Abstract
Tuber rot disease due to phytopathogen Fusarium oxysporum f. sp. cepae (Foc) infection is one of the main factors causing the decreasing global onions production. This study aims to find bacteria and fungi candidates with Foc antagonistic activity through in vitro tests using dual culture techniques. A total of three bacterial isolates and three fungal isolates isolated from the rhizosphere of healthy onion plants showed the ability to inhibit Fusarium oxysporum growth. LC648364 isolate had an average inhibitory capability of 65.93%. At the same time, LC648367 and LC648368 fungal isolates can inhibit the growth of F. oxysporum by as much as 74.82% and 67.76%, respectively. Molecular analysis based on 16S rRNA markers showed three isolates belonging to the Bacillus. The LC648364 isolates are closely related to species Bacillus sp. strain LLB-17, LC648365 is closely related to B. subtilis strain S11 and LC648366 is closely related to B. cereus strain EM6. For the fungi, based on internal transcribed spacer (ITS) gene markers, there are three isolates. The LC648367 isolate is closely related to Aspergillus tubingensis, LC648368 is closely related to Trichoderma asperellum and LC648369 is closely related to Issatchenkia orientalis. This study can be used to develop indigenous microbial consortiums as biological control agents for phytopathogenic fungi Fusarium tuber rot on onion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Onions (Allium cepa var ascalonicum L) are one of the world’s main commodities with production reaching 96.77 million tons per year. However, productivity fluctuates almost every year. In Indonesia, several regions show fluctuations in the amount of production each year (BPS 2018). Various factors, especially unfavorable environments, such as drought, salinity, climate, nutritional imbalance and plant diseases, are the main obstacles in the production of onions (Abdelrahman et al. 2016). Among a number of diseases caused by pathogens, Fusarium tuber rot or wilt disease caused by Fusarium oxysporum f. sp. cepae (Foc) is the most damaging and a serious threat to onion production worldwide (Abdelrahman et al. 2016; Chand et al. 2017; Kalman et al. 2020). Symptoms caused by Foc include plants wilting rapidly, newly formed leaves curling and turning yellow, plants almost collapsing, white fungi colonies appearing at the base of the rotting layered bulb (Brayford 1996; Taylor et al. 2016). Foc is a pathogenic fungus that can infect a very wide range of plants as the hosts (Summerell et al. 2011; Armitage et al. 2018). This fungus can form chlamydospores so that it can last a long time in the soil (Brayford 1996; Cremer 2000; Kalman et al. 2020).
Management of Fusarium tuber rot or wilt disease can be focused on integrating different prevention methods, including the use of mixed crops, crop rotation systems, use of pathogen-resistant cultivars, use of chemical fungicides and the use of biological agents (Mc Govern 2015; Gupta et al. 2020). In practice, the use of synthetic fungicides by onion farmers has not been fully effective because of the residue left on crops, environmental pollution, and killing other organisms that are not targeted. Moreover, the continuous use of synthetic fungicides can lead to the emergence of resistant pathogenic populations (Mehnaz et al. 2013; Fournier et al. 2020; Tleuova et al. 2020). Biological control using microbes that are antagonistic to pathogenic fungi is the right alternative because it does not have a negative impact on the environment (Lecomte et al. 2016; Jamil et al. 2020; Kalman et al. 2020).
Utilization of microbes as biological control agents ideally uses the potential of indigenous natural enemies with the hope that these microbes will work more effectively and are supported by appropriate environmental factors, do not cause changes in ecosystems, and are cheaper to formulate (Kalman et al. 2020). Therefore, the diversity of microbes from the root area and their propagation followed by their release back into the rhizosphere is a conservation measure that will provide promising prospects for biological disease control (Raaijmakers et al. 2009; Kandel et al. 2017.).
In the last decade, research on biocontrol and microbial metabolite products for pest and pathogen control has intensified (Jangir et al. 2018). Generally, this microbial group belongs to the genera Bacillus, Pseudomonas, Streptomyces and Trichoderma (Ramyabharathi et al. 2020; Jangir et al. 2018; Kalman et al. 2020). This group of microbes is able to act as a biocontrol agent in reducing pathogenicity through a number of mechanisms, such as antibiotic production, root colonization, induction of systemic resistance systems in the host, production of extracellular cell wall breakdown enzymes and formation of resistant spores (Ongena and Jacques 2008; Beneduzi et al. 2012). A number of studies have reported that the application of microbes, both bacteria and fungus, is effective in suppressing the growth of Fusarium pathogens, including using Bacillus sp. (Jangir et al., 2018), Pseudomonas aeruginosa DRB1 and Trichoderma harzianum CBF2 antagonist Foc Tropical Race 4 (Foc‐TR4) (Wong et al. 2019). Further, Khan et al. (2020a) report that secondary metabolites produced by Trichoderma spp., such as harzianolides, peptaibols, gliotoxin, trichokonin, and several volatile compounds, have functioned as antifungal, stimulating plant growth and increasing resistance to pathogens.
