Abstract
Summary
Despite rapidly ageing populations, data on healthcare costs associated with hip fracture in Sub-Saharan Africa are limited. We estimated high direct medical costs for managing hip fracture within the public healthcare system in SA. These findings should support policy decisions on budgeting and planning of hip fracture services.
Purpose
We estimated direct healthcare costs of hip fracture (HF) management in the South African (SA) public healthcare system.
Methods
We conducted a micro-costing study to estimate costs per patient treated for HF in five regional public sector hospitals in KwaZulu-Natal (KZN), SA. Two hundred consecutive, consenting patients presenting with a fragility HF were prospectively enrolled. Resources used including staff time, consumables, laboratory investigations, radiographs, operating theatre time, surgical implants, medicines, and inpatient days were collected from presentation to discharge. Counts of resources used were multiplied by unit costs, estimated from the KZN Department of Health hospital fees manual 2019/2020, in local currency (South African Rand, ZAR), and converted to 2020 US$ prices. Generalized linear models estimated total covariate-adjusted costs and cost predictors.
Results
The mean unadjusted cost for HF management was US$6935 (95% CI; US$6401–7620) [ZAR114,179 (95% CI; ZAR105,468–125,335)]. The major cost driver was orthopaedics/surgical ward costs US$5904 (95% CI; 5408–6535), contributing to 85% of total cost. The covariate-adjusted cost for HF management was US$6922 (95% CI; US$6743–7118) [ZAR113,976 (95% CI; ZAR111,031–117,197)]. After covariate adjustment, total costs were higher in patients operated under general anaesthesia [US$7251 (95% CI; US$6506–7901)] compared to surgery under spinal anaesthesia US$6880 (95% CI; US$6685–7092) and no surgery US$7032 (95% CI; US$6454–7651).
Conclusion
Healthcare costs following a HF are high relative to the gross domestic product per capita and per capita spending on health in SA. As the population ages, this significant economic burden to the health system will increase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osteoporotic fragility fractures pose a major clinical and economic burden due to high morbidity, mortality, and healthcare costs [1, 2]. High costs stem from multiple comorbidities present in hip fracture (HF) patients, which must be co-managed. HFs are associated with high levels of morbidity, prolonged hospital stays, and increased mortality, with 13% dying within a month of fracture [3].
Fragility fractures are an emerging healthcare problem in Sub-Saharan Africa (SSA), with significant increases projected over the next few years [4–6], largely driven by the growing (current and projected) number of older adults (age ≥60 years) [7] with lengthening life expectancy [8, 9]. Despite the reported current and projected increase in the clinical burden of fragility fractures [3, 10], including HFs, data on the economic burden attributable to fractures in SSA are limited. Data on costs associated with HFs are important for quantifying demands on healthcare services, informing accurate cost-effectiveness analyses of potential interventions, and for guiding policy decisions on budgeting and planning of future investments in clinical services.
South Africa (SA) has a dual healthcare system (public and private); however, most citizens use the public healthcare system, and only 17% have medical insurance suggesting access to private healthcare [11]. The public healthcare system is divided into primary healthcare facilities (clinics and district hospitals), larger regional and tertiary hospitals, and central hospitals. Orthopaedic surgical services are provided in regional, tertiary, and central hospitals.
eThekwini (formally known as Durban), in the province of KwaZulu-Natal (KZN), in SA, has a multi-ethnic population of approximately 3.47 million (51% Black Africans, 24% Indian/Asian, 15% White, 9% mixed ethnicity) making the municipality one of the biggest cities on the Indian Ocean coast of the African continent and third largest city in SA. In this study, we first estimated the direct healthcare system resource use and costs associated with the management of HF in the public healthcare system in eThekwini. Secondly, we evaluated the main predictors of healthcare system costs following a HF.
Methods
Study design and setting
A prospective study was conducted of incident HFs, to determine demographic profiles (Table 1), risk factors, outcomes, and healthcare costs in a cohort of patients admitted with osteoporotic HF between August 2010 and October 2011, across five public sector regional hospitals offering orthopaedic services in the city of eThekwini in SA. Study methods have previously been reported [3]. In brief, the study enrolled consecutive adult patients aged 60 years and over, admitted with a minimal trauma HF (defined as a fracture of the femur between the articular cartilage of the hip joint to 5 cm below the distal point of the lesser trochanter, following a fall from standing height or less), who provided written informed consent. Patients were followed-up for 1 year, or until death or loss to follow-up, if this occurred earlier.
