Abstract
Summary
Methodological limitations preclude determination of the association between sleep duration and bone mineral density (BMD) from existing literature. This was the first study to use objective sleep duration to determine its association with BMD. Nocturnal sleep duration, assessed objectively (actigraphy) or subjectively (questionnaire), was not independently associated with BMD in postmenopausal women.
Introduction
Both long and short self-reported sleep durations are associated with low bone mineral density (BMD) in men and women. The association between sleep duration measured by actigraphy and BMD in postmenopausal women is unknown.
Methods
The Study of Osteoporotic Fractures (SOF) ancillary sleep study was used to determine the association between sleep duration and BMD at the total hip and femoral neck in postmenopausal women ≥ 75 years old. Sleep duration was assessed by wrist actigraphy (average 4 nights) and questionnaire. BMD was compared between postmenopausal women with short (< 6 h/night) vs. NIH-recommended (7–8 h/night) sleep durations. Data were analyzed using a 2-sample t test (unadjusted) and multivariate regression model (adjusted). Simple linear regression was used to estimate the difference in BMD per additional hour of sleep when sleep duration was considered as a continuous, rather than dichotomized, variable.
Results
Total hip BMD was higher in women with actigraphically assessed shorter sleep duration in unadjusted models only. No clinically or statistically significant differences in total hip or femoral neck BMD were observed according to nocturnal sleep duration after adjusting for body mass index (BMI) in dichotomized (N = 874) or continuous (N = 1624) sleep duration models or when subjective sleep duration was used. When sleep duration included daytime naps, longer sleep duration was associated with lower total hip BMD (β = − 0.005, p = 0.04).
Conclusions
Nocturnal sleep duration, whether assessed objectively (actigraphy) or subjectively (questionnaire), was not independently associated with BMD in older postmenopausal women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One in two women 50 years of age or older will experience an osteoporotic fracture in their lifetime [1]. Unfortunately, an underlying etiology is often not identified when an evaluation for low bone mineral density (BMD) and increased fracture risk is performed [2,3,4]. Emerging data suggest that sleep and circadian disturbance may be unrecognized, potentially modifiable risk factors for impaired bone health [5, 6]. The Nurses’ Health Study [7] and the Women’s Health Initiative (WHI) [8] identified an increased risk of fracture in postmenopausal women who reported a history of rotating night shiftwork and self-reported short (≤ 5 h) sleep duration, respectively. Rats exposed to chronic sleep restriction demonstrated significantly lower bone formation and lower BMD [9, 10]. We reported that after approximately 3 weeks of cumulative sleep restriction and concurrent circadian disruption, men had significantly lower levels of the bone formation marker, propeptide of type 1 procollagen N-terminal (P1NP), despite no change in a bone resorption marker C-telopeptide of type I collagen (CTX) [11]. There have been numerous epidemiological studies of the association between sleep duration and bone mineral density (BMD) in humans, with mixed results. Methodological differences and limitations make the actual association and its direction unclear.
Prior studies performed in mostly or entirely female populations have found that both long [12,13,14,15,16,17,18,19,20] and short [14,15,16, 20,21,22,23] self-reported sleep durations have been associated with lower BMD, while others identified no association [24, 25]. The cutoffs used to define short and long sleep durations varied considerably. Short sleep was most commonly defined as < 6 h/night [12, 16, 18, 19, 22,23,24, 26, 27] but ranged from ≤ 5 [12, 18, 22] up to 7–8 h/night [14, 15]. Conversely, long sleep was most commonly defined as ≥ 8 or ≥ 9 h/day. Only one study examined sleep duration as a continuous variable and found no association with osteopenia/osteoporosis but had few individuals who reported < 6 h of sleep per night [25]. Two meta-analyses investigated the association between sleep duration and osteoporosis [17, 20]. The most recent indicated that both long (defined as ≥ 9 h/night) and short (defined as ≤ 7 h/night) self-reported sleep durations were associated with an increased risk of osteoporosis, with the lowest risk in those middle-aged and elderly adults sleeping ~ 8 h/night [20]. These conflicting data may be due to different covariate adjustments and study populations (Asian, United States, South American, and European countries), inadequate consideration for actual versus desired total sleep time, and BMD assessment by different methods (DXA, QUS) at various anatomical sites [6]. Most notably, all prior analyses used subjective (self-reported) sleep duration, with various definitions of short, reference, and long sleep times. Subjective sleep duration is variably correlated with objectively measured sleep duration and subjective estimates may be particularly inaccurate for those with shorter sleep duration or insomnia [28,29,30].