This study aims to evaluate the antagonistic activity of indigenous microbial strains isolated from onion growing areas in Enrekang Regency, South Sulawesi, Indonesia. In vitro analysis was conducted using Fusarium isolates which were isolated from onion plants showing symptoms of tuber rot. All isolates that showed potential in inhibiting the growth of the F. oxysporum pathogen were identified molecularly using specific primers for the 16S rRNA gene and the nuclear ribosomal internal transcribed spacer (ITS) region using specific primers ITS1 and ITS4. The isolates obtained are expected to be able to contribute to the inventory of genetic diversity in the region, with possible future applications for the control of Fusarium pathogens in plants, especially in onion.
Materials and methods
Isolation of Fusarium tuber rot
Fusarium tuber rot were isolated from onion rhizosphere soil samples which showed tuber rot symptoms in the onion cultivation area in Enrekang regency. The isolation was carried out based on techniques described in Miao et al. (2016) using potato dextrose agar medium (PDA, Merck) and incubated for 5 to 7 days at 25 ± 2 °C. Isolates were determined based on their microscopic morphological characteristics. Microscopic observation using the fungal slide culture method was used to observe the hyphae growth under a microscope (Harris 1986).
Fusarium-antagonist bacterial and fungal isolations
Fusarium tuber rot-antagonist bacteria and fungi were both isolated from healthy rhizosphere areas of onion plants by the serial dilution method. The rhizosphere bacteria isolation technique is based on Jangir et al. (2018) with modifications. The dilution results were grown in Nutrient Agar (Merck) medium at 30 °C for 48 h, whereas the fungal isolation technique is based on Miao et al. (2016) by growing the results of 10–3 dilution in PDA medium at 25 ± 2 °C for 5–7 days. Next, the bacterial and fungal isolates were purified in the same medium and maintained at 4 °C. Further preservation used glycerol stock (25%) and was stored at a temperature of –80 °C. All the isolates which were successfully identified were characterized based on morphological, biochemical parameters and molecular identification.
In vitro tests of Fusarium-antagonist isolates
Fusarium tuber rot-antagonist microbes screening was conducted using the dual culture method (Skidmore and Dickinson 1976). A culture block with a diameter of 8 mm from antagonist isolate and another from Fusarium isolate was placed opposite to each other in a PDA medium, 3 cm away from the edge of the Petri dish. As a control, a single Fusarium culture disk was placed alone in another Petri dish without the antagonist isolate. The Petri dish was then incubated at a temperature of 25 ± 2 °C for 5–7 days. Observation of growth inhibition (GI) was done every two days. Observation was terminated when the colony in the control reached maximum growth. The percentage of GI was calculated using the formula:
In which, R1 is the radius of radial growth to the opposite direction in the control Petri dish and R2 is the radius of radial growth in the treated petri dish. The tests were done three times to acquire the mean of the inhibition zone for each isolate.
The GI data were analyzed using one-way ANOVA with values α = 0.05 and n = 3.
DNA extraction and PCR amplification
Isolation of fungal genomic DNA was carried out using the Plant Genomic DNA Mini Kit (Geneaid) in accordance to the manufacturer's standard protocol. The nuclear ribosomal internal transcribed spacer (ITS) region was amplified using a universal primer set (ITS 1: 5’-TCC GTA GGT GAA CCT GCG G-3’ and ITS 4: 5’-TCC TCC GCT TAT TGA TAT GC-3’) (White et al. 1990). The PCR reaction consisted of 1 µl DNA template (100 ng/µl), 5 µl NZYTaq II 2× Green Master Mix, 0.25 µl ITS 1 primer (10 pmol/ µl), 0.25 µlITS 4 primer (10 pmol/ µl), 3.5 µl dH2O so that the total reagent volume was 10 µl. PCR was run with a thermal cycler for pre-denaturation at 95 °C for 5 min, for denaturation at 95 °C for 30 s, for annealing at 52 °C for 30 s, for extension at 72 °C for 30 s, the reaction being repeated for 35 cycles, and post-PCR at 72 °C for 5 min.
