Abstract
Osteoporotic fractures are one of the major problems facing healthcare systems worldwide. Undoubtedly, fragility fractures of the hip represent a far greater burden in terms of morbidity, mortality, and healthcare costs than other fracture sites. However, despite the significant impact on the health and quality of life of older adults, there is a general lack of awareness of osteoporosis, which results in suboptimal care. In fact, most high-risk individuals are never identified and do not receive adequate treatment, leading to further fragility fractures and worsening health status. Furthermore, considering the substantial treatment gap and the proven cost-effectiveness of fracture prevention programs such as Fracture Liaison Services, urgent action is needed to ensure that all individuals at high risk of fragility fracture are adequately assessed and treated. Based on this evidence, the aim of our review was to (i) provide an overview and comparison of the burden and management of fragility fractures, highlighting the main gaps, and (ii) highlight the importance of using alternative approaches, both surgical and non-surgical, with the aim of implementing early prevention of osteoporotic fractures and improving the management of osteoporotic patients at imminent and/or very high risk of fracture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Fragility fractures: epidemiology and socio-economic burden
Osteoporosis is a systemic skeletal disease characterized by a decrease in bone mineral density (BMD) and deterioration of bone microarchitectural, resulting in impaired bone strength and increased risk of fractures [1]. The prevalence of osteoporosis increases with age and is more common among women than men [2]. In fact, osteoporosis is now one of the major health risks for people aged 50 years and over, with a prevalence comparable to diseases such as hypercholesterolemia and hypertension, which affect 54% and 44% of people aged 50 years and over respectively [3]. In Western Europe, approximately 1 in 3 women and 1 in 5 men aged 50 years or older will have a fracture during their remaining lifetime [4]. In addition, a significant difference in fracture risk has been found between countries, with the highest fracture rates being observed in northern European countries [5]. Although the reasons for these discrepancies are not known, plausible factors have included differences in body mass index, low calcium intake, reduced exposure to sunlight, smoking habits, and even high socio-economic status, which in turn may be related to low levels of physical activity [6, 7].
Hip and vertebral fractures are among the most common and severe sites of osteoporotic fracture, whereas fragility fractures of the humerus, forearm, ribs, tibia, pelvis, and other femoral fractures after the age of 50 years are fractures associated with low BMD [8, 9]. Moreover, individuals who have already suffered a fragility fracture run a higher risk of further fractures either at the same site or elsewhere, and this risk is highest immediately after a fracture [10]. For women aged between 50 and 80 years, after the first fragility fracture, the risk of a subsequent fracture within the first year is five times higher than for women who have not had a previous fracture; moreover, the risk of fracture is higher in the first 2 years after an initial fracture, when there is an imminent risk of another fracture at the same or other sites [11, 12]. Thus, the existence of an imminent risk period indicates that by identifying and managing patients early after fracture, fracture prevention treatments can be optimized, and the occurrence of another fracture avoided. To identify this type of patients, the use of algorithms such as the fracture risk assessment tool (FRAX®) allows the risk of fragility fracture to be assessed over a 10-year period [13]. The variables considered are previous fractures, which represent the main risk factor, together with smoking, a family history of femoral or vertebral fracture, use of corticosteroids, alcohol consumption, and the presence of rheumatoid arthritis as the only comorbidity [14].
According to some literature data, the Derived FRAX (DeFRA) model seems to be more reliable than FRAX because it allows more detailed data entry. For example, other rheumatologic conditions can be included as comorbidities, and additional information, such as calcium intake, sun exposure, and vitamin D supplementation, falls in the previous year, and previous treatments can be included [15].
According to the EU6 report, written by the International Osteoporosis Foundation (IOF) to describe the current management and burden of osteoporosis in the five largest European Union countries (France, Germany, Italy, Spain, and the UK) and Sweden, the number of fragility fractures and cases of osteoporosis is increasing worldwide, creating a growing burden on society [16]. In fact, fragility fractures involve both short- and long-term costs for the healthcare sector and society. The costs of fracture differ substantially between countries, but also depend on the fracture sites and the severity of the fracture. For example, hip fractures are the most serious fracture site and almost always lead to hospitalization and high costs in all countries [16]. As the world’s population grows and lives longer, the hospital and societal cost of fragility fractures will continue to increase. In this regard, fracture-related costs in the EU6 are expected to increase by 27%, from a total of €37.5 billion in 2017 to €47.4 billion in 2030 [16]. The health burden was estimated using quality-adjusted life-year (QALY), a method of measuring the burden of disease in which a year of an individual’s life is weighted by the average health-related quality of life (HRQoL) that a person had during that year [17,18,19,20]. According to the EU6 report, 1.02 million QALYs were lost in 2017 due to fragility fractures, and of this loss, 66% was due to fractures occurring in women. The loss of QALYs due to fragility fractures varies across the EU6: the highest loss in absolute numbers was recorded in Germany due to the size of the population combined with a relatively high risk of fractures, while the lowest loss of QALYs was observed in Sweden due to the small population size compared to the other countries. These differences are largely due to variations in fracture risk and age distribution between countries [21, 22].
One of the main burdens caused by fragility fractures is the long-term impact on independence [16]. The fracture may result in a loss of mobility and the ability to care for oneself and may require the individual to move to long-term care (LTC) or care services [23]. The use of LTC varies considerably, depending on the fragility fracture and the age of the individual. For example, it was observed that the percentage of patients moving to LTC following a hip fracture increased significantly with age, from 2.1% at the age of 50–60 years to 35.3% at the age of 90–100 years [16].
As a result of reduced mobility and ability to complete activities of daily living, people who have suffered a fragility fracture may rely on informal caregivers, such as family members and friends. However, the continuous care provided at home can put a strain on relatives who must care for patients with osteoporotic fractures [24, 25]. Again, the hours of care provided by relatives vary considerably from one country to another. In countries where intergenerational support is more established, the impact of fragility fractures on informal caregivers is generally higher, while no significant differences were found in the care of relatives between men and women, nor between patients with and without a previous fracture [16].
Considering the epidemiological and socio-economic impact of fragility fractures, the aim of our review was to make an update on current alternative surgical and non-surgical approaches to fill existing gaps in the bone fragility management.
Gaps in the management of bone fragility
High rates of death, disability, and risk of fragility re-fracture are related to undertreatment of osteoporosis after hip fracture. According to Chau et al., only 40.3% of patients are prescribed anti-osteoporotic treatment within 1 year after a hip fracture, and only 49.7% of patients were compliant with drug treatment. Specifically, the authors claim that elderly male patients between 70 and 79 years of age were less persistent and adherent to therapy than women under 69 years of age [26]. In agreement, Rodrigues and colleagues report in their study that anti-osteoporosis treatment was prescribed to only 47.7% of 65-year-old women with fragility fracture, suggesting that undertreatment of osteoporosis and fractures represents a serious public health problem in patients over 65 [27].