Study participants
Hip fracture patients were sequentially identified from orthopaedic ward admission registers, which record date of admission, patient demographics, and admission diagnosis based on the clinical and radiological findings of the admitting doctor. Original medical records and radiographs of all patients identified from the admission registers were reviewed to verify the HF diagnosis before study enrolment.
Resource utilization
We estimated the mean cost per patient treated for a HF using a micro-costing approach (i.e. direct enumeration and valuation of each component of resource use (inputs) consumed in the treatment of a patient) [12, 13]. Standard techniques for conducting micro-cost analyses were utilized, including the prospective identification, quantification, and valuation (assigning monetary value) of all resources used in the management of HF for each individual patient studied. Firstly, a detailed stakeholder review of the HF pathway of care and of patient hospital records was performed to map out all relevant activities and resources potentially utilized in HF management (see Supplementary Table S1). The pathway of care starts with initial presentation at a primary healthcare facility followed by transfer to the outpatient department of a regional hospital or direct presentation to the emergency department of a regional hospital. This is followed by admission to an orthopaedic ward, surgery (if judged appropriate), discharge, and outpatient follow-up. Information on the types and quantities of all resources utilized in the mapped-out activities were measured from initial presentation at a primary healthcare facility/public sector regional hospital, through to discharge, and included outpatient follow-up. Data on resource use and costs for the entire episode were collected, including the referral pathway for patients initially presenting at different primary healthcare facilities.
Resource use data were collected in natural units (for example, hours and minutes for staff time and number for consumables and investigations). Staff times (for paramedics, nurses, surgeons, anaesthetists, radiographers, porters, physiotherapists, etc.) were measured in terms of minutes spent per patient, measured prospectively during the study. A researcher observed staff members performing different activities to determine the amount of time spent on a random sample of patients from different hospitals included in the study. Ambulance use was measured in terms of the distance travelled in kilometres (km) from the patient’s home or the referring primary healthcare facility to the regional hospital. The length of stay in surgical/orthopaedic wards was recorded for each patient in days. Use of ward consumables was measured by estimating average utilization per patient over a 24 h of stay and extrapolated for the entire duration of stay. Theatre time was measured as operation duration in minutes obtained from theatre notes for each patient surgically managed. Surgical implants, laboratory investigations, radiographs (X-rays), other diagnostic investigations, and medicines were measured based on actual utilization records for each patient (Table 1). Patient-level data on outpatient follow-up after discharge were not available and were not included in the analysis. Details on measurement of resources used and their valuations are shown in Supplementary Table S2.
Costs
Healthcare resources were calculated by multiplying the quantities used by the relevant unit costs (see Table 2). Unit costs for visits to different healthcare providers or departments within the hospital were estimated by calculating the cost of time spent by staff involved for each visit. For example, the unit cost for the X-rays included staff time costs for an administrative clerk, a porter, and a radiologist and the cost of radiographic materials. The average unit cost per day of inpatient ward stay was estimated in consultation with finance department of one regional hospital and then applied to all the other regional hospitals and included ward stay (cost of hospital bed per day), nursing care, and other daily ward expenses (for example, meals, once-off and daily consumables). The unit costs for staff time in theatre (orthopaedic surgeon, theatre nurse, anaesthetist) were estimated per hour of operating time.
All costs for staff times were calculated using the South African Department of Public Service and Administration’s midpoint staff salaries (averaged over the available grades) [15], including overtime pay/remuneration for work in hospital over weekends and public holidays. Unit costs for traction, operating theatre facility fees, surgical implants, pathology and laboratory investigations, radiographs, other investigations, and medicines were estimated from market-based prices published in the KZN Department of Health (DOH) hospital fees manual for 2020/2021 [16].