The National Institutes of Health (NIH) recommends 7–8 h of sleep per day for individuals 18 years of age and older [31]. The American Academy and Sleep Medicine (AASM) and Sleep Research Society (SRS) joint consensus statement recommended adults sleep “7 or more hours per night on a regular basis to promote optimal health” [32]. Despite these recommendations, over one-third of US adults report getting less than the recommended amount of sleep [33] and the Centers for Disease Control (CDC) highlighted insufficient sleep as a public health epidemic in 2014 [34]. With these recommendations in mind, we used the Study of Osteoporotic Fractures (SOF) ancillary sleep study of 3137 women to investigate the relationship between objective sleep duration measured by actigraphy and BMD in postmenopausal women. BMD was compared in older, postmenopausal women according to their sleep duration. Objective sleep duration was (a) dichotomized with short sleep duration defined as < 6 h/night and the recommended sleep duration defined as 7–8 h/night and (b) also considered as a continuous variable without any exclusions based on total sleep time. Sleep duration cutoffs for the dichotomized analysis were chosen because they were in line with the NIH recommendations [31], they facilitated comparison to some previous literature, the short sleep duration was similar to the sleep restriction imposed in our prior intervention study [11], and because the groups were sufficiently different to identify a clinically significant difference between short and recommended sleep duration groups. Based on previous interventional data from animals [9, 10] and humans [11], we hypothesized that shorter sleep duration would be associated with lower BMD.
Methods
Study design and participant selection
The study design and cohort characteristics of the Study of Osteoporotic Fractures (SOF) have been previously described [35]. In short, 9704 community-dwelling, ambulatory women ≥ 65 years were recruited using mailings to age-eligible women identified from community-based listings between September 1986 and October 1988 from 4 metropolitan areas in the USA (Baltimore, MD; Minneapolis, MN; Monongahela Valley near Pittsburgh, PA; and Portland, OR). In February 1997 through February 1998, 662 African American women were added to the original cohort for a total of 10,366 women [36].
Of the 10,366 women initially recruited for the SOF study, 3137 (66% of active survivors) participated in the ancillary SOF Sleep Study between January 2002 and April 2004. During their clinical visit, participants completed the Epworth Sleepiness Scale (ESS) and Pittsburgh Sleep Quality Index (PSQI), along with other medical questionnaires and clinical assessments. Actigraphy data were collected on 3052 women who wore the device on their non-dominant wrist for a minimum of 3 consecutive 24-h periods (average 4 nights, 5 days), except during water sports or while bathing [36]. Participants were asked to complete a sleep diary concurrent with actigraphy.
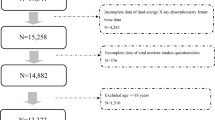
Women were included in the sleep duration/BMD analysis if they had useable actigraphy data and had a concurrent BMD assessment of the total hip and femoral neck (Fig. 1). Lumbar spine BMD was not available at this clinic visit. Women were excluded from the analysis if they had concurrent medical and/or sleep conditions that may confound the association between sleep duration and BMD including current tobacco use, current excess alcohol intake (≥ 14 drinks/week), use of bisphosphonates or oral glucocorticoids, or self-reported diabetes mellitus, hyperthyroidism, or sleep apnea diagnoses (Fig. 1). No women used non-bisphosphonate osteoporosis medications (e.g., calcitonin, denosumab, anabolic therapy) at the time the SOF sleep study was performed (2002–2004). Serum creatinine was not available on all participants at this visit. A subset of women had an assessment of renal status 5–6 years prior to this data collection. Conservatively, we excluded any woman with an eGFR < 60 ml/min/1.73m2 at that time, anticipating that renal function would decline over time (Fig. 1). Due to inconsistent results in previous sleep duration-BMD literature, we took the approach of excluding women with potential confounders to isolate the sleep duration-BMD association. Any signal detected could then be followed up in a more generalizable study population.
To examine the association between sleep duration and BMD, sleep duration was considered as a dichotomized (primary analysis) and continuous (pre-planned secondary analysis) variable. A total of 1624 women met the above eligibility criteria for the continuous sleep duration analysis (Fig. 1). Subsequently, women with 6–7 and > 8 h of sleep/night were excluded for the dichotomous sleep duration analysis leaving 874 women who were categorized as “short” (< 6 h; N = 382) or “recommended” (7–8 h; N = 492) sleep duration, respectively (Fig. 1).
The Institutional Review Board at each SOF clinical site approved the study, and all participants provided written consent. The current analysis utilized de-identified data and was deemed non-human subjects research by the Colorado Multiple Institutional Review Board.
Wrist actigraphy and objectively measured sleep duration
The Sleepwatch O (Ambulatory Monitoring, Inc., Ardsley, NY) was used to collect actigraphy data [36]. Clinic staff in charge of collecting actigraphy data underwent centralized training and certification. The San Francisco Coordinating Center at California Pacific Medical Center and the University of California, San Francisco (San Francisco, CA) scored and processed the actigraphy data centrally using Action W-2 software [36]. The proportional integration mode (PIM) was used to assess sleep duration as this mode has been reported to have the best accuracy for older cohorts [37]. Sleep duration was averaged for the nights wrist actigraphy was worn. Nocturnal sleep duration did not include daytime sleep (naps). Daytime sleep duration (naps) was measured separately and only included in analyses that used total 24-h sleep duration.
