Abstract
The need for novel and active antibiotics specially from actinomycetes is essential due to new and drug-resistant pathogens. In this study, 87 actinomycetes were isolated, and 18 strains among them characterized as thermophilic actinomycetes. Further fractionation and preliminary antibacterial activities indicated that one strain, coded as MI-S.24-3, showed good antibacterial activity. Based on the phenotypic, genomic, phylogenetic, and biochemical analyses, MI-S.24-3 was identified as Streptomyces werraensis. Results demonstrated that the ethyl acetate active fraction showed maximum antibacterial activity against Staphylococcus aureus and Escherichia coli with MIC (12.7 ± 0.1 and 18.3 ± 0.2 mg/mL), and MBC (96.5 ± 1.4 and 91.5 ± 0.7 mg/mL), respectively, with determination of time kill kinetics assay. The active fraction showed moderate-to-weak cytotoxic effects against human lung carcinoma (A549 cells), breast cancer cell line (MCF-7), and human cervical carcinoma (HELA cells) with a IC50 of (23.8 ± 1.2, 54 ± 1.8, 96.4 ± 3.2 μg/mL, respectively). Active components were characterised by different chemically volatile, ester, and lactone compounds, determined by GC–MS coupled with daughter ions of (GC–MS/MS). Notably, erucic acid and reynosin identified compounds are rare metabolites produced by Streptomyces werraensis. Our findings demonstrated that the MI-S.24-3 strain could be a potential source for active compounds of biomedical and pharmaceutical interest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The broad range of actinomycetes play important roles in soil structure and composting and are widely distributed as saprophytes, free-living, as well as spore-forming and colonising on plant material (George et al. 2010). The soil environment is the source of a large number of Streptomyces spp., which has led to the successful synthesis of multiple antibiotics (Afifi et al. 2012). Based on the refined phylogeny of the 16S rRNA gene sequences, until now 425 genera of the phylum actinomycetes divided into 6 classes, 46 orders and 79 families, including 16 new orders and 10 new families (Salam et al. 2020). One of the major groups colonising in the soil is actinomycetes, which can be different in different soil types, and some researchers have estimated that it constitute 10–33% of soil microbes (George et al. 2010; Uddin et al. 2013). Out of 23,000 bioactive microbial metabolites, 10,000 are solely produced by actinomycetes, and many actinomycetes provide valuable sources of active metabolites with antibacterial, antifungal, antiparasitic, anticancer (Berdy 2005) and immunosuppressive (Olano et al. 2009) capability, and multiple volatile compounds including herbicides and pesticides (Wilkins 1996). Of the 10,000 bioactive compounds produced by actinomycetes, 76% (7600) are produced by the genus: Streptomyces (Jackson et al. 2018). Active compounds isolated from members of Streptomyces are capable of a diverse range of antimicrobial, anticancer and antiviral activities, most of which have medical importance (Shah et al. 2016; Moaz et al. 2019). Various Streptomyces spp. produce secondary metabolites such as daptomycin, lincomycin, streptomycin and vancomycin, commonly used against microbial pathogens (Dubourg et al. 2017). They also produce the antiviral benzastatin C, a 3-chloro-tetrahydroquinolone alkaloid and methylelaiophylin used against herpes simplex types and Newcastle disease virus (Lee et al. 2011).
Actinomycetes exist in extreme environments such as glaciers, high-temperature deserts (Shah et al. 2017), and areas with high salinity or alkalinity (Goodfellow and Williams 1983; Barka et al. 2016). Actinomycetes from such environments are known to be a source of novel molecules. Earlier study has targeted actinomycetes that can survive in extreme temperatures (Jiang et al. 2012), and these thermophilic actinomycetes are well documented to produce novel antibiotics and other valuable bioactive metabolites (Xu et al. 1998), gaining the attention of microbiologists. The diverse and natural metabolites, especially antimicrobial agents from these strains, can be safely applied to synthetic antibiotics as there are fewer chances of adverse side effects (Trenozhnikova and Azizan 2018). Egyptian ecosystems are a promising source for actinomycetes with diverse biological activities, presupposing that it may be necessary to characterise such bacteria for novel and active antibiotics from archaeological sites (Reda et al. 2016). Drug-resistant pathogens considered a global threat of growing concern to human, animal, and environment health, so there is a crucial demand for new and active metabolites (Butler et al. 2017). Therefore, the main objective of the current work was the isolation and identification of Streptomyces sp. among all thermophilic actinomycetes isolated from various archaeological areas of Egypt. The strain was extracted and partially purified, and its antibacterial and cytotoxic activity assessed. The chemical constituents of the active extract were determined using (GC–MS) gas chromatography–mass spectrometry.
Materials and methods
Sampling locations and description
Soil samples were collected 2016–2018 from a sandy desert (Sahra Al-Gharbiya) at five localities in Upper Egypt (39° 30ʹ to 30° 00 N, 29° 00 to 29° 30 E), in Egypt’s western desert region (Supplementary S1). The area is characterised by very dry hot summers (42–48 °C) and cold winters (4–5 °C) with an average humidity of 76% and rare rainfall, and annually prone to sandstorms caused by the khamsin wind. Soil samples each weighing ~ 200 g were collected by inserting a spatula (sterilised with alcohol before sampling at each location) into the sediments. The soil was collected at a depth of 10–25 cm. Samples were placed into sterile poly bags, sealed tightly, and transported immediately to the laboratory. After air-drying for 4–5 h at 45 °C, the samples were crushed and sieved before use for bacterial isolation.