Adherence to anti-osteoporotic treatment
Much progress has been made in recent years, particularly in measuring BMD, assessing fracture risk, developing interventions that reduce this risk, and producing practical guidelines [25]. However, the measures available to combat the disease are under-utilized and therefore there is a need to evaluate best practices in prevention and treatment, as the adoption of these in all countries has the potential to lead to significant reductions in the burden of this disease.
Although fragility fractures cause significant mortality and morbidity in older adults, the diagnosis of osteoporosis remains very low. Moreover, most individuals who have suffered a fracture due to osteoporosis or who are at high risk of fracture are not treated and the number of patients undergoing treatment is decreasing [25]. This treatment gap is particularly problematic in hip fracture patients: in fact, most of them do not use osteoporosis drugs in the year following the fracture, and treatment rates have worsened in the past decade [28]. In a study conducted by Kim et al. in 2015, the proportion by which three cohorts of patients aged ≥ 65 years admitted to hospital for hip fracture received ≥ 1 osteoporosis drug after discharge was calculated [29]. Adherence to treatment for osteoporosis was measured as the proportion of days covered during the first year after hip fracture. Of a total of 86,202 patients, only 11 to 39% of older patients were treated with an osteoporosis drug within 3 months after fracture. Furthermore, adherence in these patients was suboptimal, with a proportion of days covered up to 1 year of less than 0.70 in all countries [29]. These data indicate that the use of drugs for the secondary prevention of osteoporotic fractures was rather low and did not increase over time in the three countries considered (the USA, Korea, and Spain) with different healthcare systems. Moreover, even after the prescription of these drugs, patient adherence to treatment was suboptimal, suggesting the urgent need for a better approach to optimize secondary prevention of osteoporotic fractures worldwide [29].
Efficacy of anti-osteoporotic treatment
Early prevention of future fractures is an important goal for people at risk. Similar fracture efficacy at 3 years is reported for most osteoporotic agents. In this regard, Inderjeeth et al. conducted a literature review to analyze data on time to onset of efficacy of commonly used treatments for morphometric vertebral fracture, clinical vertebral fracture, non-vertebral fracture, hip fracture, and any other clinical fracture [30]. Alendronate has been reported to reduce multiple morphometric vertebral fractures by 6 months; all morphometric vertebral fractures, non-vertebral fractures, and multiple other clinical fractures within 12 months; and all other clinical fractures and hip fractures within 18 months. Ibandronate reduces morphometric vertebral fracture by 12 months and non-vertebral fracture by 36 months, while raloxifene reduces clinical vertebral fracture by 3–6 months and non-vertebral fracture by 36 months. In addition, risedronate reduced clinical vertebral fracture and non-vertebral fracture by 6 months and hip fracture by 12 months; strontium ranelate reduced morphometric vertebral fracture, clinical vertebral fracture, non-vertebral fracture, and other clinical fractures by 12 months and hip fracture by 36 months; finally, zoledronic acid reduced morphometric vertebral fracture, clinical vertebral fracture, and other clinical fractures by 12 months, non-vertebral fracture by 24 months, and hip fracture by 36 months. These results indicate that risedronate, followed by alendronate, has the first onset of benefit across the range of fracture types [30]; therefore, the onset of efficacy may be an important consideration in treatment selection for some patients.
Denosumab treatment has been shown to decrease vertebral fracture, non-vertebral fracture, and hip fracture risk in post-menopausal women for up to 10 years after treatment [31]. In addition, studies have shown that the risk of wrist, forearm, and humerus fractures decreases significantly after long-term treatment with denosumab, which exerts positive effects on both trabecular and cortical bone [32, 33]. Among the bisphosphonates, zoledronic acid has been used in common clinical practice for the treatment of osteoporosis since 2007: studies show that administering less than 5 mg of this bisphosphonate per year reduces the risk of fracture [34]. In addition, Lyles and colleagues demonstrated how an annual infusion of zoledronic acid (5 mg dose) within 90 days after repair of a hip fragility fracture correlates with a reduction in the rate of re-fracture and mortality [35]. Teriparatide and abaloparatide are osteo-anabolic therapeutics. Teriparatide has been shown to decrease vertebral fracture risk more than bisphosphonates [36]. Abaloparatide has a similar mode of action to teriparatide, resulting in a higher formation-to-resorption ratio and a BMD increase at the hip [37]. Moreover, Miller and colleagues demonstrated that after 18 months of abaloparatide treatment, the risk of vertebral fracture showed a significant reduction compared to the placebo group [38]. In the FRAME phase III trial (FRActure study in postmenopausal woMen with ostEoporosis), treatment with romosozumab and then denosumab has been shown to result in a 73% reduction in the risk of vertebral fracture [39]. The efficacy of treatment with romosozumab was also demonstrated in the BRIDGE trial (placeBo-contRolled study evaluating the effIcacy anD safety of romosozumab in treatinG mEn with osteoporosis): after 12 months of treatment, an increase in BMD was found, compared to the group of patients who received placebo [40]. Unfortunately, the main issue is that many of these trials do not assess vertebral fracture at 6 months, so it remains difficult to define whether these treatments may have early effects on vertebral fracture.
Patient compliance with drug therapy for osteoporosis is often poor in clinical practice and may be associated with a higher risk of fracture [41]. In fact, a growing body of evidence suggests that anti-osteoporotic drugs are underused in clinical practice due to low prescription rates for patients at high risk of fracture and poor drug compliance among those prescribed such therapy [42,43,44]. While some of the often-cited causes for low prescription rates and poor compliance (such as the asymptomatic nature of the condition and the corresponding lack of symptomatic relief from therapy) are not modifiable, other causes (such as adverse effects, frequency of administration, and cost of the drug) are potentially modifiable [45]. In this regard, Rabenda et al. studied the proportion of patients treated with bisphosphonates or selective estrogen receptor modulators after hip fracture, evaluating, among those treated with alendronate, 12-month compliance and persistence on treatment [46]. Among a total of 23,146 patients, bisphosphonate treatment was administered to 2.6% and 3.6% of patients within 6 months and 1 year, respectively, after the occurrence of the hip fracture. Among women who received alendronate daily (n = 124) or weekly (n = 182) and were followed for at least 1 year after hip fracture, the mean drug possession ratio at 12 months was 67%. Also, at 12 months, the persistence rate was 41% and the mean duration of persistence was 40.3 weeks. Again, the data show that most hip fracture patients do not take anti-osteoporotic therapy after fracture and that adherence to treatment decreases over time and remains suboptimal [46]. In 2004, Panneman et al. conducted a retrospective study of 1654 patients aged 50 years and older who had been admitted to hospital for a non-traumatic fracture, with the aim of assessing the treatment rate of newly treated patients and the change in the treatment rate during the period 1998–2000 [47]. In total, 247 patients out of 1654 (15%) were prescribed anti-osteoporotic drugs within 1 year of hospital discharge; of these, 86 were treated primarily with bisphosphonates in the year following discharge following fracture, resulting in a new treatment rate of 5%. The probability of receiving treatment for osteoporosis following fracture did not change with the calendar year of the fracture (OR 0.95; CI 95%: 0.68–1.30). In conclusion, the results of this study show that, despite the introduction of a guideline for the treatment of osteoporosis recommending treatment for patients with fractures, most of the time, patients with fractures are not treated and, therefore, osteoporosis remains largely undertreated [47]. Thus, despite current treatment options for osteoporosis, there remains a significant risk of fracture, suggesting the need for new therapies. In addition, many physicians agree that minimally invasive surgical approaches to complement current therapies should be considered to further reduce the risk of fragility fractures in high-risk patients [48].