Bone mineral density (BMD) by dual-energy X-ray absorptiometry (DXA)
Bone mineral density (BMD) at the total hip and the femoral neck was assessed using Hologic QDR 1000 (Hologic Inc., Waltham, MA) dual-energy X-ray absorptiometry (DXA) [35]. Image analysis was performed at each clinical site, with review of a random subset and of flagged scans by the San Francisco Coordinating Center.
Subjective sleep duration and other sleep questionnaires
Each participant completed a questionnaire on sleep habits on which she answered the question “On most nights, how many hours do you sleep each night?” Other questions asked about occurrence/frequency of napping, number of hours of sleep needed to feel rested, and presence of sleep disorders. Two validated sleep questionnaires (Pittsburgh Sleep Quality Index (PSQI) and Epworth Sleepiness Scale (ESS)) were also completed. A woman was considered a poor sleeper according to the PSQI if her score was > 5 [38]. A score > 10 on ESS was considered as excessive daytime sleepiness [39].
Other measurements
BMI was calculated using body weight (kg) on a balance beam scale divided by height (m2) measured using a wall-mounted Harpenden stadiometer. Self-reported questionnaires were used to determine history of physician-diagnosed medical conditions (e.g., osteoporosis, hypertension, COPD, depression, insomnia). Medication use (e.g., estrogen, calcium, vitamin D) was determined by cataloging all medications (prescription, over-the-counter) brought in by SOF participants to their study visit. Walking speed (m/s) was determined from the 6-m walk test with participants walking at their usual pace.
Statistical analysis
A priori power analysis using preliminary data determined 53 women with short and 53 women with recommended sleep duration was required to see a 0.055 g/cm2 difference in BMD (with 80% power and 2-sided α = 0.05). This was felt to be a clinically significant difference based on data demonstrating that smaller BMD changes correlate with fracture risk reduction [40]. With our final, larger sample size, the same test would have 95% power to detect a 0.025 g/cm2 difference in BMD (N = 874) with 2-sided α = 0.05. A p value ≤ 0.05 was considered statistically significant.
For the primary analysis, total hip and femoral neck BMD were compared between women with short nocturnal sleep duration (< 6 h/night) and those getting the NIH recommended amount of sleep (7–8 h/night) using a 2-sample t test (unadjusted, model A). A multivariate regression model was used to test whether BMD differed significantly between women with short vs. recommended nocturnal sleep durations in minimally adjusted (model B, adjusted for age, race, clinic site, and body mass index (BMI)) and fully adjusted (model C, additionally adjusted for use of calcium/vitamin D/estrogen, depression, walking speed, hypertension, COPD, self-reported daily naps, and number of hours needed to feel rested) analyses. An individual was excluded from an analysis if a covariate(s) was missing (< 10% had a missing covariate); therefore, the analyzed sample size is noted for each model. Covariates were selected based on clinical relevance and significant differences identified in baseline characteristics (Table 1).
As a pre-planned analysis, the association between nocturnal sleep duration and BMD was also examined modeling sleep duration as a continuous exposure variable. A simple linear regression model was used to estimate the parameter β, representing the change in BMD (g/cm2) per each additional hour of sleep in the full SOF sleep cohort (without any exclusions for total sleep time; N = 1624). Multivariate regression models were also used to estimate β in the minimally (model B) and fully adjusted (model C) models using the same covariates as above. Although the relationship between self-reported sleep duration and BMD may be non-linear [20], visual inspection of SOF objective nocturnal sleep duration-BMD data plots and results from testing a quadratic term indicated linear models were appropriate. An interaction between BMI and sleep duration was tested by including an interaction term in model C. To facilitate comparison to prior literature, sleep duration-BMD analyses were also repeated using subjective, self-reported, sleep duration as the exposure variable. The association between BMD and objective total 24-h sleep duration including daytime naps was also examined, modeling sleep duration as a continuous exposure variable. A quadratic term was tested in model C in this 24-h total sleep duration model to investigate a non-linear association. Sensitivity analyses were performed to determine if results differed when women using bisphosphonates were included in the objective and subjective sleep duration cohorts with adjustment for bisphosphonate use. Sensitivity analyses were also performed to determine if results differed when women meeting exclusion criteria (e.g., smokers, glucocorticoid use) were included in the objective sleep duration analysis and these factors were instead adjusted for in model C.