Pre-treatment and isolation of actinomycetes
Before the isolation processes, soil samples were pre-treated to increase the concentration and proportion of actinomycetes. All samples were sieved to remove contaminating materials. Soil samples were pre-treated by heating at 70 °C for 20 min, then they were thoroughly air-dried for 7 days at ambient temperature and mixed with CaCO3 (1 g/100 g soil) for 24 h (Sengupta et al. 2015). Serial dilution was performed for the isolation and cultivation of actinomycetes. From each pre-treated soil samples, 1 g of dried soil was suspended in 10 mL sterile saline solution (0.85% NaCl) and serially diluted up to 10–5, the samples mixed by vortexing after each dilution to ensure a homogenised suspension. Of each dilution, 0.1 mL was spread over the surface of actinomycete specific agar media such as International Streptomyces Project 2 (ISP-2) medium (4 g/L yeast extract, 10 g/L malt extract, 4 g/L dextrose and 20 g/L agar in 1000 mL distilled H2O, pH 7.2 ± 0.2) supplemented with cycloheximide (25 μg/mL). All plates were incubated at 30 °C for 7–14 days. Based on the morphological properties of colonies, pure actinomycetes were selected and sub-cultured on ISP-2 medium and stored at 4 °C on slants of starch casein agar (SCA, 10 g/L starch, 2 g/L K2HPO4, 2 g/L KNO3, 0.3 g/L casein, 0.05 g/L MgSO4·7H2O, 0.02 g/L CaCO3, 0.01 g/L FeSO4·7H2O and 20 g/L agar in 1000 mL distilled H2O, pH 7.0 ± 0.2) for further investigation.
Screening for thermophilic actinomycetes and their antibacterial activity
After isolation procedures, 87 purified isolates were sub-cultured on starch nitrate agar medium (20.0 g/L soluble starch, 2.0 g/L NaNO3, 1.0 g/L K2HPO4, 0.5 g/L KCL, 0.5 g/L MgSO4⋅7H2O, 2.0 g/L CaCO3 and 20 g/L agar in 1000 mL distilled H2O, pH 7.0 ± 0.2), and incubated for 5–10 days at different temperatures, including 40, 45, 50, 55, 60 and 65 °C. Eighteen thermophilic actinomycetes were screened for qualitative antibacterial determination against multiple pathogenic clinical strains by the cross-streak method previously described (Madigan et al. 2008). Briefly, SCA plates were inoculated with all purified isolated thermophilic strains in the centre of the plate and incubated at 30 °C for 7–10 days, then inoculated with a single streak of the selected test organisms at a 90° angle to the actinomycetes isolate. The plates were observed for antagonism against tested bacteria after overnight incubation at 37 °C; the most promising strain (demonstrating the highest activity) was kept for further analyses.
Bacteria for antibacterial activity assay
Clinical bacterial strains used in this study were obtained from the Hospital of Assiut University and their Microbiological Laboratories (AUHL), including were Gram-positive (Bacillus cereus AUH 23, Staphylococcus aureus ATCC 6538P and S. epidermidis AUH 217) and Gram-negative bacteria (Pseudomonas aeruginosa ATCC 9027, Enterobacter aerogenes AUH 310, Salmonella typhi AUH 71, Klebsiella pneumoniae ATCC 43816, Serratia marcescens AUH 98 and Escherichia coli ATCC 8739). Bacteria were grown in Mueller–Hinton broth medium (2.0 g/L beef infusion solids, 1.5 g/L starch and 17.5 g/L casein hydrolysate in 1000 mL distilled water, pH 7.4 ± 0.2) under aerobic conditions with shaking at 200 rpm and overnight incubation at 37 °C.
Morphological and biochemical observations of the most active strain
The selected strain Streptomyces werraensis MI-S.24-3 was characterised according to ISP directions and confirmed using procedures provided by the Bergey’s Manual of Systematic Bacteriology (Whitman et al. 2012). Cultural features of isolated MI-S.24-3 in various media (ISP 1–7) were recorded after incubation at 30 °C for 7, 14 and 21 days. The morphology of spores with hyphae and spore chains was determined with a light microscope (×400) using the cover-slip technique in ISP medium. The following characteristics were assessed using previously published methods (Balachandran et al. 2012) cell shape, colour, Gram stain results, response to varying temperatures, pH levels and NaCl concentrations, enzyme activity, presence of pigment, and acid or gas production. Also tested were the utilisation of different carbon and nitrogen sources, tolerance to growth inhibitors, and sensitivity to antibiotics.
Molecular identification of S. werraensis strain MI-S.24-3
A genomic DNA extraction kit (Axygen, Biosciences Co., USA) was used for the isolation of the genomic DNA of S. werraensis MI-S.24-3. PCR was then used to amplify the 16S rRNA gene following a previously reported protocol (Abol Fotouh et al. 2016). Two universal primers, FC27 (5ʹ-AGAGTTTGATCCTGGCTCAG-3ʹ) and RC1492 (5ʹ-TACGGCTACCTTGTTACGACTT-3ʹ), were used. The PCR cycle protocol was (35 cycles) initial denaturation at 94 °C for 5 min, 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 90 s and a final extension at 72 °C for 10 min. The PCR products after amplification were visualised via a gel documentation system (Syngene, USA) and confirmed by 1% agarose gel electrophoresis. The PCR product was purified from the agarose gel utilising QIAquick DNA gel extraction kit (Qiagen, USA) according to the manufacturer’s instructions. Automated sequencing was performed by an automated DNA sequencer (3130X, Applied Biosystems, USA) using the enzymatic chain terminator technique following a previously published procedure (Sanger 1997). The 16S rRNA gene sequence of strain MI-S.24-3 was compared with the reference sequences available on the National Center for Biotechnology Information GenBank database using the Basic Local Alignment Search Tool (BLAST). The sequence alignment was performed by the ClustalW program (version 1.83). The phylogenetic tree was analysed and generated based on the UPGMA statistical method using MEGA software (version 5.0) through the neighbour-joining tree method with a bootstrapping value of 500 (Tamura et al. 2011).