Overheads were allocated to direct costs by raising the estimated direct costs by a 25% mark-up percentage. Direct costs, namely costs directly related to the delivery of patient care, are driven by patient type and volume and individual patient episodes of care. The proportionate value for overheads was determined by dividing the hospital-wide total annual overhead expenditure by the total annual direct expenditure in orthopaedic departments (orthopaedic clinic, surgical/orthopaedic ward, and orthopaedic operating theatre) using data obtained from Inkosi Albert Luthuli Central Hospital for the 2019–2020 financial year. All costs were measured in local currency (South African Rand [ZAR]) and reported in 2020 United States dollar (US$) prices to aid international comparison (estimated using the weighted average exchange of ZAR16.50 per US$ in 2020 obtained from the South African Reserve Bank). Unit costs for main resources used for the cohort in this analysis are presented on Table 2.
Statistical analysis
Descriptive statistics are reported for demographic and clinical characteristics, clinical outcomes, healthcare resource use, and healthcare costs. Categorical variables are summarized using frequencies (with percentages), and continuous variables are summarized using the mean and standard deviation (SD) or the median, with lower and upper quartiles, as appropriate. The total healthcare cost per patient was estimated by summing costs across all categories from initial presentation to discharge and including outpatient follow-up.
Mean unadjusted healthcare costs are grouped by main pathway of care categories (outpatient, ward stay, investigations, theatre/operation, medicines, and post-discharge follow-up) and reported by type of management (surgical versus non-surgical management). Stratified analyses, informed by literature review [17,18,19,20], were performed to investigate how costs varied between different patient groups, namely: age (<70, 70–79, ≥80 years), sex (male, female), ethnicity (as classified by SA census[14]: Black African, Indian/Asian, other ethnic groups [White and coloured]), level of education (no formal education, primary education, secondary education, higher education), employment status (receiving a pension, other employment status), residence prior to admission (formal housing, other housing), prior fragility fracture, comorbidity (no comorbidities, one comorbidity or more), and mortality at four time points (discharged alive, died by 30 days, died by 180 days, and died by 365 days).
A generalized linear model (GLM) was used to estimate predictors of total costs of HF and covariate-adjusted costs. All the variables used in the stratified analysis (age, sex, ethnicity, education, employment, residence prior to admission, prior fragility fractures, comorbidity, and mortality) were considered for the full model. The regression model evaluated the main effects (no interactions) only. The modified Park test was used to inform the choice of the GLM model family, and the Pregibon’s link test was used to inform the choice of the link functions used [21]. Different family and link functions were compared using Akaike’s information criterion. Mean unadjusted and adjusted healthcare costs are presented along with bootstrapped bias corrected and accelerated 95% confidence intervals.
Ethical and governance approvals
Ethical approval to conduct the study was granted by the Biomedical Research Ethics Committee (BREC) of the University of KwaZulu-Natal (UKZN) (BF043/09). The KwaZulu-Natal (KZN) Provincial DOH and individual hospitals each provided approvals for this study.
Results
Study population
A total of 277 adults aged 60 years and over with minimal trauma HF were admitted to the five selected hospitals, of these 200 (72%) provided informed consent and were included in the cost analysis. The demographic and clinical characteristics of these patients are shown in Table 1 and Supplementary Figure S1 (including the 77 patients who did not consent). Of those who consented, 53 patients (26%) had a prior fragility fracture, including 15 (8%) with a previous HF. The mean age was 74 (SD; 9, IQR; 68–80) years, and the majority (n = 144, 72%) were women. Most patients were either of Indian/Asian (n=110, 55%) or Black African (n=66, 33%) ethnicity. Overall, 69 (35%) were referred to the treating hospital from a primary healthcare facility (19% from another hospital, 13% from a clinic, 3% from a general practitioner, and 1% from a care home). The remainder 66% (131/200) presented directly to the treating hospital. More than 70% of patients had one or more comorbidity (n=147, 74%). The most frequent is hypertension (n=120, 60%), diabetes mellitus (n=57, 29%), and arthritis (n=55, 28%). In total, 117 (59%) patients were followed-up to 1 year, 67 (34%) died within 1 year, and 16 (8%) were lost to follow-up, resulting in an average follow-up time for the cohort of 7.7 (SD; 5.3) months.
Resource use
Among the 69 patients referred from another healthcare provider, the mean distance travelled from the site of injury in the community or facility of initial presentation to the referral hospital was 28.5 (SD; 39.4) km, and the mean time spent by attending paramedic staff on transfer was 52.9 (SD; 77.3) min. On admission to the surgical/orthopaedic ward, all patients had skin traction applied. Most patients were surgically managed (n=174, 87%), with 93% (n=162) operated under spinal anaesthesia and 7% (n=12) under general anaesthesia. Surgically managed patients mostly received a bipolar hemiarthroplasty (34%) or internal fixation (with screw and plate) (37%), and they had a mean time in surgery of 66.2 (SD; 38.3) min. The mean length of stay (LOS) in hospital was 21.4 (SD; 15.5) days (Table 2).