The difference between the reduction rates of fractures following treatment with different drugs is appreciable in some studies in the literature in which an appropriate methodology for comparison was applied. For example, the meta-analysis by Ding et al. shows that romosozumab is the only drug able to reduce the risk of clinical and vertebral fractures in post-menopausal women with or without prevalent vertebral fractures [49]. Similarly, Reginster and colleagues report in their meta-analysis that abaloparatide reduces the relative risk of vertebral and non-vertebral and wrist fractures in women with post-menopausal osteoporosis with or without a previous fracture compared to other treatment options [50].
Improving adherence to treatment can be achieved through patient education, monitoring, and supervision. It is essential that the fractured patient is actively involved in an interdisciplinary multi-specialist process to improve the physician–patient relationship and ensure persistence of drug treatment to reduce the risk of fracture and/or re-fracture [51, 52].
Adverse events related to anti-osteoporotic treatment
According to literature studies, there are adverse events generated by treatment with antiresorptive drugs. The most important reason why patients prematurely discontinue treatment with nitrogen-based bisphosphonates is side effects in the upper gastrointestinal tract, leading in the most severe cases to esophagitis or esophageal erosions. In contrast, nonnitrogen-containing bisphosphonates, such as clodronate, cause few side effects, diarrhea being the most common [53]. In addition, oral bisphosphonates such as alendronate, risedronate, and ibandronate, which are mainly used for the treatment of osteoporosis, have also been associated with adverse events, such as acute phase response, hypocalcemia and secondary hyperparathyroidism, musculoskeletal pain, and ocular events [54].
Long-term treatment with bisphosphonates, which are generally accepted as safe, effective, and well-tolerated for the treatment of post-menopausal osteoporosis, has been shown to be associated with the occurrence of atypical femoral fractures and osteonecrosis of the jaw [55]. Kharazmi and colleagues argue that such adverse events are more frequent among women due to the reduced biomechanical ability of the femur to resist stress forces [56]. Discontinuity of treatment with denosumab is also associated with adverse events. In this regard, Cummings et al. report that discontinuous treatment with denosumab leads to multiple vertebral fractures, with the highest risk in subjects with a previous vertebral fracture, suggesting the need for an alternative antiresorptive treatment [57]. Moreover, Lamy et al. report that after the second dose of denosumab there is a rebound effect with an increased risk of multiple and spontaneous vertebral fractures [58]. Noteworthy are the adverse cardiovascular effects of treatment with romosozumab, due to the expression of sclerostin in vascular smooth muscle cells. According to Langdahl et al., the incidence of severe cardiovascular damage is higher in women treated with romosozumab, compared to treatment with alendronate, suggesting that romosozumab should be used for the treatment of women with post-menopausal osteoporosis at high risk of fracture after careful evaluation of cardiovascular risks and the risk–benefit ratio [59].
System solutions to close the gaps: the Fracture Liaison Services (FLSs) model
Clinical systems have been developed worldwide to ensure appropriate management of patients following fracture. A systematic literature review showed that 65% of the reported systems include a dedicated coordinator who acts as a link between the orthopedic team, the osteoporosis and falls services, the patient, and the general practitioner [60]. Other success factors include a fracture registry and a database to monitor the care provided to the fractured patient. Such coordinator-based systems have proven their effectiveness in improving the diagnosis and treatment of osteoporosis in these patients, as well as being cost-effective [60]. In this regard, in 2012, the IOF launched the Capture the Fracture campaign [61], with the aim of substantially reducing the incidence of secondary fractures worldwide through the implementation of Fracture Liaison Services (FLSs), which are among the most exemplary coordinator-based post-fracture care models (Fig. 1). FLSs have been shown to fill the ubiquitous gap in secondary fracture prevention by ensuring that those suffering from fragility fractures receive appropriate assessment and intervention to reduce the risk of future fractures [61]. The main objectives of an FLS are inclusive case finding, evidence-based assessment (risk stratification, identification of secondary causes of osteoporosis, tailored therapy), initiating treatment in accordance with relevant guidelines, and improving long-term adherence with therapy [61].
Ganda et al. conducted a review of the scientific literature to critically evaluate the available studies on FLS models of care and to establish specific characteristics associated with effective secondary fracture prevention programs [62]. Out of 574 references, 42 articles were identified as analyzable and were grouped into four general models of care: type A (identification, assessment, and treatment of patients as part of the service); type B (like A, without initiation of treatment); type C (alerting patients plus primary care physicians); and type D (patient education only). The authors demonstrated the existence of a positive correlation between the increased effectiveness of an FLS and an increase in the intensity of the intervention. Particularly, their results suggest that a type A model of care is likely to be more effective than a type B intervention, which in turn produces better clinical outcomes than type C or D programs, whereas patient education alone had little or no impact on treatment initiation. Thus, it was concluded that currently the ideal approach to the prevention of secondary fractures is a type A care model in an integrated electronic healthcare network, supervised by a coordinator and using a dedicated database that measures performance [62]. A similar study on the effectiveness of FLS services was conducted by Huntjiers et al. in 2014, with the aim of analyzing the risk of subsequent non-vertebral fractures and mortality within 2 years after a non-vertebral fracture in patients aged 50 years and older who presented at a hospital with an FLS (FLS group) and at a hospital without an FLS (no-FLS group) [63]. Specifically, in the no-FLS group, only standard fracture care procedures were followed to address proper fracture healing, whereas, in the FLS group, dual-energy X-ray absorptiometry (DXA) scans and laboratory tests were performed. The results showed that patients in the FLS group had a significantly lower mortality and, consequently, a lower risk of non-vertebral fractures than those in the no-FLS group, with a reduction of 35% and 56%, respectively, over 2 years of follow-up. Thus, FLS appears to be an effective approach to reduce the number of subsequent fractures and premature mortality in this cohort of patients [63].