The total bacterial genome was isolated using Presto™ Mini gDNA Kit (Geneaid). According to the manufacturer's protocol, the 16S rRNA gene amplification was performed using specific primer pairs (63 F: 5’-CAG GCC TAA CAC ATG CAA GTC-3’ and 1387 R: 5’-GGG CGG WTG GTA CAA GGC-3’). The mix composition and PCR program were made the same as the ITS gene amplification procedure in fungi. PCR products were analyzed using 1% agarose gel in 1 × TAE buffer. The gel was then electrophoresed at a voltage of 100 V for 28 min and stained using ethidium bromide staining. The visualization of the electrophoresis results was carried out using a UV-Transilluminator. PT Bioneer Indonesia conducted the PCR product sequencing.
Construction of phylogenetic trees
The 16S and ITS sequences for all bacteria and fungi were constructed to determine their evolutionary relationships based on phylogenetic analysis. Multiple sequence alignments were performed using Bio Edit's CLUSTAL W program. Phylogenetic tree construction was carried out using the neighbor-joining method from the MEGA version 10.0 program. Each clade obtained was then determined using bootstrap analysis with 1000 replications and then Kimura's two-parameter model was used. The nucleotide sequences in this study have been deposited in the DNA databank of Japan (DDBJ, URL: http://www.ddbj.nig.ac.jp/) under Accession No. LC648364 through LC648369.
Results
Isolation and identification of fungal pathogens
The isolates suspected as Fusarium were isolated from the rhizosphere of the onion plants which showed tuber rot symptoms. Observation of the morphology of fungal isolates was based on the characteristics described which include parameters of color, colony, texture, and air hyphae. All parameters showed characteristics matching Fusarium oxysporum. Furthermore, the observations showed that on the upper surface, the mycelium was purple, while the lower surface was white. In addition, microscopic characteristics, such as macroconidia, microconidia and chlamydospores, were successfully observed under a microscope at magnification of 400× (Fig. 1) with the appearance of a colorless round microconidium, and a crescent-shaped macroconidium that was colorless and had 3–5 septa while chlamydospores are single-celled.
Morphological and microscopic characteristics of 7-day-old F. oxysporum isolated from the rhizosphere of onion plants. a The upper surface of the colony; b the basal surface of the colony; c microconidia (1) and macroconidia (2) microscope observation at × 400 magnification; d Chlamydospores (× 400 magnification)
Further identification was carried out by molecular method and based on the results of sequencing analysis has been confirmed that the pathogen isolated is Fusarium oxysporum which has 96.6 % similarity identity to Fusarium oxysporum strain KG_26 (Fig. 2).
In vitro tests of antagonist microbes isolates
A total of three fungi isolates and three bacterial isolates were isolated from rhizosphere soil samples. From the results of initial in vitro testing against Fusarium oxysporum, three isolates of fungi and three isolates of bacteria showed inhibitory activity reaching 50% against F. oxysporum mycelium growth. In the second test, the percentage of inhibitory potential was measured against the growth of F. oxysporum grown in dual culture with the test isolate. Tests were carried out three times to determine the average inhibition. From the results of analysis of variance (ANOVA) on all isolates, it was found that almost all tested microbes had inhibitory activity above 50%. Isolate LC648367 showed the highest inhibitory activity of F. oxysporum with an average of 74.82%, whereas the inhibitory activity of fungi against F. oxysporum was discovered to have a higher growth rate than the bacterial activity. The lowest inhibitory activity was shown by the LC648369 fungal isolate with an inhibition value of 41.12%. All data are presented in Table 1.
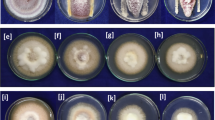
The capability of bacteria and fungi to inhibit F. oxysporum growth seems to be correlated with different growth rates. Visually, the growth of fungi in colonizing the growth medium was seen to be faster than bacteria (Fig. 3). From all isolates of bacteria and fungi, LC648364 and LC648367 can be considered to have the best potential as antagonists in suppressing F. oxysporum growth in vitro.
Further analysis was carried out to determine the capability of the isolates to suppress the growth of F. oxysporum mycelium. Microscopic observations were carried out on the outer part of the F. oxysporum mycelium growth zone. From the observations, it was found that hyphae damage occurred which is assumed to have been due to the activity of the antifungal compounds produced. In contrast to the control, hyphae in F.oxysporum were seen to undergo fragmentation (Fig. 4).