Scanning electron microscopy of MI-S.24-3
Starch nitrate agar medium was inoculated with MI-S.24-3 spores and incubated for a week at 40 °C. For electron microscopy, the culture was fixed in glutaraldehyde (2.5%, v/v). After fixation, the culture was washed with water and treated for 1 h in osmium tetroxide (1%, w/v). The sample was washed twice with water and dehydrated in ascending ethanol concentrations (30, 50, 70, 90, and 100%). Finally, it was coated with gold using a sputter coater (JFC-1100 E, JEOL, Japan) ion sputtering device and observed at 15–20 kV (JSM 5400 LV, JEOL) at the Electron Microscopy (EM) unit at Assiut University, Egypt.
Cultivation and extraction of MI-S.24-3 crude extract
Fresh MI-S.24-3 culture was scraped from SCA medium, inoculated into 100 mL of inoculation medium in a 500 mL conical flask and incubated in a rotary shaker at 120 rpm for 2 days at 30 °C. Then, a 1 L flask containing 250 mL of production medium was inoculated with the culture (10%, v/v) and incubated for 7 days at 30 °C with shaking at 120 rpm. After fermentation, mycelium and supernatant were filtered using a Whatman filter (0.45 μm), and finally, centrifuged at 10,000 rpm for 30 min at 4 °C. The culture supernatant containing extracellular compounds was extracted by liquid–liquid extraction using an equal amount of ethyl acetate in a separatory funnel. The ethyl acetate layer was concentrated by evaporating to dryness at 40 °C and the residue obtained was dissolved in HPLC grade methanol to (1.2 g) yellow crude extract.
Separation and identification of active compounds of MI-S.24-3
The separation of the active compounds from ethyl acetate extract (1.2 g), was carried out using silica gel column chromatography (CC). Methanol was applied as the eluting solvent. Silica gel (60–120 mesh size, Fisher) was packed within the column. For separation, the sample was loaded onto the prepared column and eluted with the solvent at a flow rate of 10 drops per min; numbered test tubes were used to assemble the eluted fractions. All collected fractions were analysed by thin-layer chromatography (TLC), with silica gel (G-60 F25, Merck, Kenilworth, NJ, USA). The TLC chamber with the solvent system (hexane: ethyl acetate: methanol 6:3:1) was initially incubated for 20 min until equilibration. The samples were spotted and concentrated on the silica gel sheets by a capillary tube followed by air-drying. Whole bands on TLC were detected under the UV lamp chamber at 254 and 366 nm, followed by spraying the TLC plates with vanillin/H2SO4 reagent and subsequent heating on the hot plate at 110 °C. Fractions having a similar number of spots on the TLC with the same Rf values were pooled. There were five fractions (F1–F5). The antibacterial activity was determined, and most promising active fractions were used for further bio-analysis.
Determination of biological activity
In vitro antibacterial assay
Antibacterial activity of the five fractions was determined using the standard Kirby–Bauer disc diffusion method (Bauer et al. 1966). Briefly, Petri plates were prepared with approximately 25 mL of sterile Mueller–Hinton agar (MHA, 17.5 g/L acid hydrolysate of casein, 1.5 g/L starch, 2.0 g/L beef extract and 18.0 g/L agar in 1000 mL H2O, pH 7.3 ± 0.2). Overnight nutrient broth cultures of tested bacteria were streaked on the top of the solid media and allowed to dry completely for 15–20 min. A stock fraction solution (100 mg/mL) was prepared by dissolving the extract in pure DMSO and diluting it two times. Each stock solution was sterilised and filtered using a syringe filter. Sterile blank discs (Whatman No. 1, diameter 6 mm), were impregnated in the working solution (10 mg/mL). Discs were stored at 4 °C before use. Fraction-impregnated discs (20 μL) were placed on agar plates and incubated at 37 °C for 24 h. Pure 10% DMSO (20 μL) was used as a negative control, while tetracycline discs (50 µg/mL) were used as a positive control. Then, the diameter of the inhibition zone was calculated in (mm). The experiments were performed in triplicate.
Determination of the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
A broth microdilution susceptibility assay was used for the determination of (MIC) as recommended by the National Committee for Clinical Laboratory Standards (NCCLS 2003). The stock solution of the respective active fraction was prepared as mentioned above, and the serial two-flow dilution was carried out. The bacterial suspension containing approximately 6 × 105 colony-forming units/mL was prepared from a 24 h culture plate, then 100 μL was inoculated into each well. A sterility control well and a growth control well were also studied for each strain. All microtiter plates were incubated for 24 h at 37 °C. After incubation period, 50 μL of a 0.5 mg/mL solution of INT was added to each well as an indicator of bacterial growth. The plates were incubated at 37 °C for 30 min and the MIC values visually determined. The lowest concentration of the active fraction that did not show visible growth was defined MIC. The determination of MBC was performed using the method of Ozturk and Ercisli (2006), and the MBC was defined as the lowest active concentration killing approximately 99.9% of the bacterial inocula after 24 h incubation at 37 °C. The experiments were conducted in triplicate, whereas tetracycline and 10% DMSO solution were used as a positive and negative control, respectively. The results also were represented as mean ± SD.