In a 2018 systematic review, Wu et al. reported that FLS approaches are cost-effective, regardless of program intensity [64]. Indeed, it is known that FLSs can be low-intensity interventions in which patients are identified and encouraged to seek screening or secondary prevention management; alternatively, patients can receive high-intensity interventions in which a case manager identifies patients, investigates bone density, and initiates appropriate treatment [64, 65]. In this regard, Majumdar and colleagues recently evaluated the cost-effectiveness of two models of osteoporosis care after upper limb fragility using a high-intensity FLS intervention (Case Manager) and a low-intensity FLS intervention (Active Control) and compared both with usual care [66]. The results showed that both models substantially improved osteoporosis care compared to the reported usual care rates, but that the Case Manager intervention was much more effective than the Active Control intervention. In fact, compared to Active Control, the Case Manager saved $333,000, gained seven QALYs, and prevented nine additional fractures per 1000 patients, whereas, compared to usual care, the Case Manager saved $564,000, gained 14 QALYs, and suffered 18 fewer fractures per 1000 patients. Thus, although the clinical implementation of both interventions should lead to cost savings, reduced fractures, and increased life expectancy compared to usual care, the Case Manager intervention would, by far, be the most appropriate in terms of both effectiveness and cost [66].
Surgical approaches to the treatment of fragile bones
Despite advances in the prevention and treatment of osteoporotic fractures, their prevalence continues to increase, so it is necessary to share current aspects of the management of these fractures and focus on advances in the implant design and surgical technique. There are nails, screws, and plates designed to maximize the bone-implant interface, substances that can be used locally to stimulate bone formation, and systemic therapies that can be used as adjuncts to decrease bone loss and/or improve bone formation [67].
Reduction in bone mass and especially qualitative changes in bone tissue must be considered when planning the best surgical treatment. Surgical failures generally occur because the bone-implant interface causes disruption, fracture failure, or plaque extraction. Therefore, when planning surgical fixation in osteoporotic bone, it is important to choose implants that maximize the contact surface with the remaining bone, especially in hip and proximal humeral surgery [68].
Changes in bone caused by age and osteoporosis affect the stability of osteosynthesis constructs, both mechanically and clinically. In fact, the treatment for osteoporosis fractures in the frail elderly population is generally established on the assessment of patient-specific, fracture-specific, and surgeon-specific aspects [69, 70]. The choice of therapy also requires a multidisciplinary care concept, including treatment of comorbidities, the correct timing, and technique of the operative intervention [71].
Several surgical techniques can be used to treat fragility fractures. Advanced methods of augmenting implant fixation in osteoporotic bone are currently used, including polymethylmethacrylate (PMMA), bone grafts, calcium phosphate implants, calcium phosphate cements, calcium phosphate coatings, modified implants, and pharmaceutical augmentation concepts (Table 1). The indication for these techniques should be based on bone mineral density measurements by DXA or quantitative computed tomography (QCT) [72].
Combining the right drug therapy with surgical treatment is certainly an effective strategy for treating osteoporosis fractures. In fact, anti-fracture agents typically prevent fractures by augmenting bone mass and enhancing skeletal integrity [73]. Bisphosphonates are generally considered to be antiresorptive agents, given their inhibitory effect on osteoclastic activity and bone resorption [74]. According to the literature, these drugs improve the process of fracture repair, leading to the development of a larger bone callus, increased bone mineral content, and trabecular bone volume [73]. An anabolic role of zoledronic acid has been hypothesized to improve the trabecular microarchitecture of the bone callus, promoting fracture repair in animal models [75].
Inhibitors of receptor activator of nuclear factor kappa-B ligand (RANKL) have undergone far less investigation but act on osteoclast precursors to down-regulate bone resorption [76]. The parathyroid hormone may enhance fracture repair by promoting chondrogenesis early in the healing process and osteogenesis later: the former effect improves callus geometry while the latter effect improves bone quality as well as quantity [77, 78]. The use of anti-fracture agents for the enhancement of fracture healing may ultimately depend upon high-quality evidence from well-designed, well-controlled clinical trials [79].
Treating osteoporotic bone loss in the proximal femur to improve bone mass and strength: a view to defusing the bone fragility crisis
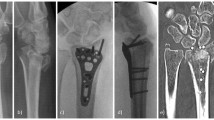
Newly local osteo-enhancement procedure (LOEP) is a targeted approach to address local bone loss, including loss due to osteoporosis: it is a minimally invasive procedure to replace the bone lost due to osteoporosis, increasing bone density and strength [80]. The treatment involves implanting several bone substitutes, one of which is OSSURE, a unique material that is resorbed and replaced by bone, immediately increasing strength of treated bone. Treatment leads to rapid formation of new bone and shows in clinical research to substantially increase bone density and strength for at least 7 years. The material is triphasic: once cured, it consists of brushite, calcium sulfate, and β-tricalcium phosphate (β-TCP) granules that are ~ 200 μm in diameter. The three phases are resorbed at different rates. The β-TCP granules are the slowest to be resorbed and are incorporated into the bone formed after the other two phases are resorbed. The procedure is conducted under fluoroscopic guidance and takes 20–30 min. Studies supporting concept of LOEP are human cadaveric femur study, canine humerus defect model, and proof-of-concept clinical study in post-menopausal women. In a study aimed to evaluate the immediate effect of LOEP on biomechanical properties of human proximal femurs in 45 pairs of cadaver femurs (77,8 years, range 60–96), 4 normal, 16 osteopenic, and 25 osteoporotic by DXA-scan, LOEP treatment improved hip strength in sideways fall loading conditions compare to un-operated control. LOEP enhanced biomechanical properties of osteopenic and osteoporotic cadaver femurs (increased failure load, increased work to failure, no change in stiffness), without deleterious effect of procedure on femoral strength [81]. In a prospective proof-of-concept clinical study on women > 55 years of age, post-menopausal (> 1 year), and with DXA femoral neck T-score < − 2.5, LOEP treatment on left hip resulted in a significant increase in BMD of the proximal femur correlated with a substantial increase in femoral strength. Specifically, the BMD of the femoral neck in the treated hip was 58% greater and femoral strength was 36% greater than in the control hip 5–7 years after treatment (two-phase follow-up, 0 to 2 years and 2 to 5–7 years) [82]. Concerning safety and adverse events, in phase 1 follow-up, procedure was well-tolerated, with no device or procedure-related serious adverse events, all patients fully weight bearing following recovery from sedation, and one fragility fracture (not procedure-related). Similarly, in phase 2 follow-up, there were no device or procedure-related serious adverse events, with six fragility fractures in four patients [82]. Based on this evidence, local augmentation of the proximal femur appears promising as a new treatment for hip fragility.
