic medium—composed by (a) glucose 1 g.L−1; inorganic salts KH2PO4 10 g.L−1; MgCl2.6H2O 6.6 g.L−1; NaCl 8 g.L−1; Na2SO4 0.28 g.L−1; NH4Cl 8 g.L−1and CaCl2.2H2O 1 g.L−1, plus the trace elements ZnSO4.7H2O 0.1 g.L−1; MnCl2.4H2O 0.03 g.L−1; H3BO3 0.3 g.L−1; CoCl2.6H2O 0.2 g.L−1; CaCl2.2H2O 0.01 g.L−1; NiCl2. 6H2O 0.02 g.L−1 and cysteine 0.05 g.L−1; (b) vitamin solution 5 ml (100 mL): PP vitamin 2 mg; B12 vitamin 1 mg; B6 vitamin 5 mg; C vitamin 2.5 mg; pantenoic acid 0.5 mg; B Bc vitamin 1 mg; biotin 3.5 mg; B2 vitamin 2.2 mg; Choline 2.5 mg; p-aminobenzoic acid 1 mg; (c) Na2HCO3 (5%) 0.05 mL; Na2S (1%) 0.05 mL; resazurin 0.5 g.L−1 2 mL (Agustini 2014; Ren et al. 2007).
Acetogenic culture medium (ACT): (a) basal medium, composed of NH4Cl 1 g.L−1; MgCl2 0.1 g.L−1; KH2PO4 0.4 g.L−1; cysteine hydrochloride 0.5 g.L−1; Na2SO4 0.5 g.L−1; NaHCO3 7 g.L−1; CaCO3 10 g.L−1; yeast extract 2 g.L−1; (b) vitamin solution (5 mL): biotin 2 mg.L−1; folic acid 2 mg.L−1; pyridoxine hydrochloride 10 mg.L−1; riboflavina 5 mg.L−1; thiamine 5 mL; nicotinic acid 5 mg.L−1; pantothenic acid 5 mg.L−1; B12 vitamin 0.1 mg.L−1; p-aminobenzoic acid 5 mg.L−1; thioethic acid 5 mg.L−1; pH 6.7; resazurin 0.5 g.L−1 2 mL (Agustini 2014; Manimegalai et al. 2014).
Methanogenic culture medium (MET): (a) K2HPO4 0.023 g.L−1; KH2PO4 0.023 g.L−1; (NH4)2SO4 0.023 g.L−1; NaCl 0.046 g.L−1; MgSO4.7H2O 0.009 g.L−1; CaCl2.2H2O 0.006 g.L−1; yeast extract 0.2 g.L−1; Na2CO3 0.4 g.L−1; cysteine hydrochloride 0.025 g.L−1; Na2S.9H2O 0.025 g.L−1. (b) vitamin solution (5 mL): biotin 2 mg.L−1; folic acid 2 mg.L−1; pyridoxine hydrochloride 10 mg.L−1; riboflavin 5 mg.L−1; thiamine 5 mg.L−1; nicotinic acid 5 mg.L−1; pantothenic acid 5 mg.L−1; B12 vitamin 0.1 mg.L−1; p-aminobenzoic acid 5 mg.L−1; thioethic acid 5 mg.L−1; pH 7.2. (c) enriched medium: C7H5NaO2 0.2 g.L−1; NH4Cl 0.075 g.L−1; K2HPO4 0.04 g.L−1; MgCl2 0.01 g.L−1; Na2CO3 0.15 g.L−1; pH 7.2; resazurin 0.5 g.L−1 2 mL (Agustini 2014; Manimegalai et al. 2014, modified).
For the ACD, ACT and MET culture media, 10% of the substrate (starter) was added. The three media were distributed in 10 mL penicillin vials in the presence of flow of nitrogen and the vials were sealed and sterilized. The sample (500 µL) was added to the vials with the aid of a 1 mL syringe and incubated at 37 ºC for 30 days (Ferry et al. 1974; Manimegalai et al. 2014;). After incubation, 50 µL aliquots were added to the solid culture media (ACD, ACT and MET—added 20 g of agar per liter), placed in an anaerobic jar with CO2 atmosphere, incubated at 37 ºC for 15 days. The isolated strains were purified and preserved at – 80 °C in 20% glycerol. The inoculum temperature on the day of collection was determined and was around 37.7 °C.
Morphological and biochemical characterization
Morphological analysis of the microbial isolates was performed by sowing each strain on plates containing the same culture media HM, ACD, ACT and MET. The characteristics of cell structures were analyzed using the Gram stain technique. Biochemical assays were performed with the isolates using the following media: i) CLED (BD) culture medium containing casein peptone 4.0 g.L−1; gelatin peptone 4.0 g.L−1; meat extract 3.0 g.L−1; lactose 10.0 g.L−1; L-cystine 0.128 g.L−1; agar 15.0 g.L−1 and bromothymol blue 0.02 g.L−1; ii) MacConkey (BD) containing peptide casein 1.5 g.L−1; meat peptone 1.5 g.L−1; gelatin peptone 17.0 g.L−1; bile salts (mixture) 1.5 g.L−1; lactose 10.0 g.L−1; sodium chloride 5.0 g.L−1; neutral red 0.03 g.L−1; crystal violet 0.001 g.L−1; agar 13.5 g.L−1. Bacteria from ACT and MET media were cultivated in the respective media, without the addition of other substances, under anaerobic conditions.
Physicochemical analysis
The physicochemical analyses were performed by evaluating the following parameters: total solids, fixed solids, volatile solids, volatile organic acids (VOA), total inorganic carbon (TIC), temperature and pH. All these parameters were determined in the CIBiogás laboratory (APHA 2017).