Results
A total of 874 women met the eligibility criteria and were included in the BMD comparison between women with short (< 6 h/night, mean = 5.2 ± 0.8 h; N = 382) and recommended (7–8 h/night, mean 7.5 ± 0.3 h; N = 492) nocturnal sleep durations (Fig. 1). They were 83.3 ± 3.4 years old. Overall, approximately 90% of women were Caucasian, although the short nocturnal sleep duration group had a significantly higher percentage of African American women (Table 1). The women with short sleep duration had a higher BMI compared with those with the recommended sleep duration (27.9 ± 4.6 kg/m2 vs. 26.3 ± 4.2 kg/m2, p < 0.001). As expected, those with shorter nocturnal sleep duration had significantly higher scores on the Epworth Sleepiness Scale (Table 1). Women with short sleep duration indicated they needed 7 h of sleep per night to feel rested and their self-reported sleep duration tended to overestimate their measured sleep duration by 1.4 ± 1.4 h. Conversely, women with the recommended sleep duration reported they needed 7.6 h of sleep per night to feel rested and slightly underestimated their sleep duration on self-report compared with on actigraphy by 0.2 ± 1.3 h.
Short sleepers had significantly higher BMD than women getting the recommended amount of sleep in the unadjusted model at the total hip (0.018 g/cm2, p = 0.054), but not femoral neck (0.005 g/cm2, p = 0.54; Table 2, model A). After adjustment for BMI and other factors, there was no statistically significant difference in BMD at the total hip or femoral neck in postmenopausal women with measured short vs. recommended nocturnal sleep duration (Table 2, models B, C). In the fully adjusted model, femoral neck BMD tended to be 0.016 g/cm2 lower in women with short sleep duration compared with those with the recommended amount of sleep but this did not reach statistical significance (p = 0.06). An interaction term between BMI and sleep duration was not significant in the fully adjusted model for total hip (p = 0.77) or femoral neck (p = 0.15). Results were essentially unchanged when the AASM and SRS’s recommended sleep duration (7–9 h/night) [32] was used to define the recommended sleep group (data not shown).
As expected, more women met the inclusion criteria when measured nocturnal sleep duration was analyzed as a continuous variable (N = 1624). Consistent with the findings based on dichotomous nocturnal sleep duration, BMD was lower at the total hip (β = − 0.009, p < 0.01) with each additional hour of sleep in the unadjusted model (Table 3, model A) but there were no significant associations between objectively measured continuous nocturnal sleep duration and BMD at the total hip or femoral neck after adjustment (Table 3, models B, C).
There were no clinically or statistically significant associations between dichotomized (< 6 h vs. 7–8 h of sleep/night) or continuous subjective (self-reported) sleep duration and BMD at the total hip or femoral neck in any model (all p ≥ 0.35; Tables 2 and 3). All results in dichotomous and continuous analyses using objective and subjective nocturnal sleep durations were unchanged when bisphosphonate users were included in the analytical cohort and adjusted for in the model. Similarly, results in dichotomous and continuous analyses using objective nocturnal sleep duration were unchanged when women meeting exclusion criteria were included in the analytical cohort and instead those factors (e.g., glucocorticoid use, smoking) were adjusted for in model C (data not shown).
Only 40 women had 9+ h of sleep per night. These women were of similar age (average 83.7 ± 3.4 years) to the other nocturnal sleep duration groups and 85% were Caucasian. On average, these women slept 9.6 ± 0.6 h per night and had a lower BMI (25.9 ± 3.7 kg/m2). The sample size was too limited for formal analysis; however, osteoporosis diagnosis (27.5%), history of falls (40%), and history of fracture (60%) were more prevalent in this group than in the other sleep duration groups.
Sleep duration was significantly longer when daytime naps were included. Average actigraphic daytime nap duration was 70 ± 60 min (range 0–479 min). Longer 24-h sleep duration was associated with significantly lower total hip BMD in model C (β = − 0.005, p = 0.04; Table 3). This relationship was non-linear. The quadratic term was significant in model C for both the total hip (p = 0.001) and femoral neck (p = 0.04). This analysis suggested that maximum total hip BMD occurs at ~ 7.26 h of sleep per 24 h, but there was a large range of sleep durations with clinically similar BMD values.
Discussion
This study was the first to examine the association between objectively measured sleep duration and BMD in postmenopausal women using the NIH sleep duration recommendations [31] to categorize nocturnal sleep duration as short (< 6 h/night) and recommended (7–8 h/night). Contrary to our hypothesis, no association between nocturnal sleep duration and BMD was identified when measured or self-reported sleep durations were considered as a dichotomized or continuous variable. In fact, total hip BMD was higher in women with shorter nocturnal sleep duration in unadjusted models but this was not significant after adjustment. The association between nocturnal sleep duration and BMD in the unadjusted model was stronger in continuous vs. dichotomized analyses, likely because of the larger sample size and in consideration of extremely short and long nocturnal sleep durations in the continuous model. However, after adjustment for BMI, age, race, and clinical site in the minimally adjusted model (model B), there was no clinically or statistically significant association between nocturnal sleep duration and BMD at the total hip or femoral neck between short (< 6 h/night) and recommended (7–8 h/night) sleepers or when sleep was considered as a continuous variable. When total sleep time included daytime naps, longer 24-h sleep duration was associated with significantly lower BMD at the total hip. The relationship between 24-h sleep duration and BMD was non-linear, suggesting that maximum total hip BMD occurs at ~ 7.26 h in older postmenopausal women, but there was a large range of sleep durations with clinically similar BMD values.