Biomaterials built for osteo-enhancement of fragile bone
The standard tissue engineering approach to provide solutions for impaired fracture healing, bone restoration, and regeneration includes the use of growth factors, scaffolds, and mesenchymal stem cells. However, although the mechanical environment is discussed and is considered as a key element in bone regeneration, its importance is often underestimated [83]. Building biomaterials for osteo-enhancement of bone provide immediate strengthening and replace lost bone with new healthy bone for a sustained increase in strength. Universe of bone graft materials include autograft, allograft, xenograft, polymeric biomaterials, metallic biomaterials, cell-based treatments, growth factors, and ceramic biomaterials. Requirements for local osteo-enhancement of patients with osteoporosis are to provide immediate, significant, and reliable mechanical strengthening; to be clinically feasible (with low risk of side effects and delivered using minimally invasive techniques); to be compatible with pharmaceutical therapies; to be ethical and demonstrate acceptable benefit/risk; and to be financially feasible. Biomaterial resorption is a dynamic process, and biomaterial should retain significant strength throughout the resorption process [72, 84].
Newly formed bone responds to stress: once biomaterial resorption is complete, newly formed bone must follow Wolff’s Law, resulting in significant and durable strength increase. Changes in femoral BMD determined by a biomaterial transformation to bone are substantial and are much greater than those from other drugs. Since bioresorbable bone substitutes are increasingly entering common clinical practice, and core decompression has been described in combination with them, the current study takes this technique into account. Two questions are often addressed by orthopedists relating to core decompression: (1) is this technique associated with a considerable lack of structural support of the bone? (2) is there an optimal region for the surgical entrance point for which the fracture risk would be lowest?
Tran and colleagues simulated and analyzed a finite element model of a femur treated by core decompression with bone substitute [85]. In vitro compression testing of femur was used to confirm finite element results. The results showed that after core decompression with standard drilling, in combination with artificial bone substitute refilling, daily activities are not risky for femoral fracture. The femoral fracture risk increased successively when the entrance point is located further distal. The critical value of the deviation of the entrance point to a more distal part is about 20 mm. The study findings demonstrate that optimal entrance point should locate on the proximal subtrochanteric region to reduce the subtrochanteric fracture risk. Furthermore, the consistent results of finite element and in vitro testing imply that the simulations are sufficient [82]. Local osteo-enhancement of patients with osteoporosis is compatible with pharmaceutical therapies: resorption rates are similar with or without alendronate, and bone formation is coupled to resorption [64, 81].
Conclusions
Fragility fractures are one of the major social and health problems affecting the elderly population, leading to loss of mobility and independence, and inevitably to a predominantly sedentary lifestyle. Given the increase in life expectancy, it is estimated that the number of fractures will increase markedly in all countries, as will the related costs. Even though fragility fractures are causing an increase in mortality and morbidity in the elderly population, the diagnosis rate is still low: individuals who have suffered a fracture due to osteoporosis or who are at high risk of fracture are not treated and, in general, the number of patients receiving adequate treatment is decreasing, even though there are numerous studies in the literature proposing guidelines for the treatment and prevention of osteoporosis. Among the tools used as a first strategy to investigate fracture risk, there are some algorithms such as FRAX® and DeFRA, which allow an early assessment of fracture risk and, consequently, to identify individuals unaware of their osteoporotic status.
The main risk factor for a fragility fracture is a previous fracture. In this regard, FLS is a model created with the aim of solving the existing gap in the management of brittle bones, proving to be an efficient approach in reducing the number of subsequent fractures. The choice of therapy must be based on a multidisciplinary approach, considering comorbidities, correct timing, and surgical techniques. It is our opinion that the treatment of fragility fractures should take into account the microarchitectural changes in bone tissue that occur with aging for the choice the most appropriate surgical strategy in combination with the right drug therapy. Currently, bone augmentation is believed to overcome the limitations of anti-osteoporosis drugs: it strengthens the bone biomechanically, is minimally invasive, and has an immediate effect. The tissue microenvironment is another important factor deciding the course of the fracture healing process. Thus, in recent years, for the treatment of fragility fractures, the use of biomaterials that allow tissue regeneration and restoration of bone strength has been considered. Finally, our work highlights the background characterizing the context of fragility fractures and the numerous aspects to be considered when establishing the best treatment. The aim of this work is also to encourage research to continue studies in this field, so that the current guidelines can be optimized to develop new protocols for the management and prevention of osteoporosis fractures.
References
Tarantino U, Iolascon G, Cianferotti L et al (2017) Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol Off J Ital Soc Orthop Traumatol 18:3–36. https://doi.org/10.1007/s10195-017-0474-7
Nuti R, Brandi ML, Checchia G et al (2019) Guidelines for the management of osteoporosis and fragility fractures. Intern Emerg Med 14:85–102. https://doi.org/10.1007/s11739-018-1874-2
Wolf-Maier K, Cooper RS, Banegas JR et al (2003) Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA 289:2363–2369. https://doi.org/10.1001/jama.289.18.2363
Kanis JA, Johnell O, Oden A et al (2000) Long-term risk of osteoporotic fracture in Malmö. Osteoporos Int J Establ Result Coop Between Eur Found Osteoporos Natl Osteoporos Found USA 11:669–674. https://doi.org/10.1007/s001980070064