Costs and cost drivers
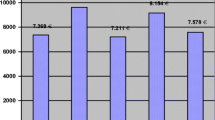
The unadjusted mean healthcare costs, following a HF, across different cost categories are shown in Table 3. Fig. 1 shows the distribution of these costs. The mean healthcare cost for management following a HF was estimated to be US$6935 (95% CI; US$6401–7620) [equivalent to ZAR114,179 (95% CI; ZAR105,468–125,335)]. The major cost driver was the surgical/orthopaedic ward stay (i.e., cost of hospital bed per day), costing on average US$5904 (95% CI; US$5408–6535) per patient, contributing 85% to the total cost (unit cost per bed day; US$239). Patients who did not receive surgery had higher mean ward costs of US$6311 (95% CI; US$4809–88,941) compared to patients managed surgically, with a mean difference in ward costs of US$473 (95% CI; −1311 to 3129) and US$395 (95% CI; −1717 to 3556) relative to patients operated under general and spinal anaesthesia, respectively. The second highest cost driver was operating theatre costs, with a mean of US$685 (95% CI; 675–696) per patient, contributing 9% of the total costs; this cost was largely driven by implant costs. The mean unit cost for an implant was US$147 (range; US$97–164).
Stratified analyses
In the unadjusted subgroup analysis, total costs of treatment were higher in females, older patients (age >70 years), and those receiving a pension, as well as patients of Black African ethnicity, patients with one or more comorbidity, patients who had surgery under spinal anaesthesia, and patients who died after 180 days. However, confidence intervals around the estimates for each subgroup were wide and overlapping (Table 4).
Adjusted costs
The full covariate-adjusted mean cost (adjusted for age, sex, ethnicity, education, employment, residence prior to admission, prior fragility fracture, comorbidity, and mortality) was US$6966 (95% CI; US$6787–7160) [ZAR114,396 (95% CI; ZAR111,745–117,931)], similar to the unadjusted cost. The adjusted total costs (Table 3) were higher in patients who had surgery under general anaesthesia [US$7295 (95% CI; US$6555–7951)] compared to patients who had surgery under spinal anaesthesia US$6925 (95% CI; US$6729–7136) and those who did not have surgery US$7072 (95% CI; US$6494–7694).
Predictors of hip fracture costs
After controlling for age, sex, ethnicity, education, employment, residence prior to admission, prior fragility fracture, comorbidity, and mortality in the multivariate analysis (Table 5), patients who died within 180 days of hip fracture had a higher cost of US$2146 [95% CI; US$389–4330] more than those discharged alive. Compared to patients of Indian/Asian ethnicity, patients of Black African ethnicity had higher costs, US$2140 [95% CI; $834–3556]).
Discussion
Hip fracture care is expensive for the South African public healthcare system. We have shown that HFs are associated with substantial healthcare resource utilization and high direct medical costs within the public healthcare system in SA. We estimated average total costs in the first-year post-HF of US$6935, with 85% attributable to on average 21-day stay in hospital. The lower contribution of theatre costs observed in this study is potentially a reflection of the fact that none of the operated patients underwent a total hip replacement, which is normally associated with higher cost. The main predictors of high costs were Black African ethnicity and mortality within 180-day post-fracture, likely reflecting a higher comorbid disease burden resulting in increased healthcare resource use and costs.