WST-1 cytotoxicity activity
Cell culture
The cytotoxicities on three human cancer cell lines, human lung carcinoma (A549 cells), breast cancer cell line (MCF-7), and human cervical carcinoma (HELA cells) were assayed. Cancer cell lines were obtained from Nawah Scientific Inc. (Mokatam, Cairo, Egypt). Tested cells were maintained in DMEM media supplemented with 100 mg/mL streptomycin, 100 units/mL penicillin, and 10% heat-inactivated foetal bovine serum in a humidified, 5% (v/v) CO2 atmosphere at 37 °C. The percentage of cell viability was calculated using the following equation:
Cytotoxicity assay
For evaluation of cytotoxicity, cell viability was assessed by WST-1 assay using Abcam® kit ab155902 WST-1 cell proliferation reagent (Alaufi et al. 2017). Aliquots of 50 μL cell suspension (3 × 103 cells) were seeded in 96-well microplates and incubated in complete media for 24 h. Then, cells were treated for 48 h with another aliquot of 50 μL media containing the extract of MI-S.24-3 at different concentrations (10, 25, 50 and 100 µg/mL). Finally, 10 μL WST-1 reagent was added to each well for a period of 1 h, and the absorbance was measured at 450 nm (BMG LABTECH®-FLUOstar Omega plate reader, Allmendgrün, Ortenberg).
Gas chromatography–mass spectrometry (GC–MS) analysis
The obtained active fraction was dissolved in methanol and dehydrated with anhydrous sodium sulphate, then filtered through a syringe filter (0.45 μm pore size) before injection. A mass spectrometer (Trace GC Ultra-ISQ, Thermo Scientific, Austin, TX, USA), was used for the chromatographic analysis, and the compounds were separated on 30 m × 0.25 mm × 0.25 µm film thickness TG–5MS capillary column. The column temperature was initially 70 °C and then increased by 5 °C/min to 280 °C withhold 2 min then increased to 300 °C at 10 °C/min. The injector and MS transfer line temperatures were kept at 300 °C. The carrier gas was used helium with a stable flow rate of 1 mL/min. The solvent delay was 3 min, and a diluted sample of 1 µL was injected automatically using an autosampler AS3000 coupled with GC in the split mode. EI mass spectra were generated at an ionisation voltage of 70 eV with a mass scan of 40–650 amu. The extract components were determined and identified with the mass spectra and retention time database of the Wiley 09 and NIST 11 library.
Time kill kinetics
Time kill assay was analyzed using the MIC values by the most sensitive tested bacteria, the selected strain(s) suspension were adjusted a bacterial serial ten-fold dilutions (10−3 and 10−4 from 1.5 × 107 cfu/mL) and tested antimicrobial activity with concentration of MIC values of 1 MIC, 2 MIC, 3 MIC and 4 MIC of EtOAc active fraction. Tetracycline at concentration of 40 mg/mL was used as a positive control. The 80 µL of each treatment was put into each well containing 100 µL MHB supplemented with 20 µL of bacterial suspension and incubated at 37 °C for 24 h. All samples were collected at 3, 6, 9, 18 and 24 h. Then, 100 µL of collected samples were sub-cultured on MHA and incubated at 37 °C for 24 h. A number of viable bacterial cell was determined.
Statistical analysis
All the experiments were performed in triplicate. Data were presented as mean ± S.D. GraphPad Prism 6.0 (GraphPad Software, San Diego, CA, USA, http://www.graphpad.com) was used to calculate one-way analysis of the variance (ANOVA) with multiple comparison tests (Tukey’s) to evaluate the effect of different fractions. Statistical significance was assigned at P > 0.05.
Results
Isolation of actinomycetes
A total of 87 actinomycetes were isolated at different archaeological locations in Egypt (Supplementary S1). There were 18 thermophilic actinomycetes, and among them, 1 thermophilic strain (MI-S.24-3) isolated from the Luxor governorate showed good antibacterial activity in preliminary screening. This strain was then selected for further studies because of its capability to produce a wide spectrum of antibacterial activities against both Gram-positive and Gram-negative bacteria (Supplementary S2 and S3).
Identification of actinomycetes
Morphological, biochemical and chemotaxonomical analyses
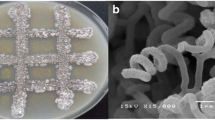
Light microscopy (400x) investigation showed that the bacterium morphology was a long-chain spiral of spore-shaped mycelium. SEM of MI-S.24-3 revealed a chain of smooth, non-branched, individual spores arranged in spiral hyphae (Supplementary S4). The physiological properties of MI-S.24-3 showed that no melamine pigments were produced on ISP1, ISP6, and ISP7. Regarding the carbon and nitrogen sources utilisation, all tested carbon and nitrogen sources were positive, except ribose and fructose. The tested isolate also showed excellent growth up to 8% NaCl, at 6–12 pH and 25–55 °C. Other biochemical, morphological, and physiological characteristics were also performed as shown in Supplementary S6. Further chemotaxonomic analysis of MI-S.24-3 were confirmed by DAP isomers and whole-cell sugar pattern. Based on the cultural and chemotaxonomic characteristics of MI-S.24-3 strain, it was found that the cells hydrolysate of MI-S.24-3 contains major amounts of LL-diaminopimelic acid (LL-DAP), and no characteristic sugars in whole-cell hydrolysates was detected. Thus, this strain, have the same composition of symbolic cellular components as the genus Streptomyces.
16S rDNA sequencing and phylogenetic analysis
The partial 16S rRNA gene sequence of MI-S.24-3 demonstrated an excellent congruence between morphology, chemotaxonomic and 16S rRNA sequence data. BLAST analysis indicated that the strain belonged to Streptomyces species, and the sequence was deposited in the NCBI database with the accession number MZ298702. The strain MI-S.24-3 showed the highest 16S rRNA gene sequence similarities (100%) with S. werraensis (JCM 4860, IIPR:KR05:01, ATCC 14424, SOT15 and S. werraensis (WS1). Based on the neighbour-joining method (Fig. 1), the phenotypic and genomic data indicated that the MI-S.24-3 strain represented a strain of the genus Streptomyces, which was referred to as S. werraensis strain MI-S.24-3. The identified actinomycetes MI-S.24-3, was deposited in the culture collection at Assiut University Mycological Center (AUMC) as Streptomyces werraensis strain MI-S.24-3 with an institutional number AUMC-B-335.