Shorter nocturnal sleep duration was associated with higher total hip BMD in the unadjusted models, likely because women with shorter nocturnal sleep duration had higher BMI and a higher percentage of African American women who tend to have higher BMD than Caucasian women [41]. There were no significant associations between nocturnal sleep duration and BMD after adjusting for these two factors, along with age and clinical site. Data from the subjective nocturnal sleep duration analyses were similar, although BMD differences were even smaller in magnitude and associations weaker than when measured sleep duration was used, despite larger sample sizes.
These data contradict prior analyses that indicated a possible U-shaped association between self-reported sleep duration and BMD [14,15,16, 20, 27]. The main difference between current and previous studies was the use of actigraphy to objectively measure sleep duration in the current study. However, there was also no association of self-reported nocturnal sleep duration, as used in previous studies, with BMD. Our contradictory findings compared with prior literature may also be due to the differences in anatomical site assessed for BMD, radiographic BMD technique (central DXA vs. QUS), age/sex/sex hormone status of the study population, and sleep duration cutoffs (rationale of which were not always provided). The average age of the current cohort was older than any other study, by > 15 years in most studies. In addition, because very few women in this postmenopausal cohort had long nocturnal sleep duration (< 5% had measured sleep duration of 9+ h/night), we may have been underpowered to detect a relationship between long nocturnal sleep duration and BMD. There were trends for a higher percentage of osteoporosis diagnosis, falls, and fractures in the small group of women who had 9+ h of nocturnal sleep. In fact, when total sleep time included daytime naps and sleep durations in the group became longer, an association was observed between longer 24-h sleep duration and lower BMD at the total hip. Samples sizes were generally larger in prior analyses; however, the current study was adequately powered to detect the magnitude of BMD difference reported in prior studies.
These data are consistent with two prior reports [24, 25] that found no association between self-reported sleep duration and BMD using (a) continuous sleep duration and similar BMD assessment sites [25] as the current study and (b) similar sleep duration cutoffs [24] as the current study but with proximal femur volumetric BMD by CT. Those studies had similar [25] or larger [24] sample sizes than the current study. Although both included younger men and women, the average age in the Marques et al. study was 77 years [24], perhaps suggesting that age modifies the effect of sleep duration on bone metabolism. Finally, this was the first study to consider the amount of sleep needed to feel rested and highlighted that those getting less sleep report needing slightly less sleep than those getting the recommended amount. Overall, the findings suggest that short nocturnal sleep duration is not associated with low BMD after adjustment for other clinically relevant factors, such as BMI.
It is likely important to include daytime naps in sleep duration analyses performed in older individuals. Stone et al. previously identified an increased risk of falls and fractures with self-reported long sleep duration (> 10 h/24 h interval, including naps) compared with women who slept 8–9 h in age-adjusted analyses but not in multivariate analyses in SOF [42]. Stone et al. also noted a significantly increased risk of falls and hip fractures in older postmenopausal women in SOF who reported daily napping, after age (hip fracture) and multivariate (falls) adjustment [42]. Although that study was based on self-reported sleep duration, current results are in line with those findings when total actigraphic 24-h sleep duration included naps. In the current analysis, an association between longer sleep duration and lower total hip BMD was only observed once daytime naps were included. Daytime naps on actigraphy were, on average, over an hour in duration, representing a significant percentage of 24-h sleep duration in these women. Although sedentary behavior in older individuals can compromise accurate actigraphic sleep assessment, 24-h sleep durations (including naps) may be more representative in these older populations than nocturnal sleep durations.
There are likely confounders or mediators in the sleep-bone relationship (e.g., muscle strength, falls, BMI, naps, comorbidities) that mitigate any apparent association between sleep duration and bone outcomes. If a small difference in BMD according to nocturnal sleep duration exists, it may be difficult to detect in older postmenopausal women, in whom other factors (e.g., prolonged estrogen deficiency) have a greater effect on bone metabolism. Furthermore, only ~ 25% of women in this cohort self-reported a high physical activity level. This level of inactivity in an elderly cohort (average age 83.3 ± 3.4 years) may have made it difficult to detect a nocturnal sleep duration-BMD association, since both older age and inactivity are associated with low BMD. More detailed information on 24-h activity patterns may help to identify sleep/wake patterns that are related to low BMD in older women. In addition, the complexity of sleep phenotype (e.g., duration, efficiency, quality) and potential for night-to-night variability in sleep may preclude detection of an association with BMD, which does not acutely change from one night to another. Moreover, the magnitude of the effect of sleep disruption on bone may vary by individual based on underlying factors (e.g., baseline BTM levels, age). Lastly, it is unclear if chronically short or long sleep durations continue to alter bone turnover or if bone metabolism is only impacted by short-term perturbations and adapts over time. If the latter is true, then no appreciable difference in BMD could be detected in observational, cross-sectional analyses. Interventional studies may be more capable of detecting sleep disruption-induced skeletal changes.