Kanis JA, Odén A, McCloskey EV et al (2012) A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int a J Establ Result Coop Between Eur Found Osteoporos Natl Osteoporos Found USA 23:2239–2256. https://doi.org/10.1007/s00198-012-1964-3
Pisani P, Renna MD, Conversano F et al (2016) Major osteoporotic fragility fractures: risk factor updates and societal impact. World J Orthop 7:171–181. https://doi.org/10.5312/wjo.v7.i3.171
Jakobsen A, Laurberg P, Vestergaard P, Andersen S (2013) Clinical risk factors for osteoporosis are common among elderly people in Nuuk. Greenland Int J Circumpolar Health 72:19596. https://doi.org/10.3402/ijch.v72i0.19596
Kanis JA, Oden A, Johnell O et al (2001) The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int J Establ Result Coop between Eur Found Osteoporos Natl Osteoporos Found USA 12:417–427. https://doi.org/10.1007/s001980170112
Warriner AH, Patkar NM, Curtis JR et al (2011) Which fractures are most attributable to osteoporosis? J Clin Epidemiol 64:46–53. https://doi.org/10.1016/j.jclinepi.2010.07.007
Johansson H, Siggeirsdóttir K, Harvey NC et al (2017) Imminent risk of fracture after fracture. Osteoporos Int J Establ Result Coop Between Eur Found Osteoporos Natl Osteoporos Found USA 28:775–780. https://doi.org/10.1007/s00198-016-3868-0
van Geel TACM, van Helden S, Geusens PP et al (2009) Clinical subsequent fractures cluster in time after first fractures. Ann Rheum Dis 68:99–102. https://doi.org/10.1136/ard.2008.092775
Kanis JA, Johansson H, Odén A et al (2018) Characteristics of recurrent fractures. Osteoporos Int J Establ Result Coop between Eur Found Osteoporos Natl Osteoporos Found USA 29:1747–1757. https://doi.org/10.1007/s00198-018-4502-0
Kanis JA, Harvey NC, McCloskey E et al (2020) Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos Int J Establ Result Coop Between Eur Found Osteoporos Natl Osteoporos Found USA 31:1–12. https://doi.org/10.1007/s00198-019-05176-3
Rocha VM, Gaspar HA, Oliveira CF de (2018) Fracture risk assessment in home care patients using the FRAX® tool. Einstein (Sao Paulo) 16:eAO4236. https://doi.org/10.1590/S1679-45082018AO4236
Bonaccorsi G, Messina C, Cervellati C et al (2018) Fracture risk assessment in postmenopausal women with diabetes: comparison between DeFRA and FRAX tools. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol 34:404–408. https://doi.org/10.1080/09513590.2017.1407308
Borgström F, Karlsson L, Ortsäter G et al (2020) Fragility fractures in Europe: burden, management and opportunities. Arch Osteoporos 15:59. https://doi.org/10.1007/s11657-020-0706-y
Svedbom A, Borgstöm F, Hernlund E et al (2018) Quality of life for up to 18 months after low-energy hip, vertebral, and distal forearm fractures-results from the ICUROS. Osteoporos Int J Establ Result Coop Between Eur Found Osteoporos Natl Osteoporos Found USA 29:557–566. https://doi.org/10.1007/s00198-017-4317-4
Ito K (2020) Cost-effectiveness of screening for osteoporosis in older men with a history of falls. JAMA Netw open 3:e2027584. https://doi.org/10.1001/jamanetworkopen.2020.27584
Svedbom A, Borgström F, Hernlund E et al (2018) Quality of life after hip, vertebral, and distal forearm fragility fractures measured using the EQ-5D-3L, EQ-VAS, and time-trade-off: results from the ICUROS. Qual Life Res Int J Qual Life Asp Treat Care Rehabil 27:707–716. https://doi.org/10.1007/s11136-017-1748-5
Borgström F, Lekander I, Ivergård M et al (2013) The International Costs and Utilities Related to Osteoporotic Fractures Study (ICUROS)–quality of life during the first 4 months after fracture. Osteoporos Int J Establ Result Coop between Eur Found Osteoporos Natl Osteoporos Found USA 24:811–823. https://doi.org/10.1007/s00198-012-2240-2
Roux C, Briot K (2017) Imminent fracture risk. Osteoporos Int J Establ Result Coop Between Eur Found Osteoporos Natl Osteoporos Found USA 28:1765–1769. https://doi.org/10.1007/s00198-017-3976-5
Bonafede M, Shi N, Barron R et al (2016) Predicting imminent risk for fracture in patients aged 50 or older with osteoporosis using US claims data. Arch Osteoporos 11:26. https://doi.org/10.1007/s11657-016-0280-5
McKercher HG, Crilly RG, Kloseck M (2000) Osteoporosis management in long-term care. Survey of Ontario physicians. Can Fam Physician 46:2228–2235
Kaffashian S, Raina P, Oremus M et al (2011) The burden of osteoporotic fractures beyond acute care: the Canadian Multicentre Osteoporosis Study (CaMos). Age Ageing 40:602–607. https://doi.org/10.1093/ageing/afr085
Hernlund E, Svedbom A, Ivergård M et al (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8:136. https://doi.org/10.1007/s11657-013-0136-1
Chau YT, Nashi N, Law LS-C et al (2020) Undertreatment of osteoporosis following hip fracture: a retrospective, observational study in Singapore. Arch Osteoporos 15:141. https://doi.org/10.1007/s11657-020-00816-2
Rodrigues AM, Eusébio M, Santos MJ et al (2018) The burden and undertreatment of fragility fractures among senior women. Arch Osteoporos 13:22. https://doi.org/10.1007/s11657-018-0430-z
Solomon DH, Johnston SS, Boytsov NN et al (2014) Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J bone Miner Res Off J Am Soc Bone Miner Res 29:1929–1937. https://doi.org/10.1002/jbmr.2202
Kim SC, Kim M-S, Sanfélix-Gimeno G et al (2015) Use of osteoporosis medications after hospitalization for hip fracture: a cross-national study. Am J Med 128:519–26.e1. https://doi.org/10.1016/j.amjmed.2015.01.014
Inderjeeth CA, Chan K, Kwan K, Lai M (2012) Time to onset of efficacy in fracture reduction with current anti-osteoporosis treatments. J Bone Miner Metab 30:493–503. https://doi.org/10.1007/s00774-012-0349-1
Bone HG, Wagman RB, Brandi ML et al (2017) 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 5:513–523. https://doi.org/10.1016/S2213-8587(17)30138-9
Bilezikian JP, Lin CJF, Brown JP et al (2019) Long-term denosumab treatment restores cortical bone loss and reduces fracture risk at the forearm and humerus: analyses from the FREEDOM Extension cross-over group. Osteoporos Int J Establ Result Coop Between Eur Found Osteoporos Natl Osteoporos Found USA 30:1855–1864. https://doi.org/10.1007/s00198-019-05020-8
Ferrari S, Butler PW, Kendler DL et al (2019) Further nonvertebral fracture reduction beyond 3 years for up to 10 years of denosumab treatment. J Clin Endocrinol Metab 104:3450–3461. https://doi.org/10.1210/jc.2019-00271
Grey A (2016) Intravenous zoledronate for osteoporosis: less might be more. Ther Adv Musculoskelet Dis 8:119–123. https://doi.org/10.1177/1759720X16650866