The neighbour-joining (NJ) phylogenetic tree based on 16S rRNA gene sequences of MI-S.24-3, with closely related strains accessed from the GenBank using BLASTN (http://www.ncbi.nlm.nih.gov/blast/). These sequences were aligned using ClustalW. Bootstrap values included 500 replicates for the NJ method using MEGA software (version 5.0). *** indicate tested strain
Fractionation of the extract and their biological activity
Antibacterial activity
Initially, the cross-streak method was used for all 18 thermophilic isolated actinomycetes for the preliminary antibacterial activity (Supplementary S2). Among these, the MI-S.24-3 strain was selected and grown on a medium-scale (10 L), and the ethyl acetate extract (1.2 g) of their fermented broth was subjected to column chromatography, followed by purification via TLC plates that resulted in five different potential fractions. Antibacterial activities of these fractions were tested against three species of Gram-positive and six species of Gram-negative bacteria and compared to the activity of the reference standard, tetracycline (Fig. 2). All the fractions were found to inhibit the tested microbes with the zones of inhibition ranging from 7 to 21 mm. Different fractions showed different degrees of inhibition, and a significant difference was observed among different fractions. Fraction 3 was found to be more potent and showed significantly higher activity against Gram-positive (S. aureus, S. epidermidis) and Gram-negative (S. typhi, S. marcescens, E. coli, and P. aeruginosa) bacteria as compared to other fractions. The sensitivity of fraction 3 against Gram-negative bacterial strains was found almost similar, while as sensitivity of E. coli was found significantly more than standard tetracycline.
Antibacterial activity by disc diffusion method of the fractions (F1–F5) from the tested strain against Gram-positive bacteria, and Gram-negative bacteria. Tet* (Tetracycline as positive control). Error bars represent standard deviations (n = 3), and asterisks indicate significant differences: *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001
Bacteriostatic and bactericidal effect
The active fraction of MI-S.24-3 was subjected to determine the MIC and MBC against all pathogens. Based on the results obtained from the in vitro antimicrobial assays, a lower MIC is observed in Table 1. The MIC test indicated that the active fraction of MI-S.24-3 exhibited minimal values of MIC ranged from 12 to 160 mg/mL against tested clinical strains. With a minimal bactericidal concentration, MBC at the concentration range of 96–730 mg/mL was determined.
WST-1 cytotoxicity activity
After isolation and partial purification of active fraction, the antitumor activities of compound-based active fraction were also evaluated using WST-1 assay. The cytotoxicity assay showed that the MI-S.24-3 active fraction at a concentration of 100 μg/mL had moderate-to-weak cytotoxic activity against human lung carcinoma (A549 cells), breast cancer cell line (MCF-7), and human cervical carcinoma (HELA cells). The IC50 values of MI-S.24-3 active fraction were 23.8 ± 1.2, 54 ± 1.8, 96.4 ± 3.2 μg/mL, respectively.
Identification of potentially bioactive compounds by GC–MS
The GC–MS analysis profile of the active fraction 3 and its constituents from the selected strain MI-S.24-3 are shown in Fig. 3 and Table 2. A total of 12 major compounds were detected and comparing those with entries in the NIST database, the nearest compound resembling those peaks were identified, as well as their molecular weight, retention time, and other characteristics. It was observed that the fraction contained various types of chemical compounds including estragole; phthalic acid 4-hydroxy-2-methoxy-6-methyl-, methyl ester; hexadecanoic acid, methyl ester; phthalic acid, butyl tetradecyl ester; 9-octadecenoic acid, methyl ester; phthalic acid, butyl hex-3-yl ester; reynosin; benzoic acid; 11-eicosenoic acid, methyl ester; erucic acid; di-n-2-propylpentylphthalate; 1,2-benzenedicarboxylic acid, 1,2-dinonyl ester; and didecyl phthalate. All the obtained compounds are known, and the peak area of each compound is directly proportional to its quantity in the extract.
Time killing kinetics assay
The time kill assay is generally used to examine the ability of an antimicrobial molecule-based extract against different pathogens. These assay was subjected over 24 h with S. aureus and E. coli being revealed to MIC (12.5 and 18.3 mg/mL), 2 MIC (24 and 36.6 mg/mL), 3 MIC (37 and 55 mg/mL) and 4 MIC (50 and 73 mg/mL, respectively) of ethyl acetate active fraction of S. werraensis MI-S.24-3. As shown in Fig. 4, the results were plotted between the logarithmic number of CFU/mL and incubation periods. At 4 MIC concentration, the active fraction of isolate MI-S.24-3 greatly exhibited. aureus and E. coli at 3 h and gradually arise to the 24 h if compared with the MIC, 2 MIC, 3 MIC and the control, respectively (Fig. 4).