This study represents the largest analysis of the association between objectively determined sleep duration and BMD in postmenopausal older women. However, there were limitations. BMD at the lumbar spine was not available in the cohort. Results may differ at skeletal sites that have higher trabecular bone content (such as the lumbar spine) as compared to those with a higher cortical bone content (such as the total hip and femoral neck). In addition, serum 25-hydroxy vitamin D was not measured, which may modify the effect of sleep duration on BMD. It is possible that excluding those on osteoporosis therapy biased our results towards the null by eliminating those with the lowest BMD. However, results were unchanged when bisphosphonate users were included in the cohort with adjustment for drug therapy. This study assessed two-dimensional BMD and therefore could not evaluate differences in bone quality or microarchitecture that may be influenced by sleep duration. Although we excluded women who reported having sleep apnea, the high prevalence of sleep-disordered breathing in older adults [43] means there may have been undiagnosed sleep apnea, particularly in the short sleep duration group. We expect this would have increased the risk of a type 1 error and, therefore, was unlikely to have changed the results. A longer actigraphy assessment period may provide a more accurate assessment of sleep duration. Women in this cohort wore their wrist actigraphy device for 4 nights, on average, and sleep duration was averaged over the duration of use so first night effect was minimized [44]. Although women were excluded for current bisphosphonate use, ~ 3% of the BMD cohorts were taking estrogen or raloxifene. This small number of users is not expected to have significantly influenced the overall results.
Conclusion
Nocturnal sleep duration, whether assessed objectively with actigraphy or subjectively by questionnaire, was not associated with BMD in older postmenopausal women after adjusting for BMI. Longer 24-h sleep duration (including naps) was associated with lower total hip BMD.
Abbreviations
- BMD:
-
Bone mineral density
- P1NP:
-
Propeptide of type 1 procollagen N-terminal
- CTX:
-
C-telopeptide of type I collagen
- AASM:
-
American Academy of Sleep Medicine
- SRS:
-
Sleep Research Society
- SOF:
-
Study of Osteoporotic Fractures
- QUS:
-
Quantitative ultrasound
- DXA:
-
Dual-energy X-ray absorptiometry
- BMI:
-
Body mass index
- PIM:
-
Proportional integration mode
- h:
-
hour
References
(NOF) NOF About NOF. https://www.nof.org/about-us/about-nof/ Accessed July 9 2018
Hudec SM, Camacho PM (2013) Secondary causes of osteoporosis. Endocr Pract 19:120–128
Diab DL, Watts NB (2013) Secondary osteoporosis: differential diagnosis and workup. Clin Obstet Gynecol 56:686–693
Painter SE, Kleerekoper M, Camacho PM (2006) Secondary osteoporosis: a review of the recent evidence. Endocr Pract 12:436–445
Swanson CM, Shea SA, Stone KL, Cauley JA, Rosen CJ, Redline S, Karsenty G, Orwoll ES (2015) Obstructive sleep apnea and metabolic bone disease: insights into the relationship between bone and sleep. J Bone Miner Res 30:199–211
Swanson CM, Kohrt WM, Buxton OM, Everson CA, Wright KP Jr, Orwoll ES, Shea SA (2018) The importance of the circadian system & sleep for bone health. Metabolism 84:28–43
Feskanich D, Hankinson SE, Schernhammer ES (2009) Nightshift work and fracture risk: the Nurses’ Health Study. Osteoporos Int 20:537–542
Cauley JA, Hovey KM, Stone KL, et al. (2018) Characteristics of self-reported sleep and the risk of falls and fractures: the Women’s Health Initiative (WHI). J Bone Miner Res 34:464–474. https://doi.org/10.1002/jbmr.3619
Everson CA, Folley AE, Toth JM (2012) Chronically inadequate sleep results in abnormal bone formation and abnormal bone marrow in rats. Exp Biol Med 237:1101–1109
Xu X, Wang L, Chen L, Su T, Zhang Y, Wang T, Ma W, Yang F, Zhai W, Xie Y, Li D, Chen Q, Fu X, Ma Y, Zhang Y (2016) Effects of chronic sleep deprivation on bone mass and bone metabolism in rats. J Orthop Surg Res 11:87
Swanson C, Shea SA, Wolfe P, Cain SW, Munch M, Vujovic N, Czeisler CA, Buxton OM, Orwoll ES (2017) Bone turnover markers after sleep restriction and circadian disruption: a mechanism for sleep-related bone loss in humans. J Clin Endocrinol Metab 102:3722–3730
Niu J, Sahni S, Liao S, Tucker KL, Dawson-Hughes B, Gao X (2015) Association between sleep duration, insomnia symptoms and bone mineral density in older Boston Puerto Rican adults. PLoS One 10:e0132342
Tian Y, Shen L, Wu J, Xu G, Yang S, Song L, Zhang Y, Mandiwa C, Yang H, Liang Y, Wang Y (2015) Sleep duration and timing in relation to osteoporosis in an elderly Chinese population: a cross-sectional analysis in the Dongfeng-Tongji cohort study. Osteoporos Int 26:2641–2648