Molecular identification of bacterial and fungal isolates
The three bacterial isolates were analyzed molecularly to identify species based on their evolutionary relationships. The phylogenetic tree construction from the alignment results of 16S gene amplification products with the GenBank database showed that all F. oxysporum antagonist bacteria were related to the genus Bacillus and all of them belong to different species evolutionarily (Fig. 5). There are three isolates belonging to the genus Bacillus. The LC648364 isolate is closely related to species Bacillus sp. strain LLB-17 with a gene similarity rate of 96%, LC648365 is closely related to B. subtilis strain S11 with the 97 % similarity identity and LC648366 showed a closer relationship with species B. cereus strain EM6 with level of 97 %.
The results of the BLAST analysis were different for each fungal sample. From the results of phylogenetic constructs, it was found that the LC648367, LC648368, and LC648369 samples were of different species. The LC648367 isolate is closely related to Aspergillus tubingensis with a similarity rate of 99.6%, LC648368 is closely related to Trichoderma asperellum with a similarity 99.2% and LC648369 is closely related to Issatchenkia orientalis with a similarity level of 99.2% (Fig. 6).
Discussion
Fusarium oxysporum f. sp. cepae (Foc) is one of the most severe diseases (Cramer 2000; Wang et al. 2019) which affects all phases of plant development at pre- and post-harvest stages from damping off and delayed seedling emergence to bulb rot (Galeano et al. 2014; Wang et al. 2019). Warm temperatures (28–32 °C optimum) can induce infection and climate change will predict increase it (Cramer 2000). Fusarium oxysporum was white floccose mycelia. Some isolates produced dark violet pigment in the agar (this character was observed for 1H (less virulent) and 13 M (avirulent) isolates). Microconidia were formed in false heads on short monophialides. Thin-walled macroconidia were approximately straight and slightly tapered at the ends (Ghanbarzadeh et al. 2014).
Fusarium wilt or tuber rot in onion plants due to Foc infection causes enormous losses annually to global agriculture. It can survive in the soil for many years because like many other Fusarium species, Foc produces resilient, long-lived chlamydospores (Brayford 1996; Cramer 2000; Armitage et al. 2018) so that the treatment using synthetic fungicide is not entirely effective (Fig. 1d) which is resistant to extreme environmental stress (Gupta et al. 2020). This is also considered uneconomical and a source of environmental pollution, so that alternative pathogen control with antagonistic microbes (biocontrol) is more promising and sustainable (Abbey et al. 2018; Fournier et al. 2020; Tleuova et al. 2020). In this study, a number of indigenous microbes showed antagonistic activity against F. oxysporum growth in vitro (Fig. 3).
Three bacterial isolates and three fungal isolates showed inhibitory activity of F. oxysporum mycelium growth. LC648364 (Bacillus sp. strain LLB-17) was significantly (p < 0.05) able to inhibit the growth of radial mycelium F. oxysporum when compared to controls with inhibition percentages of 65.93%. When compared with bacterial isolates, the F. oxysporum inhibition capability of fungal isolates was much higher. Although the rate of bacterial cell proliferation is faster, the expansion capability of fungal hyphae in the test medium is much faster, so this is thought to be correlated with its antagonistic activity in suppressing the growth of F. oxysporum mycelium. Kalman et al. (2020) reported that the Foc growth rate reached 0.83–0.87 cm/day. The activity of rhizosphere bacteria in suppressing pathogen growth can be through a number of mechanisms of action, including synthesis of hydrolytic enzymes, such as chitinase, β-1,3-glucanase, and proteases, that can lyse pathogenic fungal cells (Lopez et al. 2020), (2) competition for nutrition and colonization of the rhizosphere niche (Rana et al. 2019), and (3) production of siderophores and antibiotics (Kumar et al. 2018; Panchami et al. 2020). But generally, the mechanism of inhibitory action by bacteria occurs due to the synthesis of a number of bioactive compounds, particularly antibiotics (Jangir et al. 2018; Panchami et al. 2020; Ramyabharathi et al. 2020).
From the results of molecular analysis using 16S rRNA markers, it was found that the three bacterial isolates were included in the genus Bacillus (Fig. 5). The isolate with the highest inhibitory capability, LC648364 has evolutionary similarity to Bacillus sp. strain LLB-17. The interesting thing is that isolate LC648364 has a percent identity of 94% when compared to Bacillus sp. strain LLB-17, where both share the same branch. A number of studies have reported the capability of Bacillus to suppress the growth of various phytopathogenic fungi so that it is commonly used as a biocontrol agent in both monoculture and consortium forms (Khan et al. 2017). Cucu et al. (2019) reported that B. subtilis QST713 was able to suppress the growth of F. oxysporum f. sp. lycopersici (Fol). Bacillus sp. B44 Anatagonist Fol (Jangir et al. 2018). In contrast to bacteria, of the three antagonistic fungi isolates tested with the dual culture method, isolates LC648367 and LC648368 showed significant inhibitory activity while isolate LC648369 was the lowest among the three (Table 1) with an inhibitory percentage of 41%. The results of molecular analysis showed that the LC648367 isolate had high homology (99.4% –100% similarity) (Gupta et al. 2020) with Aspergillus tubingensis strain ND8, whereas LC648368 and LC648369 are identical to Trichoderma asperellum strains CHI3 and Issatchenkia orientalis.