The HF-specific costs identified in this study equate to 58% of the GDP per capita (US$12,096 in 2020) [22] and are six times greater than the mean per capita health expenditure for SA (US$1187 in 2019) [23], indicating the substantial economic burden HFs have on the public healthcare system in SA. Comparison to local cost estimates in SA is limited by lack of relevant data on costs of other major acute healthcare conditions and by differences in methodological approaches used to derive estimates. Our total cost estimate is higher but closer to an estimate of the cost of an uncomplicated primary hip arthroplasty, US$5015 in 2021 prices, reported from a single public hospital in Johannesburg, South Africa [24]. However, there is a stark difference in the distribution of costs. Unlike in our study where ward stay was the largest component of cost (85%), Sekeitto and Aden [24] reported the major contributors to their cost as prosthesis (54%), theatre (23%), and inpatient admission (18%). This difference is largely due to the different prosthesis/implant unit costs as well as differences in LOS. The average unit cost for a prosthesis in our study [US$147 (range; US$97–164)] is 18 times lower than that applied in Sekeitto and Aden (US$ 2725)[24], potentially reflecting a high level of subsidization. Therefore, our total estimate could well be an underestimate, and more work is required to establish the true cost. The low contribution of inpatient costs in Sekeitto and Aden [24] can be explained by the reported low LOS of 7.5 days compared to 21.5 days in our study. Adjusted for inflation to 2021 prices, the public-sector average cost per general admission was previously estimated to be US$888 [25]. This public-sector average cost per admission is relatively low and not comparable to our HF estimate because it is adjusted for a mix of hospital types, case-mix, and different comorbidities [25]. In another study, the direct cost of stroke care per patient (inpatient only) was estimated to be US$12,113 [26], based on 2021 prices. The cost of managing stroke is relatively high because it includes often lengthy rehabilitation and speech therapy costs but importantly uses different costing approaches compared to our methods.
To the best of our knowledge, ours is the first study in the SSA region, to systematically and comprehensively estimate the costs associated with HF care. Existing evidence on the economic burden of HF is largely derived from HICs, where high costs are consistently reported. In 2017 a systematic review and meta-regression analysis estimated the cost of initial hospitalization for HF in a HIC setting, to be US$11,549 (95% CI US$9540–13,559) [27], adjusted for inflation to 2021 prices. Although directly comparing international cost estimates is challenging, due to several differences in methodological approaches, chosen cost categories, resource utilization, unit cost prices, pathways of care, and patient populations, our estimates are consistent with these figures. Putting these HIC costs into the context of our estimates, the systematic review cost estimate represented 17% of the GDP per capita and is approximately equal to the annual per capita health expenditure in the USA in 2021. In Europe, the 2021 average percentage of healthcare spending on osteoporotic fractures was 3.5%, ranging from 1.3% in Luxembourg to 6.2% in Greece [28]. Multiplying our estimate of the average cost per HF by the estimated annual number of HFs for SA in 2020 (11,000) [6] results in a total cost of US$76,285,000, representing 0.5% of healthcare spending for SA in 2020 (ZAR229.7 billion or US$13.9 billion). In SA, the annual number of HFs is projected to rise to 26,400 by 2050 [6] and will result in substantial increases in costs and in the economic burden to SA.
In HICs, the same systematic review and meta-regression analysis reported the total health and social care costs in the 12 months following HF to be U$50,540 (95% CI; US$36,372–64,707), adjusted for inflation to 2021 prices. More recently, Talevski et al. [29] reported a mean cost per patient in class 6 HF care pathway (the model we considered to have close similarities to HF care as provided in KZN public hospitals) of $48,999 (95% CI; 39,704–58,294) Australian Dollars [approximately US$30,387 (95% CI; 24,622–36,151)]. Healthcare resource utilization and costs following fractures reflect the pathways of care. The direct cost component of these international cost estimates often includes community and residential care. High-income countries often have highly developed multidisciplinary orthogeriatric units, with hospital discharges supported by intermediate care services so that HF patients can be discharged home with community-based rehabilitation teams or, if necessary, transferred to step-down inpatient facilities for ongoing care and rehabilitation prior to home discharge supported as needed by social care services. By contrast, step-down and social care services are not widely available in SA especially for patients accessing public healthcare nor across SSA. Hence, most patients return directly home on discharge irrespective of rehabilitation needs. Residual disability, dependency, and loss of income may represent a significant indirect cost of an illness, potentially causing a healthcare expenditure catastrophe (defined as financial hardship caused by health-related payments) [31]. Our healthcare costs largely reflect in-hospital care and do not include community care, which contributes substantially to the total cost. These indirect costs are difficult to identify and estimate, which limits our analysis.