Lyles KW, Colón-Emeric CS, Magaziner JS et al (2007) Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 357:1799–1809. https://doi.org/10.1056/NEJMoa074941
Kendler DL, Marin F, Zerbini CAF et al (2018) Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet (London, England) 391:230–240. https://doi.org/10.1016/S0140-6736(17)32137-2
Leder BZ, O’Dea LSL, Zanchetta JR et al (2015) Effects of abaloparatide, a human parathyroid hormone-related peptide analog, on bone mineral density in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 100:697–706. https://doi.org/10.1210/jc.2014-3718
Miller PD, Hattersley G, Riis BJ et al (2016) Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA 316:722–733. https://doi.org/10.1001/jama.2016.11136
Cosman F, Crittenden DB, Adachi JD et al (2016) Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 375:1532–1543. https://doi.org/10.1056/NEJMoa1607948
Lewiecki EM, Dinavahi RV, Lazaretti-Castro M et al (2019) One year of romosozumab followed by two years of denosumab maintains fracture risk reductions: results of the FRAME extension study. J bone Miner Res Off J Am Soc Bone Miner Res 34:419–428. https://doi.org/10.1002/jbmr.3622
Weycker D, Macarios D, Edelsberg J, Oster G (2007) Compliance with osteoporosis drug therapy and risk of fracture. Osteoporos Int J Establ Result Coop Between Eur Found Osteoporos Natl Osteoporos Found USA 18:271–277. https://doi.org/10.1007/s00198-006-0230-y
Andrade SE, Majumdar SR, Chan KA et al (2003) Low frequency of treatment of osteoporosis among postmenopausal women following a fracture. Arch Intern Med 163:2052–2057. https://doi.org/10.1001/archinte.163.17.2052
Caro JJ, Ishak KJ, Huybrechts KF et al (2004) The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos Int J Establ Result Coop Between Eur Found Osteoporos Natl Osteoporos Found USA 15:1003–1008. https://doi.org/10.1007/s00198-004-1652-z
McCombs JS, Thiebaud P, McLaughlin-Miley C, Shi J (2004) Compliance with drug therapies for the treatment and prevention of osteoporosis. Maturitas 48:271–287. https://doi.org/10.1016/j.maturitas.2004.02.005
Recker RR, Gallagher R, MacCosbe PE (2005) Effect of dosing frequency on bisphosphonate medication adherence in a large longitudinal cohort of women. Mayo Clin Proc 80:856–861. https://doi.org/10.4065/80.7.856
Rabenda V, Vanoverloop J, Fabri V et al (2008) Low incidence of anti-osteoporosis treatment after hip fracture. J Bone Joint Surg Am 90:2142–2148. https://doi.org/10.2106/JBJS.G.00864
Panneman MJM, Lips P, Sen SS, Herings RMC (2004) Undertreatment with anti-osteoporotic drugs after hospitalization for fracture. Osteoporos Int J Establ Result Coop between Eur Found Osteoporos Natl Osteoporos Found USA 15:120–124. https://doi.org/10.1007/s00198-003-1544-7
Ferrari S, Reginster J-Y, Brandi ML et al (2016) Unmet needs and current and future approaches for osteoporotic patients at high risk of hip fracture. Arch Osteoporos 11:37. https://doi.org/10.1007/s11657-016-0292-1
Ding L-L, Wen F, Wang H et al (2020) Osteoporosis drugs for prevention of clinical fracture in white postmenopausal women: a network meta-analysis of survival data. Osteoporos Int J Establ Result Coop between Eur Found Osteoporos Natl Osteoporos Found USA 31:961–971. https://doi.org/10.1007/s00198-019-05183-4
Reginster J-Y, Bianic F, Campbell R et al (2019) Abaloparatide for risk reduction of nonvertebral and vertebral fractures in postmenopausal women with osteoporosis: a network meta-analysis. Osteoporos Int J Establ Result Coop Between Eur Found Osteoporos Natl Osteoporos Found USA 30:1465–1473. https://doi.org/10.1007/s00198-019-04947-2
Cornelissen D, de Kunder S, Si L et al (2020) Interventions to improve adherence to anti-osteoporosis medications: an updated systematic review. Osteoporos Int J Establ Result Coop Between Eur Found Osteoporos Natl Osteoporos Found USA 31:1645–1669. https://doi.org/10.1007/s00198-020-05378-0
Hiligsmann M, Cornelissen D, Vrijens B et al (2019) Determinants, consequences and potential solutions to poor adherence to anti-osteoporosis treatment: results of an expert group meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeleta. Osteoporos Int J Establ Result Coop between Eur Found Osteoporos Natl Osteoporos Found USA 30:2155–2165. https://doi.org/10.1007/s00198-019-05104-5
Abrahamsen B (2010) Adverse effects of bisphosphonates. Calcif Tissue Int 86:421–435. https://doi.org/10.1007/s00223-010-9364-1
Papapetrou PD (2009) Bisphosphonate-associated adverse events. Hormones (Athens) 8:96–110. https://doi.org/10.14310/horm.2002.1226
Won Y, Lim J-R, Kim Y-H et al (2014) Atypical femoral fracture combined with osteonecrosis of jaw during osteoporosis treatment with bisphosphonate. J bone Metab 21:155–159
Kharazmi M, Hallberg P, Michaëlsson K (2014) Gender related difference in the risk of bisphosphonate associated atypical femoral fracture and osteonecrosis of the jaw. Ann Rheum Dis 73:1594
Cummings SR, Ferrari S, Eastell R et al (2018) Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J bone Miner Res Off J Am Soc Bone Miner Res 33:190–198. https://doi.org/10.1002/jbmr.3337
Lamy O, Stoll D, Aubry-Rozier B, Gonzalez Rodriguez E (2020) Correction to: Stopping denosumab. Curr Osteoporos Rep
Langdahl BL, Hofbauer LC, Forfar JC (2021) Cardiovascular safety and sclerostin inhibition. J Clin Endocrinol Metab 106:1845–1853. https://doi.org/10.1210/clinem/dgab193
Marsh D, Akesson K, Beaton DE et al (2011) Coordinator-based systems for secondary prevention in fragility fracture patients. Osteoporos Int J Establ Result Coop between Eur Found Osteoporos Natl Osteoporos Found USA 22:2051–2065. https://doi.org/10.1007/s00198-011-1642-x
Akesson K, Marsh D, Mitchell PJ et al (2013) Capture the fracture: a best practice framework and global campaign to break the fragility fracture cycle. Osteoporos Int J Establ Result Coop Between Eur Found Osteoporos Natl Osteoporos Found USA 24:2135–2152. https://doi.org/10.1007/s00198-013-2348-z
Ganda K, Puech M, Chen JS et al (2013) Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int J Establ Result Coop Between Eur Found Osteoporos Natl Osteoporos Found USA 24:393–406. https://doi.org/10.1007/s00198-012-2090-y
Huntjens KMB, van Geel TACM, van den Bergh JPW et al (2014) Fracture liaison service: impact on subsequent nonvertebral fracture incidence and mortality. J Bone Joint Surg Am 96:e29. https://doi.org/10.2106/JBJS.L.00223