Molecular analysis
DNA extraction
For DNA extraction, performed based on the protocol of Aamir et al. (2015), 10 morphologically distinct isolates were selected, 1 from each different culture medium and 3 from the methanogenic medium (MET). Cells were extracted with 900 μL of phenol in a tube containing a small amount of glass beads followed by incubation at 65 °C for 20 min. Samples were centrifuged at 16,000×g for 10 min at 4 °C. The supernatants were added with 800 μL of phenol, briefly homogenized and centrifuged at 16,000xg for 5 min at 4 °C. Phenol, in a 1:1 ratio, was added to the supernatant, followed by brief homogenization and centrifugation at 16,000×g for 5 min at 4 °C. A volume of 600 μL of isopropanol was added to the supernatants, followed by homogenization and incubation at − 20 °C for 20 min. Samples were centrifuged at 16,000×g for 10 min at 4 °C and supernatants were discarded. A volume of 100 μL of 70% ethanol was added to the pellets and, after 1 min, the ethanol was discarded. Pellets were dried at room temperature and then suspended in 50 μL of sterile MilliQ water. The extracted DNA was quantified in 0.8% agarose gel and visualized in a photo documenter.
PCR and purification
The DNAs of the isolates were subjected to PCR for amplification of the 16S rRNA gene. Reactions were performed with Buffer Solution 1 X, MgCl2 solution 1.5 mM, primer pair 0.5 μM, dNTP's 0.2 mM, Taq DNA polymerase 2.0 U and genomic DNA 2–25 ng, for a total volume of 25 μL. PCR conditions were: an initial cycle of 5 min at 95 °C; 40 sequential amplification cycles of 30 s of 95 °C, 30 s of 63 °C and 60 s of 72 °C; plus 1 final cycle of 10 min at 72 °C. Amplicons were purified using the GFX Gel Band Purification Kit column kit and were visualized in 0.8% agarose gel (Aamir et al. 2015). The set of primers used was 16S 10f (AGTTTGATCCTGGCTC) e 1100r (GGGTTGCGCTCGTTG) (Belgini et al. 2014).
Sequencing and phylogenetic analysis
Amplified products purified were sequenced using Big Dye Terminator Cycle Sequencing Kit (Applied Biosystems™) for ABI 3500 Genetic Analyzer (Applied Biosystems™), according to the manufacturer’s guideline. Partial gene sequences obtained from isolates were assembled in a consensus sequence using the BioEdit program and further compared to sequences obtained from reference and type strains in the public database GenBank (www.ncbi.nlm.nih.gov). Sequence alignment was performed using the BioEdit program and analyzed with MEGA X software by using the Kimura Evolutionary distances substitution model (Kimura 1980). Phylogenetic reconstruction was performed using the neighbor-joining (NJ) algorithm (Saitou and Nei 1987) with bootstrap values calculated from 1000 replicate runs.
Sequence accession numbers of the 16S rDNA strains
The sequences obtained from the isolates sequencing were deposited in GenBank under the following accession codes: D2 (OK570084), AM1 (OK570085), RB1 (OK570086), C3 (OK570087), OL2 (OK570088), D1 (OK570089), L3 (OK570090), D3 (OK570091), CMC1 (OK570092) and NA1 (OK570093). D1-D3: methanogenic medium.
Metagenomic DNA extraction and 16S ribosomal gene sequencing.
Metabarcoding analyses were performed by an outsourced company, MicrobiomeX. DNA extraction was performed using the DNeasy Powersoil Pro kit (Qiagen), following the manufacturer's recommendations. The integrity and purity of the extracted DNA were verified in 0.8% agarose gel electrophoresis and in a NanoDrop1000 spectrophotometer (Thermo Fischer Scientific, Waltham, MA, USA), respectively. The hypervariable V4 region of the 16S gene was amplified using primers 515F (5ʹ GTGYCAGCMGCCGCGGTAA) and 806R (5ʹ GGACTACNVGGGTWTCTAAT) (Caporaso et al. 2010) and submitted to large-scale sequencing using the Illumina MiSeq platform (2 × 250 bp).
Bioinformatic analysis
The quality of raw sequences was checked using the FASTQ program version 0.11.5 (Andrews 2010). Primer sequences were removed by the Cutadapt tool (Martin 2011). Microbiome analyses were performed using the DADA2 tool version 1.18.0 (Callahan et al. 2016), comprising: removal of low-quality reads (phread < 20) and noise (denoising), joining of R1 sequences (forward) and R2 (reverse) removal of chimeras (using the consensus method) and assembly of representative sequences based on amplicon sequence variants (ASVs). Subsequently, the taxonomic classification was assigned using the SILVA ribosomal RNA gene database version 138 (Quast et al. 2013).
Statistical analysis
Statistical analyses were performed in the R statistical environment (v. 3.6.1) (R Development Core Team, 2014). The taxonomic table containing the count was imported along with the “metadata” file for analysis in the R Phyloseq package (McMurdie and Holmes 2013). Sequencing coverage was assessed by rarefaction analysis. Alpha diversity indices based on the Chao1 richness estimator (Chao 1984), the observed species and the Shannon–Wiener H' index were calculated by the R Phyloseq package. The microbial composition was expressed in relative abundance for all taxonomic levels.
Accession numbers
The metabarcoding raw sequence data are deposited in the European Nucleotide Archive under accession numbers: Sample ERS7624265 (SAMEA9945945) Inoculum.
Results and discussion
Isolation and phylogenetic analysis
The results of the present study showed the existence of a multiple bacterial community in the studied sample. A total of 30 bacteria were isolated from all culture media used (Table 1) except for the guaiacol-containing (which did not show bacterial growth). Among the culture media to isolate hydrolytic bacteria, the NA medium had the highest number of bacterial colonies (n = 10), followed by the LE medium (n = 5). Regarding the culture medium for isolation of anaerobic bacteria, the acidogenic medium (ACD) did not recover any bacteria, while the acetogenic (ACT) and methanogenic (MET) recovered 2 and 3 isolates, respectively. Biochemical analyses in CLED culture medium and the use of the 4 distinct groups of culture media, simulating the 4 phases of anaerobic digestion (hydrolytic, acidogenic, acetogenic and methanogenic), suggested the presence of 16 distinct ribotypes from the 30 isolates recovered from the starter, with the vast majority being Gram-positive bacteria (Table 1).