Discussion
Exploring the diversity of thermophilic actinomycetes is important for detecting new isolates that could be a promising source of novel antibiotics (Müller and Wink 2013). Therefore, we researched thermophilic actinomycetes from unexplored ancient and archaeological sites in Egypt. The chemical and physical properties of Egyptian soil and, consequently, the soil populations varied based on environment and location (Abdel-Haliem et al. 2013). In this study, the most promising strain was MI-S.24-3. The morphological and biochemical properties of strain MI-S.24-3 were consistent with those of the genus Streptomyces (Williams et al. 1989). Similarly, Boudemagh et al (2005) reported the isolation and molecular identification of actinomycetes microflora from some Saharan soils of southeast Algeria and tested them for antimicrobial activities. The results were similar to those previously reported by many authors for Moroccan and Jordanian Streptomyces isolates (Saadoun and Gharaibeh 2002) and Bangladesh Streptomyces isolates (Rahman et al. 2011). Furthermore, the cultural characteristics of the strains in the above locations were close to those of the strain we obtained from Egyptian soil. The morphological description by SEM played a major role in distinguishing the MI-S.24-3 spore surface, and these results were consistent with those of a previously published study (Latha et al. 2015). Our results demonstrated that the MI-S.24-3 strain belonged to the genus Streptomyces, and it was showing 100% similarity with S. werraensis, on the basis of molecular, morphological, physiological, biochemical, and cultural parameters. In a previous study by Gopikrishnan et al. (2019), strain Streptomyces PM33 was reported to show similar nutritional and environmental requirements; besides, strain MI-S.24-3 also had ll-diaminopimelic acid and glycine residues in whole-cell hydrolysates, like other streptomycetes. The physiological and biochemical properties of a thermophilic Streptomyces strain Al-Dhabi-1 revealed that this strain is similar to the current strain and can grow at temperatures between 37 and 55 °C, and pH between 6 and 11 (Naif et al. 2016). Strain MI-S.24-3 resembles the type strain of S. werraensis ATCC 14424 in the appearance of aerial and substrate mycelia, development of spiral spore chains, and dark grey colonies on SCA. These results confirmed that the stain MI-S.24-3 belongs to Streptomyces werraensis which was further confirmed by DNA sequence analyses.
MI-S.24-3 showed promising activity against a panel of pathogens. These antibacterial results were comparable to a previous study in which actinomycetes were isolated from different soil samples and screened against Gram-positive and Gram-negative bacteria (Zhao et al. 2011). The antibacterial results of MI-S.24-3 revealed that Gram-negative bacterial pathogens were more sensitive than Gram-positive strains, that was similar as reported by Sutyak et al. (2008). Among the most significant findings is that MI-S.24-3 has moderate-to-weak cytotoxic effects against A549 cells, MCF-7, and HELA cell lines. The strain was subjected to GC analysis to determine the chemical constituents responsible for its activity (Claeson and Sunesson 2005). The GC–MS analysis profile of the active fraction revealed 12 major compounds including estragole; phthalic acid 4-hydroxy-2-methoxy-6-methyl-, methyl ester; hexadecanoic acid, methyl ester; phthalic acid, butyl tetradecyl ester; 9-octadecenoic acid, methyl ester; phthalic acid, butyl hex-3-yl ester; reynosin; benzoic acid; 11-eicosenoic acid, methyl ester; erucic acid; di-n-2-propylpentylphthalate; 1,2-benzenedicarboxylic acid, 1,2-dinonyl ester; and didecyl phthalate. Some of these known compounds, such as phthalate and phthalate derivatives, also demonstrated antimicrobial activity after isolation from Streptomyces bangladeshiensis (Chairman et al. 2012). 9-Octadecenoic acid methyl ester is well known for cancer-preventive, anti-inflammatory, and antimicrobial activity (Yunfeng et al. 2007). Studies investigating reynosin bioactivity discovered that this compound demonstrated mycobactericidal effects and cytotoxicity against MCF-10A, the myeloid leukaemia HL-60 and U937 cell lines (Eliana et al. 2014; Coronado-Aceves et al. 2016). Another study has identified erucic acid as an antibacterial agent with broad-spectrum antiviral activity (Alam et al. 2007). Finally, by GC–MS analysis, benzoic acid was identified and then investigated for anticancer activity. It was discovered to be a potent agent in inducing cancer cell death (Anantharaju et al. 2017). The results of the current research confirmed that the antimicrobial and cytotoxic activity of the active fraction might be due to the presence of these compounds.
Conclusions
A thermophilic Streptomyces werraensis (MI-S.24-3) was isolated, screened, and identified from the soil samples of ancient archaeological sites, Luxor governorate, Egypt. The isolated strain demonstrated significant antibacterial activities and moderate cytotoxicity. GC–MS analysis of the bioactivity guided active fraction of the ethyl acetate led to the identification of 12 chemical compounds with antibacterial properties. Therefore, isolation and identification of economically important active metabolites from Streptomyces in Egyptian soil and characterisation of the isolated strain may assist in the exploration of antibacterial and cytotoxic compounds from natural sources. The results of our study can assist in future drug development and their application.
Availability of data and materials
All obtained data analysed during this study are included in this published article except the results of the 16S rRNA gene sequence which was deposited to Gen Bank (NCBI) with accession number, MZ298702.
Code availability
Not applicable.