The application of fungi in controlling the growth of the F.oxysporum pathogen is not only related to its high proliferation capability so that it is able to colonize the environment quickly, especially habitats exposed to pathogens (rhizosphere, phyllosphere, and plant organs) but is also related to its capability to produce bioactive compounds (Ghorbanpour et al. 2018). A number of previous studies have reported that A. tubingensis has antifungal activity. Zhao et al. (2018) reported that A. tubingensis QF05 was able to inhibit the activity of the pathogenic fungus Botrytis cinerea in tomato plants, whereas Kriaa et al. (2015) reported that the activity of glucose oxidase (β-d-glucose: oxygen-oxidoreductase EC 1.1.3.4) which was partially purified from A. tubingensis CTM 507 effectively inhibited F. solani. This enzyme activity causes the mycelium to undergo lysis, cytoplasmic vacuolization, premature formation of chlamydospores, and mycelium induction through anastomosis between hyphal filaments.
The inhibitory activity of F. oxysporum by the fungus LC648368 with a percentage of 41.12% was strong. The results of molecular analysis showed that LC648368 had an evolutionary relationship with T. asperellum with a similarity percentage reaching 99.2% with T. asperellum strain CHI13. The mechanism of inhibitory action by Trichoderma can be either direct contact or the result of diffusion of the compound being excreted into the environment. Trichoderma species have antagonistic activity which are production of anti-microbial metabolites, faster physiological conformation, spatial and nutrient competition, mycoparasitism, and antibiosis by enzymes and secondary metabolites (Verma et al. 2007). Trichoderma is one of the fungi that has the capability to produce a number of metabolites that can inhibit or kill pathogenic fungi, so it is the most common biocontrol agent (Ghorbanpour et al. 2018). A number of bioactive compounds with the antifungal activity of Trichoderma have been reported, such as 3-octanone and 1-octen-3-ol, which are both fungistatic and strong fungicides (Okkull et al. 2003), 6-pentyl-2H-pyran-2-one produced by T. koningii, T. harzianum, T. virens, and T. viride (Worsatit et al. 1994) and sesquiterpenes from T. harzianum (Lee et al. 2016).
De la cruz-Quiroz et al. (2018) reported that there are two mechanisms to inhibit the activity of Phytophthora capsica and Colletotrichum gloeosporioides by Trichoderma, namely the production of antibiotic compounds, which work during the growth of Trichoderma hyphae to touch the phytopathogenic biomass, and the second is the mycoparasitic mechanism, which works when these organisms come into contact. Furthermore, Das et al. (2019) reported that T. asperellum was able to effectively inhibit the growth of Ralstonia solani and Phytophthora capsica through mycelium colonization of pathogens. T. asperellum was also reported to be able to suppress the growth of F. oxysporum f. sp. cucumerinum (May et al. 2019). Cotxarrera et al. (2002) also reported that T. asperellum was able to effectively inhibit the growth of Fusarium oxysporum f. sp. lycopersici by antibiosis, mycoparasitism and competition for nutrients in wilt. In addition, Khan et al. (2020b) reported that the inhibition of pathogenic fungi growth by Trichoderma spp. includes interactions between secondary metabolites and hydrolytic enzymes can induce expansion of cell death, competition for nutrients, and inhibition of enzymes that play a role in the synthesis of the cell wall of pathogenic fungi.
From this research, all tested isolates have great potential to be applied as a field biocontrol to suppress F. oxysporum. However, the capability for antifungal activity by both bacteria and fungi can be further optimized through bioformulation in the form of a consortium. A large number of studies have stated that the application of fungi and a number of bacteria, especially Bacillus, are able to inhibit or even kill the growth of phytopathogens through a number of mechanisms (Cucu et al. 2019; Karuppiah et al. 2019; Jangir et al. 2018). Furthermore, Wong et al. (2019) stated that a BCA consortium (biological control agents) is more effective in controlling plant pathogens than single strains due to the involvement of various modes of action of antagonists in suppressing phytopathogens. Apart from acting as a biocontrol agent against phytopathogens, the application of fungi and bacteria as biocontrol agents is also correlated with supporting plant growth through the mechanism of action of providing metabolites synthesized by bacteria, for example phytohormones, or facilitating the absorption of certain nutrients from the environment (Beneduzi et al. 2012; Jangir et al. 2018). However, further testing is still needed to obtain a more comprehensive understanding of all isolates obtained.