Ten of the 30 isolates recovered were sequenced. From the hydrolytic culture media, the following genera and/or species were identified: Lisinibacillus capsici., Luteimonas sp., Priestia sp., Bacillus sp., Rhodococcus sp., Niallia circulans, for the mediums LE, NA, RBBR, AM, CMC and OL, respectively. Regarding the culture medium presenting acetogenic conditions (ACT), a Robertmurraya siralis strain was identified, while the culture medium showing methanogenic conditions (MET) recovered the strains Vagococcus acidifermentans, Bacillus sp., and Proteinclasticum sp. (Table 1, Fig. 1).
The taxonomic groups identified in this study have already been reported in the literature, with strains involved in hydrolysis processes of compounds present in the metabolism and production of biogas. The genus Lysinibacillus have already been identified in a study of the characterization of the methanogenic microbial community in brewery wastewater samples (Murunga et al. 2016) as well as in samples of digestate associated with digestion processes using animal manure and food waste (Sun et al. 2020). The specie Lysinibacillus sphaericus has been reported as a strain capable of breaking down the complex structure of lignin (Persinoti et al. 2018; Rashid et al. 2017). Luteimonas species have been reported with the activity of Esterase (C4), β-Galactosidase, α and β-Glucosidase as well as strains were present in samples of biogas waste and organic manure (Pu et al. 2018; Roh et al. 2008).
Several Bacillus species including B. megaterium (currently known as Priestia megaterium), B. licheniformis, B. pumilus, B. brovis and B. alvei, have already been recovered from samples obtained of anaerobic digestion processes for biogas production (Biedendieck et al. 2021; Rabah et al. 2010). Bacillus genus is represented by mandatory or facultative aerobic species, and the species B. halodurans has been reported as a carbohydrate fermenter in high-temperature environments in an anaerobic biodigestion process in acidic phases (Ali Shah et al. 2014).
Yoon (2021), suggested a new potential species of the genus Bacillus (or proposed new genus Niallia), with the new species Niallia circulans, and no reports were found of the association of this new species with processes of anaerobic digestion and biogas production. Likewise, according to Gupta et al. (2020), representatives of the new genera Niallia gen. nov., Priestia gen. nov., Robertmurraya gen. nov, were reclassified from several Bacillus species after strong phylogenetic and molecular evidence using multiple phylogenetic trees on a genomic scale. Proteiniclasticum sp. and Clostridium sp. were observed in a study involving the use of peat soil, digested sludge, and ruminal fluid for simultaneous consumption of carbon dioxide and production of acetic acid in a biogas production process (Chaikitkaew et al. 2021).
The species Rhodococcus opacus PD630 has catabolic pathways and tolerance mechanisms for aromatic compounds present in ligninocellulosic material, including hexoses and pentoses, and can be considered a good candidate for hydrolysis of the material found in the starter (Anthony et al. 2019). Representatives of the genus Vagococcus were identified in a study addressing the genomic analysis of 16S rRNA in anaerobic digestion processes and were correlated with ammonia inhibition (Poirier et al. 2020). In addition, the first-time reported species Vagococcus acidifermentans was isolated from an acidogenic fermentation bioreactor in Naju province, South Korea, with the ability to ferment different sugars (Wang et al. 2011).
Metabarcoding analysis
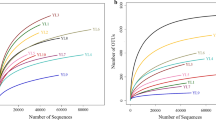
The initial metabarcoding analyses of the sample resulted in a total of 20.652 reads and, after quality processing (filtering, denoising, reads merging, and chimera removal), 16.377 final sequences were obtained. The rarefaction curve of the observed ASVs richness showed that it reached saturation, indicating that the sampling was efficient and capable of revealing almost all the prokaryote microbial species of the samples (Fig. 2). Shannon and Simpson’s index values 97 and 0.96, respectively, indicated great diversity in the samples.
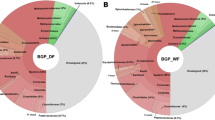
Metabarcoding results revealed great prokaryotic diversity and showed the presence of representatives of 16 different phyla, 14 from Bacteria and 2 from Archaea domain (Fig. 3). The most abundant phylum was Firmicutes (42.60%), followed by Bacteroidota (32.41%), Chloroflexi (11.38%) and Synergistota (4.07%). The archaea domain was represented by Halobacterota (77.71%) and Euryarchaeota (22.29%). The low archaeal diversity observed may be a result of the inability of prokaryotic primers to amplify archaea, as they are specific for the bacterial rRNA genes.
The main phyla found in our work have already been reported in other studies that evaluated the diversity in anaerobic digestion processes. In the work carried out by Nordgård et al. (2017), the authors observed that the Firmicutes phylum was more abundant in swine manure samples. Brandt et al. (2020), observed a greater abundance of representatives of the phyla Bacteroidota and Firmicutes in complex microbial communities associated with anaerobic digestion processes in different biogas and wastewater treatment plants. Representatives of the phylum Chloroflexi were found in large-scale anaerobic digesters with excess sludge capture from wastewater treatment plants (Petriglieri et al. 2018), while in processes used to understand the regulatory role of H2 in methane production in anaerobic digestion processes the presence of members of the phylum Synergistota was described (Kakuk et al. 2021). Representatives of methanogenic archaea are well known in biogas production processes. The archaeal phyla found in our study, Halobacterota and Euryarchaeota, have also been reported in other works involving anaerobic digestion and biogas production (Heitkamp et al. 2021; Zhang et al. 2020).