Wu C-H, Kao I-J, Hung W-C et al (2018) Economic impact and cost-effectiveness of fracture liaison services: a systematic review of the literature. Osteoporos Int a J Establ as result Coop between Eur Found Osteoporos Natl Osteoporos Found USA 29:1227–1242. https://doi.org/10.1007/s00198-018-4411-2
Wu C-H, Tu S-T, Chang Y-F et al (2018) Fracture liaison services improve outcomes of patients with osteoporosis-related fractures: a systematic literature review and meta-analysis. Bone 111:92–100. https://doi.org/10.1016/j.bone.2018.03.018
Majumdar SR, Lier DA, McAlister FA et al (2019) Cost-effectiveness of osteoporosis interventions to improve quality of care after upper extremity fracture: results from a randomized trial (C-STOP Trial). J bone Miner Res Off J Am Soc Bone Miner Res 34:1220–1228. https://doi.org/10.1002/jbmr.3699
Giannoudis PV, Schneider E (2006) Principles of fixation of osteoporotic fractures. J Bone Joint Surg Br 88:1272–1278. https://doi.org/10.1302/0301-620X.88B10.17683
Iolascon G, Resmini G, Tarantino U (2013) “Osteoporotic fragility fractures: medical and surgical approaches” II National Congress of the Italian Orthopedic Group for the Study of Severe Osteoporosis (GISOOS). Aging Clin Exp Res 25(Suppl 1):S1-2
Lems WF, Dreinhöfer KE, Bischoff-Ferrari H et al (2017) EULAR/EFORT recommendations for management of patients older than 50 years with a fragility fracture and prevention of subsequent fractures. Ann Rheum Dis 76:802–810. https://doi.org/10.1136/annrheumdis-2016-210289
Mak JCS, Cameron ID, March LM (2010) Evidence-based guidelines for the management of hip fractures in older persons: an update. Med J Aust 192:37–41. https://doi.org/10.5694/j.1326-5377.2010.tb03400.x
von Rüden C, Augat P (2016) Failure of fracture fixation in osteoporotic bone. Injury 47(Suppl 2):S3–S10. https://doi.org/10.1016/S0020-1383(16)47002-6
Moroni A, Hoang-Kim A, Lio V, Giannini S (2006) Current augmentation fixation techniques for the osteoporotic patient. Scand J Surg SJS Off organ Finnish Surg Soc Scand Surg Soc 95:103–109. https://doi.org/10.1177/145749690609500205
Vannucci L, Brandi ML (2016) Healing of the bone with anti-fracture drugs. Expert Opin Pharmacother 17:2267–2272. https://doi.org/10.1080/14656566.2016.1241765
Morris CD, Einhorn TA (2005) Bisphosphonates in orthopaedic surgery. J Bone Joint Surg Am 87:1609–1618. https://doi.org/10.2106/JBJS.D.03032
Türker M, Aslan A, Çırpar M et al (2016) Histological and biomechanical effects of zoledronate on fracture healing in an osteoporotic rat tibia model. Eklem Hastalik Cerrahisi 27:9–15. https://doi.org/10.5606/ehc.2016.03
Einhorn TA (2010) Can an anti-fracture agent heal fractures? Clin cases Miner bone Metab Off J Ital Soc Osteoporosis, Miner Metab Skelet Dis 7:11–14
Alkhiary YM, Gerstenfeld LC, Krall E et al (2005) Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1–34). J Bone Joint Surg Am 87:731–741. https://doi.org/10.2106/JBJS.D.02115
Kakar S, Einhorn TA, Vora S et al (2007) Enhanced chondrogenesis and Wnt signaling in PTH-treated fractures. J bone Miner Res Off J Am Soc Bone Miner Res 22:1903–1912. https://doi.org/10.1359/jbmr.070724
Varga P, Hofmann-Fliri L, Blauth M, Windolf M (2016) Prophylactic augmentation of the osteoporotic proximal femur-mission impossible? Bonekey Rep 5:854. https://doi.org/10.1038/bonekey.2016.86
Kanis JA, Cooper C, Rizzoli R, Reginster J-Y (2019) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int J Establ Result Coop between Eur Found Osteoporos Natl Osteoporos Found USA 30:3–44. https://doi.org/10.1007/s00198-018-4704-5
Stroncek JD, Shaul JL, Favell D et al (2019) In vitro injection of osteoporotic cadaveric femurs with a triphasic calcium-based implant confers immediate biomechanical integrity. J Orthop Res Off Publ Orthop Res Soc 37:908–915. https://doi.org/10.1002/jor.24239
Howe JG, Hill RS, Stroncek JD et al (2020) Treatment of bone loss in proximal femurs of postmenopausal osteoporotic women with AGN1 local osteo-enhancement procedure (LOEP) increases hip bone mineral density and hip strength: a long-term prospective cohort study. Osteoporos Int a J Establ as result Coop between Eur Found Osteoporos Natl Osteoporos Found USA 31:921–929. https://doi.org/10.1007/s00198-019-05230-0
Giannoudis PV, Einhorn TA, Marsh D (2007) Fracture healing: the diamond concept. Injury 38(Suppl 4):S3-6. https://doi.org/10.1016/s0020-1383(08)70003-2
Trost M, Schmoelz W, Wimmer D et al (2020) Local osteo-enhancement of osteoporotic vertebra with a triphasic bone implant material increases strength-a biomechanical study. Arch Orthop Trauma Surg 140:1395–1401. https://doi.org/10.1007/s00402-020-03382-x
Tran TN, Warwas S, Haversath M et al (2014) Experimental and computational studies on the femoral fracture risk for advanced core decompression. Clin Biomech (Bristol, Avon) 29:412–417. https://doi.org/10.1016/j.clinbiomech.2014.02.001
Funding
This work was supported by F.I.R.M.O. Foundation through a grant from AgNovos.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
K-E. Akesson reports consultations and lecture fees from Amgen, Astellas Pharma, Chugai, RenaPharma, and UCB. M. Bouxsein serves on the Advisory Board for Keros Therapeutics and Beryl Health. M-L. Brandi has received honoraria from Amgen, Bruno Farmaceutici, Calcilytix, Kyowa Kirin, and UCB; reports grants and/or speaker for Abiogen, AgNovosAlexion, Amgen, Bruno Farmaceutici, Echolight, Eli Lilly, Kyowa Kirin, SPA, Theramex, and UCB; and reports consultations for Aboca, Alexion, Amolyt, Bruno Farmaceutici, Calcilytix, Kyowa Kirin, and UCB. I. Cariati has no disclosures. R. Civinini reports personal fees and consultancy or lecture fees from Orthofix, Smith &Nephew, and Microport. F. Falez reports consultations for Lima, Microport, Adler, G21, and external collaborations for Menarini. E. Gasbarra has no disclosures. C. Greggi has no disclosures. G. Iolascon serves on the Advisory Board for UCB pharma, AMGEN, Eli-Lilly. R. Iundusi has no disclosures. A. Kurth serves on the advisory board for UCB, Amgen, and AgNovos; speaker fees from Amgen, Alexion, UCB, Eli-Lilly, Theramex, Ratiopharm, and AgNovos. P. Tranquilli Leali has no disclosures. U. Tarantino serves on the Advisory Board for Abiogen, AMGEN, Eli-Lilly.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tarantino, U., Cariati, I., Greggi, C. et al. Gaps and alternative surgical and non-surgical approaches in the bone fragility management: an updated review. Osteoporos Int 33, 2467–2478 (2022). https://doi.org/10.1007/s00198-022-06482-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-022-06482-z