Conclusion
A total of three bacterial isolates and three fungal isolates isolated from the rhizosphere of healthy onion plants showed the ability to inhibit Fusarium oxysporum growth. Based on the molecular study, LC648364 isolates are closely related to species Bacillus sp. strain LLB-17, LC648365 is closely related to B. subtilis strain S11, LC648366 is closely related to B. cereus strain EM6, LC648367 is closely related to Aspergillus tubingensis, LC648368 is closely related to Trichoderma asperellum and LC648369 is closely related to Issatchenkia orientalis. The study shows that LC648364 and LC648367 can be considered to have the best potential as antagonists in suppressing F. oxysporum growth. The microbial consortium used in this study could be developed as a biological control agent for F. oxysporum on onion.
References
Abbey JA, Percival D, Abbey L, Asiedu SK, Prithiviraj B, Schilder A (2019) Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)—prospects and challenges. Biocontrol Sci Tech 29(3):207–228
Abdelrahman M, Abdel-Motaal F, El-Sayed M, Jogaiah S, Shigyo M, Ito SI, Tran LSP (2016) Dissection of Trichoderma longibrachiatum-induced defense in onion (Allium cepa L.) against Fusarium oxysporum f. sp. cepae by target metabolite profiling. Plant Sci 246:128–138
Armitage AD, Taylor A, Sobczyk MK, Baxter L, Greenfeld BPJ, Bates HJ, Wilson F, Jackson AC, Ott S, Harrison RJ, Clarkson JP (2018) Characterisation of pathogen specific regions and novel effector candidates in Fusarium oxysporum f. sp. Cepae. Sci Rep 8(13530):1–19
Beneduzi A, Ambrosini A, Passaglia LMP (2012) Plant growth-promoting rhizobacteria (PGPR): their potential as antagonists and biocontrol agents. Genet Mol Biol 35:1044–1051
Brayford D (1996) IMI descriptions of fungi and bacteria set 127. Mycopathologia 133:35–63
Chand SK, Nanda S, Mishra R, Joshi RK (2017) Multiple garlic (Allium sativum L.) microRNAs regulate the immunity against the basal rot fungus Fusarium oxysporum f. sp. Cepae. Plant Sci 257:9–21
Cotxarrera L, Trillas-Gay MI, Steinberg C, Alabouvette C (2002) Use of sewage sludge compost and Trichoderma asperellum isolates to suppress fusarium wilt of tomato. Soil Biol Biochem 34:467–476
Cramer CS (2000) Breeding and genetics of Fusarium basal rot resistance in onion. Euphytica 115(3):159–166
Cucu MA, Gilardi G, Pugliese M, Gullino ML, Garibaldi A (2020) An assessment of the modulation of the population dynamics of pathogenic Fusarium oxysporum f. sp. lycopersici in the tomato rhizosphere by means of the application of Bacillus subtilis QST 713, Trichoderma sp. TW2 and two composts. Biol Control 142:104–158
Das MM, Haridas M, Sabu A (2019) Biological control of black pepper and ginger pathogens, Fusarium oxysporum, Rhizoctonia solani and Phytophthora capsici, using Trichoderma spp. Biocatal Agric Biotechnol 17:177–183
De la Cruz-Quiroz R, Roussos S, Rodríguez-Herrera R, Hernandez-Castillo D, Aguilar CN (2018) Growth inhibition of Colletotrichum gloeosporioides and Phytophthora capsici by native Mexican Trichoderma strains. Karbala Int J Mod Sci 4(2):237–243
Fournier B, Dos Santos SP, Gustavsen JA, Imfeld G, Lamy F, Mitchell EA, Heger TJ (2020) Impact of a synthetic fungicide (fosetyl-Al and propamocarb-hydrochloride) and a biopesticide (Clonostachys rosea) on soil bacterial, fungal, and protist communities. Sci Total Environ 20:139–635
Galeano P, González PH, Fraguas LF, Galván GA (2014) Age-related resistance to Fusarium oxysporum f. sp. cepae and associated enzymatic changes in seedlings of Allium cepa and A. fistulosum. Tropical Plant Pathol 39(5):374–383
Ghanbarzadeh B, Goltapeh EM, Safaie N (2014) Identification of Fusarium species causing basal rot of onion in East Azarbaijan province, Iran and evaluation of their virulence on onion bulbs and seedlings. Arch Phytopathol Plant Protect 47(9):1050–1062
Gupta V, Kumar K, Fatima K, Razdan VK, Sharma BC, Mahajan V, Hussain R (2020) Role of biocontrol agents in management of corm rot of saffron caused by Fusarium oxysporum. Agronomy 10(9):1398