Firmicutes phylum has been well described in the literature as an important taxonomic group associated with anaerobic digestion processes as its representatives can express metabolic pathways involved in this process, such as at acetogenic phase, in the degradation of cellulosic compounds, with the formation of volatile acetic acid, CO2 and hydrogen (Mukhuba et al. 2020; Nordgård et al. 2017; Zhou et al. 2017). The most abundant genera belonging to the Firmicutes phylum observed in the sample were Enterococcus (17.27%), HN-HF0106 (15.32%), Clostridium sensu stricto 1 (15.28%), Syntrophomonas (4.4%) and a large abundance of unaffiliated bacteria NA (23.67%) (Fig. 4).
Watcharasukarn et al. (2009), performed a study where the ability to reduce pathogens from biogas plants was evaluated. In this study, Enterococcus species were used as biological indicators in treatments where the temperature exceeds 55 °C. The authors concluded that Enterococcus spp. can be resistant to different types of waste treatments, serving as biological indicators in biogas plants. Regarding the genus HN-HF0106, members of this group have been associated with cellulolytic activity, being able to use cellulose as a substrate with the production of H2 and acetate (Xie et al. 2021). In a work by Hahnke et al. (2014), carried out from a biogas production reactor fed with corn silage and wheat straw, found a new anaerobic hydrogen-producing mesophilic bacterium affiliated to the genus Clostridium sensu stricto (cluster I of the clostridia). This strain, cultivated in the presence of glucose, was able to produce H2, CO2, formate, lactate, and propionate, which are intermediate compounds to produce methane. In another work, the structures of the microbial community in biogas digesters with different types of waste, including cow, pig, sheep manure and human feces, were evaluated. Clostridium sensu stricto 1 represented the highest abundance in the digester with mixed raw materials including dairy cattle manure, sheep manure, and human feces (Han et al. 2021).
Wongfaed et al. (2020) evaluated the effect of the presence of oil and its derivatives (long-chain fatty acids) in palm oil factory effluent destined for methane production, as well as the structure of the microbial community. The authors observed cooperation between fatty acid degrading bacteria including Syntrophomonas sp. (strain capable of using long-chain fatty acids with more than 12 carbon atoms) and Acinetobacter sp., with H2- consuming methanogenic bacteria, including Methanococcus sp. and Methanogenium sp. The authors point out that the occurrence of this association in the normal AD process plays an important role in the degradation of oil and derivatives present in palm oil mill effluent.
The most abundant genus belonging to the phylum Bacteroidota was Ruminofilibacter (20.87%), while the main Chloroflexi genus was Longilinea (54%) and, for the phylum Synergistota, the most abundant group was Acetomicrobium (100%). There are no reports in the literature on the association between these genera in the production of biogas. In a work developed by Dong et al. (2019), genes from representatives of the genus Ruminofilibacter (related to cellulose degradation) were found in large quantities in the digestate after anaerobic digestion of cattle manure for biogas production. Yıldırım et al. (2017) evaluated the effects of bioaugmentation using anaerobic ruminal fungi on biogas production in anaerobic digesters fed with animal manure. In the study, the genera Clostridium and Longilinea were some of the most abundant observed in digesters, and the genus Clostridium has been reported to be important in the production of butanol, butyric acid, acetone and iso-propanol, intermediate compounds in this bioprocess. The authors also reported that these two genera were the ones with the greatest capacity to degrade animal waste, which provided higher methane yields.
Zhao et al. (2013) evaluated the dynamics of the microbial community in composting systems using biogas slurry compost and cow manure compost for biogas production. The authors adopted the denaturing gradient gel electrophoresis (DGGE) and gene clone library approaches, finding sequences associated with the Acetomicrobium genus after sequencing the clones. Representatives of the Acetomicrobium genus were reported as dominant in a dark fermentation process of fats and protein, using proteins as substrate (Litti et al. 2020). However, it is important to highlight that a large quantity of bacteria was not affiliated to any taxonomic group (NA = 42.16%), showing that a lot of information remains unknown and reinforcing the need for further studies to characterize the taxonomic groups associated with the starter studied here.
Regarding the archaeal sequences, representatives were found for the genera Methanosaeta and Methanobacterium, respectively, of the phyla of the Halobacterota and Euryarchaeota phyla in the starter, which have already been related to other processes of anaerobic digestion and biogas production. Representatives of the Methanosaeta genus maintained their dominance over other methanogenic groups in a study where acetoclastic methanogen groups able to act at low pH were acclimated to replace the use of NaOH to regulate buffer pH, a procedure that can inhibit methanogenic microorganisms (Ali et al. 2019). The acetoclastic methanogenic genus Methanosaeta has also been observed in other studies to improve biogas production (Zamorano et al. 2020; Chen et al. 2017). Concerning the genus Methanobacterium, representatives of this group were reported in a study that evaluated the production of biogas containing hydrogen and methane using Microbial Electrolysis Cell (He et al. 2021). In this work, the authors observed that through hydrogenotrophic methanogenesis, the group could synthesize CH4 using H2 and CO2.
The diversity of the microbial community found in anaerobic digestion processes is very diverse, and a large group of bacteria can be found in the organic substrates used in the system. From the beginning of the process, with the anaerobic degradation of organic substances, to the formation of biogas, there is the participation of a diverse microbial consortium, which includes fermentative bacteria, hydrogen-producing acetogenic bacteria, hydrogen-consuming acetogenic bacteria, carbon dioxide-reducing methanogens and acetoclastic methanogenic archaea (Lohani and Havukainen 2018).