Strengths and limitations
This study presents the first systematically derived estimate of the costs of managing acute HFs in SA. The study draws major strength from the bottom-up costing approach using “real-world” patient-level data collected prospectively from all the five public hospitals providing the majority of orthopaedic services in the eThekwini municipality, which are likely to be representative of practice in the province. The approach was based on a detailed review of the pathway of care typically followed by HF patients, from admission to discharge allowing for meticulous gathering of data on resource utilization and estimation of costs. The prospective design of this study ensured that all relevant resources were carefully identified and quantified resulting in accurate estimates of resource utilization and costs.
Despite these strengths, the interpretation of our findings requires consideration of potential limitations. Firstly, we analysed resource use data collected from a cohort of patients back in 2010/2011 (12 years ago), which may not correctly reflect current practice and resource utilization. However, our review of local guidelines on the management of hip fractures in South Africa and consultation with in-country musculoskeletal experts suggests that there has not been a major shift in clinical practice since the time of resource use data collection. More so, these first estimates are relevant in the context of the reported current and projected clinical burden of fragility fractures (Hawley et al. 2022) and a dearth of data on resource utilization and costs in SSA. Secondly, our study focused on the public healthcare system, and cost estimates do not reflect private sector spending, largely characterized by high and rising healthcare costs [30]. For example, in SA the annual per capita health expenditure was approximately US$140 in the public sector and US$1400 in the private sector in 2014 [32]. However, the private healthcare sector provides only the minority of the population with healthcare [11]. Thirdly, we estimated direct healthcare costs incurred by the provider, excluding direct non-medical and indirect costs, despite evidence of their impact on the total economic burden of HF [33]. These costs falling upon patients and their families can be catastrophic [31]. Attending health facilities, especially higher-level facilities that may be at a great distance from the patient’s home, require transportation and may necessitate patient and/or caregiver loss of earnings. We acknowledge the importance of these costs to families and society, and we highlight it as an essential area for future research. The data collected and analysed in this study could, however, be used alongside appropriate data on societal costs to produce a wider analysis of the societal impact of HF in SA. Fourthly, although our estimates represent the first estimates for SA, these costs may not be generalisable across the rest of the country as patient care seeking [11, 34] and hospital care practices, and unit costs are variable [25]. We lacked data to stratify patients according to the fracture subtypes (neck, intertrochanteric and subtrochanteric); hence, further research is needed. Audits of the resources used in the management of HF and costs in other SA provinces are required to validate these estimates.
Conclusion
Our results demonstrate that the management of HFs are associated with significant direct medical costs in the public healthcare system of SA, which far exceeds the annual South African per capita health expenditure. These cost estimates should enable resource planning and allocation decisions in public healthcare facilities, which will be important given the ageing South African population and predicted rise in HF incidence. Further research is required to evaluate direct non-medical, and the indirect costs patients incurred post-HF.
References
Kanis JA, Oden A, Johnell O et al (2001) The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int 12(5):417–427
Lorentzon M, Johansson H, Harvey NC et al (2021) Osteoporosis and fractures in women: the burden of disease. Climacteric:1–7
Paruk F, Matthews G, Gregson CL et al (2020) Hip fractures in South Africa: mortality outcomes over 12 months post-fracture. Arch Osteoporos 15(1):76
Conradie M, Conradie MM, Scher AT et al (2015) Vertebral fracture prevalence in black and white South African women. Arch Osteoporos 10:203
Paruk F, Matthews G, Cassim B (2017) Osteoporotic hip fractures in Black South Africans: a regional study. Arch Osteoporos 12(1):107
Hawley S, Dela S, Burton A et al (2022) Incidence and number of fragility fractures of the hip in South Africa: estimated projections from 2020 to 2050. Osteoporos Int
United Nations Department of Economic and Social Affairs Population Division (2019) World Population Prospects 2019, custom data acquired via website
Salami D, Shaaban AN, Oliveira Martins MDR (2019) Africa rising, a narrative for life expectancy gains? Evidence from a health production function. Ann Glob. Health 85(1)