Harris JL (1986) Modified method for fungal slide culture. J Clin Microbiol 24:460–461
Jamil A, Musheer N, Ashraf S (2020) Antagonistic potential of Trichoderma harzianum and Azadirachta indica against Fusarium oxysporum f. sp. capsici for the management of chilli wilt. J Plant Dis Protect. https://doi.org/10.1007/s41348-020-00383-1
Jangir M, Pathak R, Sharma S, Sharma S (2018) Biocontrol mechanisms of Bacillus sp., isolated from tomato rhizosphere, against Fusarium oxysporum f. sp. lycopersici. Biol Control 123:60–70
Kalman B, Abraham D, Graph S, Perl-Treves R, Meller Harel Y, Degani O (2020) Isolation and identification of Fusarium spp, the causal agents of onion (Allium cepa) basal rot in northeastern Israel. Biology 9(4):69
Kandel SL, Firrincieli A, Joubert PM, Okubara PA, Leston ND, McGeorge KM, Doty SL (2017) An in vitro study of bio-control and plant growth promotion potential of Salicaceae endophytes. Front Microbiol 8:386
Karuppiah V, Sun J, Li T, Vallikkannu M, Chen J (2019) Co-cultivation of Trichoderma asperellum GDFS1009 and Bacillus amyloliquefaciens 1841 causes differential gene expression and improvement in the wheat growth and biocontrol activity. Front Microbiol 10:1068
Khan N, Maymon M, Hirsch AM (2017) Combating Fusarium infection using Bacillus-based antimicrobials. Microorganisms 5(4):75
Khan RAA, Najeeb S, Hussain S, Xie B, Li Y (2020a) Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms 8(6):817. https://doi.org/10.3390/microorganisms8060817
Khan RAA, Najeeb S, Mao Z, Ling J, Yang Y, Li Y, Xie B (2020b) Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic bacteria and root-knot nematode. Microorganisms 8(3):401. https://doi.org/10.3390/microorganisms8030401
Kriaa M, Hammami I, Sahnoun M, Azebou MC, Triki MA, Kammoun R (2015) Purification, biochemical characterization and antifungal activity of a novel Aspergillus tubingensis glucose oxidase steady on broad range of pH and temperatures. Bioprocess Biosyst Eng 38(11):2155–2166
Kumar A, Singh VK, Tripathi V, Singh PP, Singh AK (2018) Plant growth-promoting rhizobacteria (PGPR): perspective in agriculture under biotic and abiotic stress. In: Crop improvement through microbial biotechnology, pp 333–342. Elsevier, New York
Lecomte C, Alabouvette C, Edel-Hermann V, Robert F, Steinberg C (2016) Biological control of ornamental plant diseases caused by Fusarium oxysporum : a review. Biol Control 101:17–30
Lee S, Yap M, Behringer G, Hung R, Bennett JW (2016) Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biol Biotechnol 3(1):1–14
Lopez CG, Castellanos LNM, Ortiz NAF, González JAG (2019) Control of powdery mildew (Leveillula taurica) using Trichoderma asperellum and Metarhizium anisopliae in different pepper types. Biocontrol 64(1):77–89
McGovern RJ (2015) Management of tomato diseases caused by Fusarium oxysporum. Crop Prot 73:78–92
Mehnaz S, Saleem RSZ, Yameen B, Pianet I, Schnakenburg G, Pietraszkiewicz H, Valeriote F, Josten M, Sahl HG, Franzblau SG, Harald G (2013) Lahorenoic acids A-C, ortho-dialkyl-substituted aromatic acids from the biocontrol strain Pseudomonas aurantiaca PB-St2. J Nat Prod 76:135–141
Miao CP, Mi QL, Qiao XG, Zheng YK, Chen YW, Xu LH, Zhao LX (2016) Rhizospheric fungi of Panax notoginseng: diversity and antagonism to host phytopathogens. J Ginseng Res 40(2):127–134
Okull DO, Beelman RB, Gourama H (2003) Antifungal activity of 10-oxo-trans-8-decenoic acid and 1-octen-3-ol against Penicillium expansum in potato dextrose agar medium. J Food Prot 66(8):1503–1505
Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16(3):115–125
Panchami PS, Thanuja KG, Karthikeyan S (2020) Isolation and characterization of indigenous plant growth-promoting rhizobacteria (PGPR) from cardamom rhizosphere. Curr Microbiol 77(10):2963–2981
Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moënne-Loccoz Y (2009) The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321:341–361