The hydrolytic metabolism performed by enzymes such as amylases, lipases, ligninases, cellulases and proteases breaks down organic matter into simpler compounds, including sugars, amino acids, fatty acids, and peptides. This hydrolysis is generally carried out by the metabolic activity of anaerobic bacteria associated with the genera Streptococcus and enterobacteria (Kunz et al. 2019; Ali Shah et al. 2014), and these groups were found in our work, Enterococcus representing the most abundant enterobacteria, and Streptococcus in lesser abundance (0.42% of Firmicutes).
Metabolites formed by enzymatic hydrolysis are converted to other compounds in the acidogenic step. Glucose can be converted into lactic acid by Lactobacillus, and fatty acids can be degraded by Acetobacter species via β-oxidation, forming acetate. Likewise, amino acids are degraded by Clostridium species to form acetate, ammonia, carbon dioxide and hydrogen sulfide (Kunz et al. 2019). In our study, we found Clostridium, but it was not possible to observe Lactobacillus and Acetobacter. However, a relative abundance of Acetomicrobium was found, which can ferment glucose to acetate, CO2 and H2 (Hania et al. 2016), as well as the genus gene HN-HF0106 (Xie et al. 2021).
During the acidogenic step, further short-chain organic acids can be formed including formic, acetic, propionic, butyric and pentanoic acids, as well as alcohols (methanol, ethanol), aldehydes, carbon dioxide and hydrogen (Ali Shah et al. 2014). In our work, it was possible to isolate 3 distinct lactose fermenting morphotypes, two isolates recovered from the culture medium enriched with starch (01 Bacillus sp.) and one isolated from the culture medium enriched with olive oil, which proves that they are bacteria capable of fermenting simpler sugars and lipids via enzymatic hydrolysis. According to Westerholm and Schnürer (2019), the degradation of proteins and amino acids in anaerobic digesters has been shown to be carried out by several genera within the Firmicutes phylum (predominant in our work), which include Gram-positive bacilli.
In the methanogenesis stage (strictly anaerobic), the carbon contained in the biomass is converted into carbon dioxide and methane by methanogenic archaea. Acetoclastic methanogenic archaea, such as the genus Methanosarcina, convert acetate to methane, and the hydrogenotrophic methanogenic archaea, such as the genus Methanobacterium and Methanospirillum, convert hydrogen and carbon dioxide to methane (Kunz et al. 2019). Our findings corroborate those reported by Kunz et al. (2019) in view of the methanogenic representatives, including Methanobacterium in the inoculum sample studied in the present work.
The analysis of parameters found for volatile solids, volatile organic acids (FOS) and total inorganic carbon (TAC) show the rich nutritional composition of the evaluated substrate (carbon sources) for the development of the microbial community studied (Cerqueira et al. 2011). The concentrations of volatile solids, FOS, and TAC, found in the inoculum were 659.10 g kg−1, 717.70 g kg−1, 70,005.0 g kg−1, respectively, which correspond to a large amount of material, including volatile organic acids (acetic, propionic, and butyric acids) and inorganic carbon (Cerqueira et al. 2011). pH can influence microbial growth inside the biodigester. On the day of inoculum collection, the pH was 7.6, which may favor the growth of methanogenic archaea, whose optimal pH for development is 6.7 to 7.5. However, fermentative bacteria can adapt to pH variations between 4.0 and 8.5 (Ali Shah et al. 2014).
Thus, we can say that the methodology adopted in this study was able to recover hydrolytic bacteria, such as proteolytic, ligninolytic, amylolytic and cellulolytic bacteria, capable of hydrolyzing protein, lignin, starch, and cellulose that may be present in the inoculum composition, as well as bacteria of the acetogenic phase. However, it was not possible to isolate methanogenic archaea using the media defined for this purpose. This limitation was overcome by using the combination of culture-dependent (enrichment and isolation) and culture-independent (metabarcoding) methods, which allowed access to a greater amount of information about the microbial diversity associated with the anaerobic digestion process (starter). The methods were complementary, as with culture-dependent methods it was possible to isolate representative strains of AD, which were not observed in the culture-independent method and vice versa. Thus, we can conclude that the adoption of both approaches to characterize the microbial community in samples of AD processes is integrative and provides information of great relevance for understanding the microbial function and dynamics in the different stages of biogas production.
References
Aamir S, Sutar S, Sk S, Baghela A (2015) A rapid and efficient method of fungal genomic DNA extraction, suitable for PCR based molecular methods. Plant Pathol Quarant 5:74–81. https://doi.org/10.5943/ppq/5/2/6
Agustini CB (2014). Isolamento microbiano na biodegradação de resíduos de curtumes.