Chen G, Chen L, Wen J, Yao J, Li L, Lin L, Tang K, Huang H, Liang J, Lin W, Chen H, Li M, Gong X, Peng S, Lu J, Bi Y, Ning G (2014) Associations between sleep duration, daytime nap duration, and osteoporosis vary by sex, menopause, and sleep quality. J Clin Endocrinol Metab 99:2869–2877
Wang K, Wu Y, Yang Y, Chen J, Zhang D, Hu Y, Liu Z, Xu J, Shen Q, Zhang N, Mao X, Liu C (2015) The associations of bedtime, nocturnal, and daytime sleep duration with bone mineral density in pre- and post-menopausal women. Endocrine 49:538–548
Cunningham TD, Di Pace BS (2015) Is self-reported sleep duration associated with osteoporosis? Data from a 4-year aggregated analysis from the National Health and Nutrition Examination Survey. J Am Geriatr Soc 63:1401–1406
Moradi S, Shab-Bidar S, Alizadeh S, Djafarian K (2017) Association between sleep duration and osteoporosis risk in middle-aged and elderly women: a systematic review and meta-analysis of observational studies. Metabolism 69:199–206
Kim N, Choi HR, Kim SW, Kim BS, Won CW, Kim SY (2014) Association between bone mineral density and sleep duration in the Korean elderly population. Korean J Fam Med 35:90–97
Saint Martin M, Labeix P, Garet M, Thomas T, Barthelemy JC, Collet P, Roche F, Sforza E (2016) Does subjective sleep affect bone mineral density in older people with minimal health disorders? The PROOF cohort. J Clin Sleep Med 12:1461–1469
Wang D, Ruan W, Peng Y, Li W (2018) Sleep duration and the risk of osteoporosis among middle-aged and elderly adults: a dose-response meta-analysis. Osteoporos Int 29:1689–1695
Specker BL, Binkley T, Vukovich M, Beare T (2007) Volumetric bone mineral density and bone size in sleep-deprived individuals. Osteoporos Int 18:93–99
Fu X, Zhao X, Lu H, Jiang F, Ma X, Zhu S (2011) Association between sleep duration and bone mineral density in Chinese women. Bone 49:1062–1066
Kuriyama N, Inaba M, Ozaki E, Yoneda Y, Matsui D, Hashiguchi K, Koyama T, Iwai K, Watanabe I, Tanaka R, Omichi C, Mizuno S, Kurokawa M, Horii M, Niwa F, Iwasa K, Yamada S, Watanabe Y (2017) Association between loss of bone mass due to short sleep and leptin-sympathetic nervous system activity. Arch Gerontol Geriatr 70:201–208
Marques EA, Figueiredo P, Gudnason V, Lang T, Sigurdsson G, Sigurdsson S, Aspelund T, Siggeirsdottir K, Launer L, Eiriksdottir G, Harris TB (2017) Associations of 24-hour sleep duration and CT-derived measurements of muscle and bone: the AGES-Reykjavik study. Exp Gerontol 93:1–6
Lucassen EA, de Mutsert R, le Cessie S, Appelman-Dijkstra NM, Rosendaal FR, van Heemst D, de n Heijer M, Biermasz NR, group NEOs (2017) Poor sleep quality and later sleep timing are risk factors for osteopenia and sarcopenia in middle-aged men and women: the NEO study. PLoS One 12:e0176685
Kobayashi D, Takahashi O, Deshpande GA, Shimbo T, Fukui T (2012) Association between osteoporosis and sleep duration in healthy middle-aged and elderly adults: a large-scale, cross-sectional study in Japan. Sleep Breath 16:579–583
Lima MG, Bergamo Francisco PM, de Azevedo Barros MB (2012) Sleep duration pattern and chronic diseases in Brazilian adults (ISACAMP, 2008/09). Sleep Med 13:139–144
Means MK, Edinger JD, Glenn DM, Fins AI (2003) Accuracy of sleep perceptions among insomnia sufferers and normal sleepers. Sleep Med 4:285–296
Bianchi MT, Wang W, Klerman EB (2012) Sleep misperception in healthy adults: implications for insomnia diagnosis. J Clin Sleep Med 8:547–554
Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC (2001) Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med 2:389–396
(2012) Explore sleep deprivation and deficiency: how much sleep is enough? National Heart, Lung, and Blood Institute. http://www.nhlbi.nih.gov/health/health-topics/topics/sdd/howmuch
Watson NF, Badr MS, Belenky G et al (2015) Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 38:843–844
Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB (2016) Prevalence of healthy sleep duration among adults - United States, 2014. MMWR Morb Mortal Wkly Rep 65:137–141
Centers for Disease C (2014) Insufficient sleep is a public health epidemic. http://www.cdc.gov/features/dssleep/index.html#References
Cummings SR, Black DM, Nevitt MC et al (1990) Appendicular bone density and age predict hip fracture in women. The study of osteoporotic fractures research group. Jama 263:665–668
Blackwell T, Ancoli-Israel S, Gehrman PR, Schneider JL, Pedula KL, Stone KL (2005) Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep 28:1599–1605