Ramyabharathi S, Meena KS, Rajendran L et al (2020) Potential of a rhizobacterium Bacillus subtilis (Bbv 57) on Fusarium oxysporum f. sp. gerberae and Meloidogyne incognita infecting Gerbera grown in protected cultivation. Eur J Plant Pathol 158:615–632
Rana KL, Kour D, Sheikh I, Yadav N, Yadav AN, Kumar V, Saxena AK (2019) Biodiversity of endophytic fungi from diverse niches and their biotechnological applications. In: Advances in endophytic fungal research, pp 105–144. Springer, Cham
Skidmore AM, Dickinson CH (1976) Colony interactions and hyphal interference between Septoria nodorum and phylloplane fungi. Trans Br Mycol Soc 66:57–64
Summerell BAJF, Leslie EC, Liew MH, Laurence S, Bullock T, Petrovic AR, Bentley CG, Howard SA, Peterson JL (2011) Fusarium species associated with plants in Australia. Fungal Divers 46:1–27
Taylor A, Vágány V, Jackson AC, Harrison RJ, Rainoni A, Clarkson JP (2016) Identification of pathogenicity-related genes in Fusarium oxysporum f. sp. cepae. Mol Plant Pathol 17(7):1032–1047
Tleuova AB, Wielogorska E, Talluri VP, Štěpánek F, Elliott CT, Grigoriev DO (2020) Recent advances and remaining barriers to producing novel formulations of fungicides for safe and sustainable agriculture. J Control Release 17(7):1032–1047
Verma M, Brar SK, Tyagi RD, Surampalli RY, Valero JR (2007) Antagonistic fungi, Trichoderma spp.: panoply of biological control. Biochem Eng J 37(1):1–20
Wang A, Nahidul IMd, Anders J, Minna H, Satu L, Merete E (2019) Pathogenic Fusarium oxysporum f. sp. cepae growing inside onion bulbs emits volatile organic compounds that correlate with the extent of infection. Postharvest Biol Technol 152:19–28
White TJ, Bruns T, Lee SJWT, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc Guide Methods Appl 18(1):315–322
Wong CKF, Saidi NB, Vadamalai G, Teh CY, Zulperi D (2019) Effect of bioformulations on the biocontrol efficacy, microbial viability and storage stability of a consortium of biocontrol agents against Fusarium wilt of banana. J Appl Microbiol 127(2):544–555
Zaim S, Bekkar AA, Belabid L (2018) Efficacy of Bacillus subtilis and Trichoderma harzianum combination on chickpea Fusarium wilt caused by F. oxysporum f. sp. ciceris. Arch Phytopathol Plant Protect 51(3–4):217–226
Acknowledgements
This work was funded by Universitas Negeri Makassar PNBP Research Grant No. 2399/UN36.11/LP2M/2020.
Funding
This study was funded by Universitas Negeri Makassar PNBP Research Grant No. 2399/UN36.11/LP2M/2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical statement
Hereby, I Dr. Hilda Karim consciously assure that for the manuscript Antagonistic Activity and Characterization of Indigenous Soil Isolates of Bacteria and Fungi Against Onion Wilt Incited by Fusarium sp. the following is fulfilled: (1) This material is the authors' own original work, which has not been previously published elsewhere. (2) The paper is not currently being considered for publication elsewhere. (3) The paper reflects the authors' own research and analysis in a truthful and complete manner. (4) The paper properly credits the meaningful contributions of co-authors and co-researchers. (5) The results are appropriately placed in the context of prior and existing research. (6) All sources used are properly disclosed (correct citation). Literally copying of text must be indicated as such using quotation marks and giving proper reference. (7) All authors have been personally and actively involved in substantial work leading to the paper and will take public responsibility for its content. I agree with the above statements and declare that this submission follows the policies of Solid-State Ionics as outlined in the Guide for Authors and in the Ethical Statement.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karim, H., Azis, A.A. & Jumadi, O. Antagonistic activity and characterization of indigenous soil isolates of bacteria and fungi against onion wilt incited by Fusarium sp.. Arch Microbiol 204, 68 (2022). https://doi.org/10.1007/s00203-021-02663-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-021-02663-2