Ali S, Hua B, Jeanne J et al (2019) Bioresource Technology Effect of different initial low pH conditions on biogas production, composition, and shift in the aceticlastic methanogenic population. Bioresour Technol 289:121579. https://doi.org/10.1016/j.biortech.2019.121579
Ali Shah F, Mahmood Q, Maroof Shah M, Pervez A, Ahmad Asad S (2014) Microbial ecology of anaerobic digesters: the key players of anaerobiosis. Sci World J
Andrews S (2017) FastQC: a quality control tool for high throughput sequence data
Anguita-Maeso M, Olivares-García C, Haro C et al (2020) Culture-dependent and culture-independent characterization of the olive xylem microbiota: effect of sap extraction methods. Front Plant Sci. https://doi.org/10.3389/fpls.2019.01708
Anthony WE, Carr RR, Delorenzo DM et al (2019) Development of Rhodococcus opacus as a chassis for lignin valorization and bioproduction of high-value compounds. Biotechnol Biofuels 12:1–14. https://doi.org/10.1186/s13068-019-1535-3
APHA. American Publish Health Association (2017) Standard methods for the examination of water and wastewater, 23rd edn. American Water Works Association, Washington
Belgini DRB, Dias RS, Siqueira VM et al (2014) Culturable bacterial diversity from a feed water of a reverse osmosis system, evaluation of biofilm formation and biocontrol using phages. World J Microbiol Biotechnol 30:2689–2700. https://doi.org/10.1007/s11274-014-1693-1
Biedendieck R, Knuuti T, Moore SJ, Jahn D (2021) The “beauty in the beast”—the multiple uses of Priestia megaterium in biotechnology. Appl Microbiol Biotechnol 105:5719–5737. https://doi.org/10.1007/s00253-021-11424-6
Agustini CB (2014) Isolamento microbiano na biodegradação deresíduos de curtumes
Borges AC (2014) Isolamento microbiano na biodegradação de resíduos de cortume Dissertação. Universidade Federal do Rio Grande do Sul, Escola de Engenharia
Brandt C, Bongcam-Rudloff E, Müller B (2020) Abundance tracking by long-read nanopore sequencing of complex microbial communities in samples from 20 different biogas/wastewater plants. Appl Sci 10:1–14. https://doi.org/10.3390/app10217518
Callahan BJ, McMurdie PJ, Rosen MJ et al (2016) DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. https://doi.org/10.1038/nmeth.3869
Caporaso JG, Kuczynski J, Stombaugh J et al (2010) correspondence QIIME allows analysis of high- throughput community sequencing data Intensity normalization improves color calling in SOLiD sequencing. Nat Publ Gr 7:335–336. https://doi.org/10.1038/nmeth0510-335
Cerqueira MBR, Dias AN, Caldas SS et al (2011) Validação de método para determinação de ácidos orgânicos voláteis em efluentes de reatores anaeróbios empregando cromatografia líquida. Quim Nova 34:156–159. https://doi.org/10.1590/s0100-40422011000100029
Chaikitkaew S, Seengenyoung J, Mamimin C et al (2021) Simultaneous biogas upgrading and acetic acid production by homoacetogens consortium enriched from peatland soil. Bioresour Technol Reports 15:100701. https://doi.org/10.1016/j.biteb.2021.100701
Chao A (1984) Nonparametric estimation of the number of classes in a population. Scand J Stat 11:265–270. https://doi.org/10.2307/4615964
Chen S, Cheng H, Liu J et al (2017) Unexpected competitiveness of Methanosaeta populations at elevated acetate concentrations in methanogenic treatment of animal wastewater. Appl Microbiol Biotechnol 101:1729–1738. https://doi.org/10.1007/s00253-016-7967-9
Chernicharo CAL. Reatores anaeróbios. 2.ed. Belo Horizonte: DESA, UFMG, 2007. 380p. (Princípios do Tratamento Biológico de Águas Residuárias, v.5)
Covey KR, Megonigal JP (2019) Methane production and emissions in trees and forests. New Phytol 222(1):35–51. https://doi.org/10.1111/nph.15624
Dong L, Cao G, Guo X et al (2019) Efficient biogas production from cattle manure in a plug flow reactor: a large scale long term study. Bioresour Technol 278:450–455. https://doi.org/10.1016/j.biortech.2019.01.100
Ferry JG, Smith PH, Wolfe RS (1974) Methanospirillum, a new genus of methanogenic bacteria, and characterization of Methanospirillum hungatii sp.nov. Int J Syst Bacteriol 24:465–469. https://doi.org/10.1099/00207713-24-4-465
Gielen D, Boshell F, Saygin D et al (2019) The role of renewable energy in the global energy transformation. Energy Strateg Rev 24:38–50. https://doi.org/10.1016/j.esr.2019.01.006
Gulhane M, Pandit P, Khardenavis A et al (2017) Study of microbial community plasticity for anaerobic digestion of vegetable waste in Anaerobic Baffled Reactor. Renew Energy 101:59–66. https://doi.org/10.1016/j.renene.2016.08.021
Gupta RS, Patel S, Saini N, Chen S (2020) Robust demarcation of 17 distinct Bacillus species clades, proposed as novel Bacillaceae genera, by phylogenomics and comparative genomic analyses: Description of Robertmurraya kyonggiensis sp. nov. and proposal for an emended genus Bacillus limiting it only to the members of the Subtilis and Cereus clades of species. Int J Syst Evol Microbiol 70:5753–5798. https://doi.org/10.1099/ijsem.0.004475
Hahnke S, Striesow J, Elvert M et al (2014) Clostridium bornimense sp. nov., isolated from a mesophilic, two-phase, laboratory-scale biogas reactor. Int J Syst Evol Microbiol 64:2792–2797. https://doi.org/10.1099/ijs.0.059691-0
Han R, Liu L, Meng Y et al (2021) Archaeal and bacterial community structures of rural household biogas digesters with different raw materials in Qinghai Plateau. Biotechnol Lett 43:1337–1348. https://doi.org/10.1007/s10529-021-03105-1