Spira AP, Stone KL, Redline S, Ensrud KE, Ancoli-Israel S, Cauley JA, Yaffe K (2017) Actigraphic sleep duration and fragmentation in older women: associations with performance across cognitive domains. Sleep 40
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28:193–213
Johns MW (2000) Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res 9:5–11
Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW Jr, Dequeker J, Favus M (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 333:1437–1443
Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, Berger ML, Santora AC, Sherwood LM (2001) Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. Jama 286:2815–2822
Stone KL, Ewing SK, Lui LY, Ensrud KE, Ancoli-Israel S, Bauer DC, Cauley JA, Hillier TA, Cummings SR (2006) Self-reported sleep and nap habits and risk of falls and fractures in older women: the study of osteoporotic fractures. J Am Geriatr Soc 54:1177–1183
Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O (1991) Sleep-disordered breathing in community-dwelling elderly. Sleep 14:486–495
Tamaki M, Bang JW, Watanabe T, Sasaki Y (2016) Night watch in one brain hemisphere during sleep associated with the first-night effect in humans. Curr Biol 26:1190–1194
Acknowledgments
The authors thank Lily Lui for her assistance with this analysis. In addition, the authors thank the Investigators in the Study of Osteoporotic Fractures Research Group: San Francisco Coordinating Center (California Pacific Medical Center Research Institute and University of California San Francisco): SR Cummings (co-principal investigator), K Yaffe (co-principal investigator), DC Bauer (co-investigator), DM Black (co-investigator), PM Cawthon (co-investigator), N Lane (co-investigator), C McCulloch (co-investigator), A Schwartz (co-investigator), G Tranah (co-investigator), D Evans (co-investigator), R Benard, T Blackwell, L Concepcion, S Ewing, SL Harrison, D Kriesel, N Parimi, K Peters, C Schambach, and J Ziarno.
Funding
CMS is supported by NIH grant T32DK007674-20, NIH grant T32DK007446-34, and 1K23AR070275-01.
The Study of Osteoporotic Fractures (SOF) is supported by the National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, R01 AG027576, and R01 AG026720.
The research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number 1K23AR070275-01, P50 HD073063 (Kohrt), and the Eastern Colorado VA Geriatric, Research, Education, and Clinical Center (GRECC). The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. This research is also supported by NIH/NCATS Colorado CTSA Grant Number UL1 TR002535. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
The Institutional Review Board at each SOF clinical site approved the study, and all participants provided written consent.
Conflict of interest
In the interest of full disclosure, we report the following; however, we do not believe any of these pertain to the current work. PJB, JAC, HAF, MEW, WMK have nothing to disclose. CMS did consulting for Radius Health, Inc. ESL was PI on an unrelated Merck, Inc. grant given to her institution. This funding does not reflect a conflict of interest. KPW reports research support from the NIH, Office of Naval Research, Pac-12, Philips Inc., CurAegis Technologies (formerly known as Torvec Inc.), Somalogics; Financial relationships: consulting fees from or served as a paid member of scientific advisory boards for NIH (Sleep Disorders Research Advisory Board - National Heart, Lung and Blood Institute), CurAegis Technologies, Circadian Therapeutics, LTD, Kellogg Company; Board of Directors: Sleep Research Society; Speaker/educational consultant honorarium fees: American Academy of Sleep Medicine, American College of Chest Physicians, American Diabetes Association. ESO consults for and has received research support from Radius, Mereo, and Bayer. ESO, for The Osteoporotic Fractures in Men (MrOS) Study, is supported by the National Institutes of Health funding via the following institutes: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research, under the following grant numbers: U01AG027810, U01 AG042124, U01 AG042139, U01 AG042140,U01 AG042143, U01 AG042145, U01 AG042168, U01AR066160, and UL1 TR000128. KLS has received grant funding from Merck.
Ethical approval
The current analysis utilized de-identified data and was deemed non-human subjects research by the Colorado Multiple Institutional Review Board.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Swanson, C., Blatchford, P., Orwoll, E. et al. Association between objective sleep duration and bone mineral density in older postmenopausal women from the Study of Osteoporotic Fractures (SOF). Osteoporos Int 30, 2087–2098 (2019). https://doi.org/10.1007/s00198-019-05007-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-019-05007-5