United Nations Department of Economic and Social Affairs Population Division (2019) World population prospects 2019
Dela SS, Paruk F, Brown SL et al (2020) Ethnic and gender-specific incidence rates for hip fractures in South Africa: a multi-centre study. Bone 133:115253
Statistics South Africa (2020) General household survey 2019. Statistics South Africa, Pretoria, p 113
Potter S, Davies C, Davies G et al (2020) The use of micro-costing in economic analyses of surgical interventions: a systematic review. Health Econ Rev 10(1):3
Drummond MF, Sculpher MJ, Claxton K et al (2015) Methods for the economic evaluation of health care programmes. Oxford university press
Statistics South Africa (2016) Community survey 2016, Statistical release P0301
Republic of South Africa Department of Public Service and Administration (DPSA) (2019) Salary scales. DPSA
KwaZulu-Natal Department of Health (KZN DoH) (2020) Hospital Fees Manual Republic of South Africa: KZN DoH
Glynn J, Hollingworth W, Bhimjiyani A et al (2020) How does deprivation influence secondary care costs after hip fracture? Osteoporos Int 31(8):1573–1585
Lambrelli D, Burge R, Raluy-Callado M et al (2014) Retrospective database study to assess the economic impact of hip fracture in the United Kingdom. J Med Econ 17(11):817–825
Leal J, Gray AM, Prieto-Alhambra D et al (2016) Impact of hip fracture on hospital care costs: a population-based study. Osteoporos Int 27(2):549–558
Kanters TA, van de Ree CLP, de Jongh MAC et al (2020) Burden of illness of hip fractures in elderly Dutch patients. Arch of Osteopo 15(1):11
Jones AM (2010) Models for health care University of York, Centre for Health Economics
World Bank (2020) World development indicators 2020, in GDP per capita, PPP (current international $) - South Africa. The World Bank
World Bank (2018) World development indicators 2018, in Current health expenditure per capita, PPP (current international $) - South Africa. The World Bank
Sekeitto AR, Aden AA (2021) Costing total hip arthroplasty in a South African state tertiary hospital. S Afr Med J 111(3):250–254
Ramjee S (2013) Comparing the cost of delivering hospital services across the public and private sectors in South Africa. University of Cape Town, The Hospital Association of South Africa
Matizirofa L, Chikobvu D (2021) Analysing and quantifying the effect of predictors of stroke direct costs in South Africa using quantile regression. BMC Public Health 21(1):1560
Williamson S, Landeiro F, McConnell T et al (2017) Costs of fragility hip fractures globally: a systematic review and meta-regression analysis. Osteoporos Int 28(10):2791–2800
Kanis JA, Norton N, Harvey NC et al (2021) SCOPE 2021: a new scorecard for osteoporosis in Europe. Arch of Osteopo 16(1):82
Talevski J, Sanders K, Lal A et al (2022) A micro-costing analysis of post-fracture care pathways: results from the International Costs and Utilities Related to Osteoporotic Fractures Study (ICUROS). Osteopo Int 33(9):1895–1907
Competition Commission of South Africa (2019) Health market inquiry final findings and recommendations report. Government Gazette No. 42861
World Health Organization (2021) Tracking universal health coverage: 2021 global monitoring report. World Health Organization
de Villiers K (2021) Bridging the health inequality gap: an examination of South Africa’s social innovation in health landscape. Infect Dis Poverty 10(1):19
Veronese N, Kolk H, Maggi S (2021) Epidemiology of fragility fractures and social impact, in orthogeriatrics: the management of older patients with fragility fractures. In: Falaschi P, Marsh D (eds) . Springer Copyright 2021, Cham (CH), pp 19–34
Jim A (2010) Health seeking behaviours in South Africa: a household perspective using the general households survey of 2007. University of the Western Cape
Funding
This work was supported by the National Institute for Health Research (NIHR) (using the UK’s Official Development Assistance (ODA) Funding) and the Wellcome (217135/Z/19/Z; 2020-25) under the NIHR-Wellcome Partnership for Global Health Research. The views expressed are those of the authors and not necessarily those of the Wellcome, the NIHR, or the Department of Health and Social Care. FP was funded through an unrestricted educational grant from Servier® PTY (LTD) (2010) and the University of KwaZulu-Natal competitive grant (2010). For the purpose of Open Access, the author has applied a CC-BY public copyright licence to the Author Accepted Manuscript version arising from this submission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nyashadzaishe Mafirakureva and Farhanah Paruk are the joint first authors.
Supplementary information
ESM 1
(DOCX 528 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mafirakureva, N., Paruk, F., Cassim, B. et al. The healthcare system costs of hip fracture care in South Africa. Osteoporos Int 34, 803–813 (2023). https://doi.org/10.1007/s00198-022-06664-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-022-06664-9