Hania WB, Bouanane-Darenfed A, Cayol JL et al (2016) Reclassification of Anaerobaculum mobile, Anaerobaculum thermoterrenum, Anaerobaculum hydrogeniformans as Acetomicrobium mobile comb. nov., Acetomicrobium thermoterrenum comb. nov. and Acetomicrobium hydrogeniformans comb. nov., respectively, and emendation of the genus Acetomicrobium. Int J Syst Evol Microbiol 66:1506–1509. https://doi.org/10.1099/ijsem.0.000910
He C, Zhang B, Jiang Y et al (2021) Microbial electrolysis cell produced biogas as sustainable electron donor for microbial chromate reduction. Chem Eng J 403:126429. https://doi.org/10.1016/j.cej.2020.126429
Heitkamp K, Latorre-Pérez A, Nefigmann S et al (2021) Monitoring of seven industrial anaerobic digesters supplied with biochar. Biotechnol Biofuels 14:1–14. https://doi.org/10.1186/s13068-021-02034-5
Kakuk B, Wirth R, Maróti G et al (2021) Early response of methanogenic archaea to H2 as evaluated by metagenomics and metatranscriptomics. Microb Cell Fact. https://doi.org/10.1186/s12934-021-01618-y
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. https://doi.org/10.1007/BF01731581
Knoblauch C, Beer C, Liebner S et al (2018) Methane production as key to the greenhouse gas budget of thawing permafrost. Nat Clim Chang. https://doi.org/10.1038/s41558-018-0095-z
Kunz A, Steinmetz RLR, do Amaral AC (2019) Fundamentos da digestão anaeróbia, purificação do biogás, uso e tratamento do digestato- Concórdia. Embrapa Suínos e Aves, Sbera, p 209
Litti YV, Kovalev DA, Kovalev AA, Merkel AY, Vishnyakova AV, Russkova YI, Nozhevnikova AN (2021) Auto-selection of microorganisms of sewage sludge used as an inoculum for fermentative hydrogen production from different substrates. Int J Hydrogen Energy 46(58):29834–29845. https://doi.org/10.1016/j.ijhydene.2021.06.174
Lohani SP, Havukainen J (2018) Anaerobic digestion: factors affecting anaerobic digestion process. Energy Environ Sustain. https://doi.org/10.1007/978-981-10-7413-4_18
Manimegalai R, Gopinath LR, Christy PM, Divya D (2014) Isolation and identification of acetogenic and methanogenic bacteria from anoxic black sediments and their role in biogas production. Int J Plant Anim Environ Sci 4(3):156–164
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet J 17(1):10–12
Mcmurdie PJ, Holmes S (2013) phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE. https://doi.org/10.1371/journal.pone.0061217
Mukhuba M, Roopnarain A, Moeletsi ME, Adeleke R (2020) Metagenomic insights into the microbial community and biogas production pattern during anaerobic digestion of cow dung and mixed food waste. J Chem Technol Biotechnol 95:151–162. https://doi.org/10.1002/jctb.6217
Murunga SI, Duncan OM, Ayub NG et al (2016) Isolation and characterization of methanogenic bacteria from brewery wastewater in Kenya. African J Biotechnol 15:2687–2697. https://doi.org/10.5897/ajb2016.15551
Muturi SM, Muthui LW, Njogu PM et al (2021) Metagenomics survey unravels diversity of biogas microbiomes with potential to enhance productivity in Kenya. PLoS ONE 16:e0244755. https://doi.org/10.1371/journal.pone.0244755
Nordgård ASR, Bergland WH, Vadstein O et al (2017) Anaerobic digestion of pig manure supernatant at high ammonia concentrations characterized by high abundances of Methanosaeta and non-euryarchaeotal archaea. Sci Rep 7:1–14. https://doi.org/10.1038/s41598-017-14527-1
Orhorhoro EK, Ebunilo PO, Sadjere GE (2018) Effect of organic loading rate (OLR) on biogas yield using a single and three-stages continuous anaerobic digestion reactors. Int J Eng Res Africa 39:147–155. https://doi.org/10.4028/www.scientific.net/JERA.39.147
Persinoti GF, Paixão DAA, Bugg TDH, Squina FM (2018) Genome sequence of Lysinibacillus sphaericus, a lignin-degrading bacterium isolated from municipal solid waste soil. Genome Announc 6:1–2. https://doi.org/10.1128/genomeA.00353-18
Petriglieri F, Nierychlo M, Nielsen PH, McIlroy SJ (2018) In situ visualisation of the abundant Chloroflexi populations in full-scale anaerobic digesters and the fate of immigrating species. PLoS ONE 13:1–14. https://doi.org/10.1371/journal.pone.0206255
Poirier S, Déjean S, Midoux C et al (2020) Integrating independent microbial studies to build predictive models of anaerobic digestion inhibition by ammonia and phenol. Bioresour Technol 316:123952. https://doi.org/10.1016/j.biortech.2020.123952
Pu C, Liu L, Yao M et al (2018) Responses and successions of sulfonamides, tetracyclines and fluoroquinolones resistance genes and bacterial community during the short-term storage of biogas residue and organic manure under the incubator and natural conditions. Environ Pollut 242:749–759. https://doi.org/10.1016/j.envpol.2018.07.063
Quast C, Pruesse E, Yilmaz P et al (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:590–596. https://doi.org/10.1093/nar/gks1219
Rabah A, Baki A, Hassan L et al (2010) Production of biogas using abattoir waste at different retention time. Sci Wold J 5:23–26
Rashid GMM, Durán-Peña MJ, Rahmanpour R et al (2017) Delignification and enhanced gas release from soil containing lignocellulose by treatment with bacterial lignin degraders. J Appl Microbiol 123:159–171. https://doi.org/10.1111/jam.13470
Ren NQ, Chua H, Chan SY et al (2007) Assessing optimal fermentation type for bio-hydrogen production in continuous-flow acidogenic reactors. Bioresour Technol 98:1774–1780. https://doi.org/10.1016/j.biortech.2006.07.026
Roh SW, Kim KH, Do NY et al (2008) Luteimonas aestuarii sp. nov., isolated from tidal flat sediment. J Microbiol 46:525–529. https://doi.org/10.1007/s12275-008-0189-9