References
Abdel-Haliem ME, Ali MF, Ghaly MF, Sakr AA (2013) Efficiency of antibiotics and gamma irradiation in eliminating Streptomyces strains isolated from paintings of ancient Egyptian tombs. J Cult Herit 14:45–50
Abol Fotouh DM, Bayoumi RA, Hassan MA (2016) Production of thermo-alkaliphilic lipase from geo Bacillus thermoleovorans DA2 and application in leather industry. Enzyme Res. https://doi.org/10.1155/2016/9034364
Afifi MM, Atta HM, Elshanawany AA, Abdoul-raouf UM, El-Adly AM (2012) Biosynthesis of Hygromycin-B Antibiotic by Streptomyces crystallinus AZ151 Isolated from Assuit. Egypt Bacteriol J 2:46–65
Alam MS, Kaur G, Jabbar Z, Javed K, Athar M (2007) Eruca sativa seeds possess antioxidant activity and exert a protective effect on mercuric chloride induced renal toxicity. Food Chem Toxicol 45:910–920
Alaufi OM, Noorwali A, Zahran F, Al-Abd AM, Al-Attas S (2017) Cytotoxicity of thymoquinone alone or in combination with cisplatin (CDDP) against oral squamous cell carcinoma in vitro. Sci Rep 7(1):13131
Anantharaju PG, Reddy BD, Padukudru MA, Kumari CM, Vimalambike MG, Madhunapantula SV (2017) Naturally occurring benzoic acid derivatives retard cancer cell growth by inhibiting histone deacetylases (HDAC). Canc Biol Ther 18:492–502
Balachandran C, Duraipandiyan V, Balakrishna K, Ignacimuthu S (2012) Petroleum and polycyclic aromatic hydrocarbons (PAHs) degradation and naphthalene metabolism in Streptomyces sp. (ERI-CPDA-1) isolated from oil contaminated soil. Bioresour Technol 112:83–90
Barka EA, Vatsa P, Sanchez L, Gaveau-Vaillant N, Jacquard C, Meier-Kolthoff JP, Klenk HP, Clement C, Ouhdouch Y, van Wezel GP (2016) Taxonomy, physiology, and natural products of actinobacteria. Microbiol Mol Biol Rev 80(1):1–43
Bauer AW, Kirby WM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45(4):493–496
Berdy J (2005) Bioactive microbial metabolites. J Antibiot 58:1–26
Boudemagh A, Kitouni M, Boughachiche F, Hamdiken H (2005) Isolation and molecular identification of Actinomycete microflora, of some Saharian soils of southeast Algeria (Biskra, EL-Oued and Ourgla) study of antifungal activity of isolated strains. J Mycol Med 15:39–44
Butler M, Blaskovich M, Cooper M (2017) Antibiotics in the clinical pipeline at the end of 2015. J Antibiot 70:3–24
Chairman K, Ranjit Singh AJA, Alagumuthu G (2012) Cytotoxic and antioxidant activity of selected marine sponges. Asian Pac J Trop Dis 2(3):234–238
Claeson AS, Sunesson AL (2005) Identification using versatile sampling and analytical methods of volatile compounds from Streptomyces albidoflavus grown on four humid building materials and one synthetic medium. Indoor Air 15:41–47
Coronado-Aceves EW, Velázquez C, Robles-Zepeda RE (2016) Reynosin and santamarine: two sesquiterpene lactones from Ambrosia confertiflora with bactericidal activity against clinical strains of Mycobacterium tuberculosis. Pharm Biol 54(11):2623–2628
Dubourg G, Abat C, Raoult D (2017) Why new antibiotics are not obviously useful now? Int J Antimicrob Agents 49:49–553
Eliana MM, Daniel S, Stina MO, Olov S (2014) Cytotoxic Sesquiterpene Lactones from Kauna lasiophthalma Griseb. Sci Pharm 82(1):147–160
George M, George G, Hatha AM (2010) Diversity and antibacterial activity of actinomycetes from wetland soil. S Pac J Nat Appl Sci 28:52–57
Goodfellow M, Williams ST (1983) Ecology of Actinomycetes. Annu Rev Microbiol 37:189–216
Gopikrishnan V, Radhakrishnan M, Shanmugasundaram T, Ramakodi MP, Balagurunathan R (2019) Isolation, characterization and identification of antibiofouling metabolite from mangrove derived Streptomyces sampsonii PM33. Sci Rep 9(1):12975
Jackson SA, Crossman L, Almeida EL, Margassery LM, Kennedy J, Dobson AW (2018) Diverse and abundant secondary metabolism biosynthetic gene clusters in the genomes of marine sponge derived Streptomyces spp. isolates. Mar Drugs 16:1–18
Jiang H, Dong CZ, Huang Q, Wang G, Fang B, Zhang C, Dong H (2012) Actinobacterial diversity in microbial mats of five hot springs in Central and Central-Eastern Tibet. China Geomicrobiol 29(6):520–527
Latha S, Vinothini G, Dhanasekaran D (2015) Chromium [Cr(VI)] biosorption property of the newly isolated actinobacterial probiont Streptomyces werraensis LD22. 3 Biotech 5:423–432
Lee SD, Lee DW, Ko YH (2011) Marmoricola korecus sp. nov. Int J Syst Evol Microbiol 61(7):1628–1631
Madigan MT, Martinko JM, Dunlap PV, Clark DP (2008) Brock biology of microorganism, 12th edn. Int Microbiol Pearson Education, Inc, pp 416–417
Moaz M, Hamed L, Nayer M (2019) Antimicrobial activity of marine actinomycetes and the optimization of culture conditions for the production of antimicrobial agent(s). J Pure Appl Microbiol 13(4):2177–2188
Müller R, Wink J (2013) Future potential for anti-infectives from bacteria—how to exploit biodiversity and genomic potential. Int J Med Microbiol 304:3–13
Naif AA, Galal AE, Veeramuthu D, Mariadhas VA, Mounir MS (2016) Isolation, identification and screening of antimicrobial thermophilic Streptomyces sp. Al-Dhabi-1 isolated from Tharban hot spring. Saudi Arabia Extremophiles 20:79–90
National Committee for Clinical Laboratory Standards (2003) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 6th edn. Approved Standard M7-A6, NCCLS, Wayne
Olano C, Méndez C, Salas JA (2009) Antitumor compounds from marine actinomycetes. Mar Drugs 7:210–248
Ozturk S, Ercisli S (2006) Chemical composition and in vitro antibacterial activity of Seseli libanotis. World J Microbiol Biotechnol 22:261–265
Rahman M, Islam M, Islam M (2011) Antibacterial activities of actinomycete isolates collected from soils of Rajshahi, Bangladesh. Biotechnol Res Int. https://doi.org/10.4061/2011/857925