Saitou N, Nei M (1987) ESCALA CIWA-AR Escala CIWA-Ar (Clinical Institute Withdrawal Assesment for Alcohol) Evaluación del Síndrome de Abstinencia Alcohólica. Mol Biol Evol 4:406–425
Soares CMT, Feiden A, Tavares SG (2017) Fatores que influenciam o processo de digestão anaeróbia na produção de biogás Factors that influence the anaerobic digestion process in biogas production. Rev Nativ 5:522–528
Sun H, Bjerketorp J, Levenfors JJ, Schnürer A (2020) Isolation of antibiotic-resistant bacteria in biogas digestate and their susceptibility to antibiotics. Environ Pollut 266:115265
The Scientific World Journal (2017) Retracted: microbial ecology of anaerobic digesters: the key players of anaerobiosis. Sci World J 2017:3852369. https://doi.org/10.1155/2017/3852369
Vale M, Mateus MM, Galhano dos Santos R et al (2019) Replacement of petroleum-derived diols by sustainable biopolyols in one component polyurethane foams. J Clean Prod 212:1036–1043. https://doi.org/10.1016/j.jclepro.2018.12.088
Valijanian E, Meisam T, Mortaza A, Alawi S, Yusuf C (2018) Biogas production systems. In: Tabatabaei M, Ghanavati H (eds) Biogas: fundamentals, process and operation. Springer, Cham, pp 95–116
Vrieze J, Verstraete W (2016) Perspectives for microbial community composition in anaerobic digestion: from abundance and activity to connectivity. Environ Microbiol 18:2797–2809. https://doi.org/10.1111/1462-2920.13437
Wang L, Cui YS, Kwon CS, Lee ST, Lee JS, Im WT (2011) Vagococcus acidifermentans sp. nov., isolated from an acidogenic fermentation reactor. Int J Syst Evol Microbiol 61(5):1123–1126. https://doi.org/10.1099/ijs.0.022087-0
Watcharasukarn M, Kaparaju P, Steyer JP, Krogfelt KA, Angelidaki I (2009) Screening Escherichia coli, Enterococcus faecalis, and Clostridium perfringens as indicator organisms in evaluating pathogen-reducing capacity in biogas plants. Microb Ecol 58(2):221–230. https://doi.org/10.1007/s00248-009-9497-9
Wei Y, Wang F, Gao J, Huang Y, Ren W, Sheng H (2021) Culture-dependent and culture-independent characterization of bacterial community diversity in different types of sandy lands: the case of Minqin County, China. BMC Microbiol 21(1):1–15. https://doi.org/10.1186/s12866-021-02150-0
Westerholm M, Schnürer A (2019) Microbial responses to different operating practices for biogas production systems. Anaerob Dig. https://doi.org/10.5772/intechopen.82815
Wongfaed N, Kongjan P, Prasertsan P, Sompong O (2020) Effect of oil and derivative in palm oil mill effluent on the process imbalance of biogas production. J Clean Prod 247:119110. https://doi.org/10.1016/j.jclepro.2019.119110
Xie Z, Meng X, Ding H, Cao Q, Chen Y, Liu X, Li D (2021) The synergistic effect of rumen cellulolytic bacteria and activated carbon on thermophilic digestion of cornstalk. Biores Technol 338:125566. https://doi.org/10.1016/j.biortech.2021.125566
Xu Q, Luo TY, Wu RL, Wei W, Sun J, Dai X, Ni BJ (2021) Rhamnolipid pretreatment enhances methane production from two-phase anaerobic digestion of waste activated sludge. Water Res 194:116909. https://doi.org/10.1016/j.watres.2021.116909
Yan BH, Selvam A, Wong JW (2020) Bio-hydrogen and methane production from two-phase anaerobic digestion of food waste under the scheme of acidogenic off-gas reuse. Biores Technol 297:122400. https://doi.org/10.1016/j.biortech.2019.122400
Yıldırım E, Ince O, Aydin S, Ince B (2017) Improvement of biogas potential of anaerobic digesters using rumen fungi. Renewable Energy 109:346–353. https://doi.org/10.1016/j.renene.2017.03.021
Yoon YM, Kim M, Kim ET, Park SJ (2021) Draft genome sequence of Bacillus sp. strain B1–b2 isolated from meconium. Microbiol Soc Korea 57(1):55–57
Zamorano-López N, Borrás L, Seco A, Aguado D (2020) Unveiling microbial structures during raw microalgae digestion and co-digestion with primary sludge to produce biogas using semi-continuous AnMBR systems. Sci Total Environ 699:134365. https://doi.org/10.1016/j.scitotenv.2019.134365
Zhang J, Zhang R, He Q, Ji B, Wang H, Yang K (2020) Adaptation to salinity: response of biogas production and microbial communities in anaerobic digestion of kitchen waste to salinity stress. J Biosci Bioeng 130(2):173–178. https://doi.org/10.1016/j.jbiosc.2019.11.011
Zhao HY, Jie LI, Liu JJ, Lü YC, Wang XF, Cui ZJ (2013) Microbial community dynamics during biogas slurry and cow manure compost. J Integr Agric 12(6):1087–1097. https://doi.org/10.1016/S2095-3119(13)60488-8
Zhou J, Yang J, Yu Q, Yong X, Xie X, Zhang L, Jia H (2017) Different organic loading rates on the biogas production during the anaerobic digestion of rice straw: a pilot study. Biores Technol 244:865–871. https://doi.org/10.1016/j.biortech.2017.07.146
Acknowledgements
We thank the International Center for Renewable Energies—Biogás supported by Itaipu Binacional (supported by Itaipu Technological Park Foundation), EDITAL PRPPG Nº 105/2020—Latin America and the Caribbean Priority Institutional Program and EDITAL PRPPG n° 80/2019—Researcher Integration Assistance Program (PAIP).
Funding
Financial support granted by International Center for Renewable Energies – Biogás, supported by Itaipu Binacional; Latin American and the Caribbean Priority Institutional Program (PRPPG 105/2020), Researcher Integration Assistance Program (PAIP)(Edital PRPPG nº80/2019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ottoni, J.R., Bernal, S.P.F., Marteres, T.J. et al. Cultured and uncultured microbial community associated with biogas production in anaerobic digestion processes. Arch Microbiol 204, 340 (2022). https://doi.org/10.1007/s00203-022-02819-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-02819-8