Reda FM, Shafi SA, Ismail M (2016) Efficient inhibition of some multi-drug resistant pathogenic bacteria by bioactive metabolites from Bacillus amyloliquefaciens S5I4 isolated from archaeological soil in Egypt. Appl Biochem Microbiol 52:593–601
Saadoun I, Gharaibeh R (2002) The Streptomyces flora of Jordan and its’ potential as a source of antibiotics active against antibiotic-resistant Gram-negative bacteria. World J Microbiol Biotechnol 18(5):465–470
Salam N, Jiao JY, Zhang XT, Li WJ (2020) Update on the classification of higher ranks in the phylum Actinobacteria. Int J Syst Evol Microbiol 70(2):1331–1355
Sanger F, Nicklen S, Coulson AR (1997) DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Sengupta S, Pramanik A, Ghosh A, Bhattacharyya M (2015) Antimicrobial activities of actinomycetes isolated from unexplored regions of Sundarbans mangrove ecosystem. BMC Microbiol 15:170
Seong CN, Choi JH, Baik K (2001) An improved selective isolation of rare actinomycetes from forest soil. J Microbiol 39:17–23
Shah AM, Wani A, Qazi PH, Rehman SU, Mushtaq S, Ali SA, Hussain A, Shah A, Qazi AK, Makhdoomi US, Hamid A, Kumar A (2016) Isolation and characterization of alborixin from Streptomyces scabrisporus: a potent cytotoxic agent against human colon (HCT-116) cancer cells. Chem Biol Interact 256:198–208
Shah AM, Shakeel-U-Rehman HA, Mushtaq S, Rather MA, Shah A, Ahmad Z, Khan IA, Bhat KA, Hassan QP (2017) Antimicrobial investigation of selected soil actinomycetes isolated from unexplored regions of Kashmir Himalayas. India Microb Pathog 110:93–99
Sutyak KE, Wirawan RE, Aroutcheva AA, Chikindas ML (2008) Isolation of the Bacillus subtilis antimicrobial peptide subtilosin from the dairy product-derived Bacillus amyloliquefaciens. J Appl Microbiol 104(4):1067–1074
Tamura K, Peterson D, Peterson N, Stecher G, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Trenozhnikova L, Azizan A (2018) Discovery of actinomycetes from extreme environments with potential to produce novel antibiotics. Central Asian J Glob Health 7:1–15
Uddin M, Mahmud N, Anwar N, Manchur MA (2013) Bioactive metabolite production by Streptomyces albolongus in favourable environment. J Microbiol Infect Dis 3:75–82
Whitman W, Goodfellow M, Kämpfer P, Busse HJ, Trujillo M, Ludwig W, Suzuki KI, Parte A (2012) Bergey’s manual of systematic bacteriology. The Actinobacteria, 2nd edn. Springer, New Delhi, India XLVIII, p 2083
Wilkins K (1996) Volatile metabolites from actinomycetes. Chemosphere 32:1427–1434
Williams ST, Sharpe ME, Holt JG (1989) Bergey’s manual of systematic bacteriology, vol. 4. Williams and Wilkins Company, Baltimore, p 2648
Xu LH, TiangY ZY, Zhao L, Jiang C (1998) Streptomyces thermogriseus, a new species of the genus Streptomyces from soil, lake and hot-spring. Int J Syst Bacteriol 48:1089–1093
Yunfeng Z, Dong W, Siyuan G, Xuewu Z, Mingfu W, Feng C (2007) Chemical components and antioxidant activity of the volatile oil from Cassia tora L. seed prepared by supercritical fluid extraction. J Food Lipids 14(4):411–423
Zhao K, Penttinen P, Xiao TG, Chen Q, Xu J (2011) The Diversity and antimicrobial activity of endophytic actinomycetes isolated from medicinal plants in Panxi Plateau, China. Curr Microbiol 62:182–190
Acknowledgements
The authors would like to extend their appreciation to Prof. Dr Yuanda Song for helping and technical support during the research work, and we also thank the Botany and Microbiology Department, Faculty of Science, Al-Azhar University for providing lab and instrumental facilities.
Funding
This study was funded by the National Natural Science Foundation of China (Grants Nos. 31972851 and 31670064) and TaiShan Industrial Experts Programme (No.tscy20160101).
Author information
Authors and Affiliations
Contributions
Conceptualization, HM and YS; methodology, HM; software, HM, AH and MR; validation, ME, UAR, and AG; formal analysis, HM; resources, AG; data curation, AMS, HA; writing—original draft preparation, HM; writing—review and editing, HM, and YS; supervision, UAR, and MR; funding acquisition, YY. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
203_2021_2487_MOESM1_ESM.docx
Supplementary file 1 (DOCX 1634 KB). Figure S1. The geographic location of sampling sites at different Egyptian governorates. a) El-Minia (40 isolates); b) Assiut (21 isolates); c) Sohag (8 isolates); d) Qena (4 isolates); and E) Luxor (14 isolates), during the period 2016–2018, Table S2. The temperature profile of all isolated actinomycetes, Table S3. Preliminary antibacterial screening of thermophilic actinomycetes isolates using cross streaking method, Figure S4. Streak plate showing the pure culture of Streptomyces sp. MI-S.24-3 on starch casein agar medium at 50 °C for 7 days (a) and light microscopy 400x: showing spiral-shaped mycelium of Streptomyces sp. MI-S.24-3 growing on starch nitrate agar at 50°C for 3 days (b). Scanning electron micrographs of isolate, MI-S.24-3 (c): long, none or moderately branched spiral hyphae (12000x), (d): aerial mycelium dividing into rod-shaped spores and spiny-surfaced spores (14000x), Table S5. Physiological and biochemical characteristics of the MI-S.24-3., Table S6. Cultural characteristics of MI-S.24-3 isolate, allowed to grow on different.
Rights and permissions
About this article
Cite this article
Mohamed, H., Hassane, A., Rawway, M. et al. Antibacterial and cytotoxic potency of thermophilic Streptomyces werraensis MI-S.24-3 isolated from an Egyptian extreme environment. Arch Microbiol 203, 4961–4972 (2021). https://doi.org/10.1007/s00203-021-02487-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-021-02487-0