Abstract
Summary
Dietary patterns may interfere with the efficacy of herbal intervention. Our results demonstrated the protective effects of Salvia miltiorrhiza aqueous extract (SMA) on bone metabolism were influenced by levels of dietary fat and sucrose in ovariectomized (OVX) rats through its actions on attenuating lipid deposition and oxidative stress in rats.
Introduction
Salvia miltiorrhiza (SM), also known as Danshen, has been tested as an osteoporosis treatment in a series of small, short human trials that generally report improvements in bone property. However, dietary patterns may interfere with the effects of herbal intervention. We hypothesized that dietary fat and sucrose levels could influence the effects of SM supplementation on bone in estrogen-deficient animals.
Methods
Six-month-old Sprague-Dawley sham or OVX rats were fed either a low-saturated fat-sucrose (LFS, a diet that was similar in composition to normal rat chow) or a high-fat-sucrose (HFS) diet and OVX rats were treated (8 rats/group) with SM aqueous extract (SMA, 600 mg/kg/day), 17β-estradiol (1 mg/kg/day), or vehicle for 12 weeks.
Results
SMA significantly improved bone properties as revealed by the increase in trabecular bone mineral density and decrease in trabecular separation at proximal metaphysis of the tibia (PT) in HFS-fed OVX rats, but not in LFS-fed OVX rats. SMA greatly reduced lipid deposition and malondialdehyde levels, improved the activities of superoxide dismutase, catalase, and glutathione peroxidase in the livers of HFS-fed OVX rats. SMA could directly improve the proliferation and differentiation in vitro in an H2O2-induced preosteoblast cell model by attenuating cellular reactive oxygen species levels.
Conclusions
The protective effects of SMA on bone metabolism were influenced by dietary fat and sucrose levels in OVX rats. The ability of SMA to reduce bone loss in HFS-fed OVX rats was associated with the attenuation of lipid deposition and oxidative stress levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a metabolic disease characterized by fragility fractures of the spine and hip, in which an imbalance between ostoblasts and osteoclasts develops with age and subsequently leads to increased risk of bone fracture. [1]. A growing amount of evidence indicates that oxidative stress (OS) induced by reactive oxygen species (ROS), which increases with age, can adversely affect bone homeostasis and OS is speculated to be a pathogenic mechanism of the age-related bone loss and strength [2, 3]. Cross-sectional or case-control studies have indicated that high OS levels in women and men were along with reduced bone mineral density and increased risk of osteoporosis [4,5,6,7]. In vitro mechanistic study showed that OS decreases the lifespan of osteoblast in bone and inhibits the Wnt/β-catenin signaling pathway involved in osteoblast differentiation [8]. OS also promotes osteoclast formation and activity [3]. Thus, OS modulation by antioxidants might be useful for preventing or delaying the process of osteoporosis. However, studies on the efficacy of antioxidant supplementation for osteoporosis or bone loss prevention give inconsistent results [9,10,11,12,13]. Such inconsistency might be due to the weak efficacy of these antioxidants in comparison to commonly prescribed agents such as estrogens, bisphosphonates, and RANK ligand antibodies; or it might be due to differences in genetic makeup and eating habits among individuals.
High-fat-sucrose (HFS) diet has been demonstrated to increase ROS and lipid peroxidation and alter the antioxidant defense status in various tissues in mice [14]. Studies using hyperlipidemic mice models [15, 16] indicated that low-density lipoprotein particles, upon modification by OS, would accumulate in both vascular and bone tissues, theoretically triggering the pathogenesis of atherosclerosis and osteoporosis. Estrogen deficiency was found to be the cause of low antioxidant levels in post-menopausal women [7], and it might lead to increased risk of osteoporosis. Both HFS diets and estrogen deficiency are contributing factors for developing abnormality in bone metabolism. Our recently published study [17] indicated that HFS diet consumption caused the same bone mass/microarchitecture changes in sham-operated and ovariectomized (OVX) rats as low-fat-sucrose (LFS) diet consumption did. The negative synergistic actions of HFS and estrogen deficiency on bone properties occurred in cancellous bones and were characterized by elevated OS and accelerated bone resorption [17].
Danshen (Salvia miltiorrhiza, SM), the dried root of Salvia miltiorrhiza Bunge, is one of the best-known Chinese traditional herbs that has been used to manage cardiovascular diseases for more than 2000 years. In traditional Chinese medicine theory, SM can promote blood flow, remove blood stasis and nourish blood in menstruation, and reduce pain and mental restlessness [18]. SM, in combination with other herbs, was tested in more than 30 clinical trials for treatment of post-menopausal, senile, and secondary osteoporosis in different regions of China [19]. However, most of these clinical trials were not well designed, with either too small patient sample sizes, a lack of dietary record, and/or unclear clinical endpoints, making it difficult to conclude if SM was effective for management of osteoporosis. In particular, the lack of dietary control or inconsistency in dietary intake might be one of the major confounding factors that interfere with the efficacy of herbal intervention.
It is hypothesized that SM exerts its protective effects on the cardiovascular system mainly via removing free radicals and preventing lipid oxidation [20]. Indeed, our previous studies reported that aqueous extract of Danshen (SMA) could prevent HFS-fed OVX rats from endothelial dysfunction [21]. In the present study, we investigated whether daily SMA consumption would ameliorate the aggravated cancellous bone loss in OVX rats induced by HFS diet and estrogen deficiency. Specifically, we hypothesized that a higher dietary fat and sucrose consumption would alter the protective effects of SMA on bone in estrogen-deficient rats.
In the present study, SMA was administrated to both LFS-fed and HFS-fed OVX rats. Bone properties, tissue and blood lipids, and antioxidant status of rats were measured. Hydrogen peroxide (H2O2)-induced MG63 preosteoblast cells were used as an in vitro model in order to determine if the action mechanism of SMA on osteoblastogenesis could occur through suppression of OS.
Materials and methods
Preparation of Danshen aqueous extract
Danshen (SM, the dried root of S. miltiorrhiza Bunge) was obtained from the Beijing Guancheng Pharmaceutical Co., Ltd. of China. A voucher specimen was deposited at the Department of Applied Biology and Chemical Technology of the Hong Kong Polytechnic University. The dried herb (33 kg) was ground into powder by a herb grinder (R-14L, Buyamag Inc., Carlsbad, CA, USA) and extracted twice with distilled water at 100 °C for 30 min and precipitated with 60% alcohol. The preparation was filtered and concentrated to produce a condensed plaster. Then the extract was freeze-dried for a week. A yield of powder weighing a total of 4.3 kg was obtained, and the yield (4.3-kg extract powder/dried herb 33 kg) was about 13.03%. The extract was stored at − 20 °C and dissolved in distilled water before administration. The dosage of Danshen aqueous extract (SMA) was 600 mg/kg once daily by oral gavage. Standardization of the extract was performed as described in our previously published paper [21]. Salvianolic acid B and danshensu, two major active ingredients, were identified in SMA by using high-performance liquid chromatography analysis. SMA applied in the present study was found to contain 0.19% ± 0.01% danshensu and 3.93% ± 0.10% salvianolic acid B [21].
Materials and reagents
All reagents for cell culture were purchased from Life Technologies, Inc., USA. 17β-Estradiol (E2) (Cat. E8875) and 2′,7′-dichlorodihydrofluorescin diacetate (DCFH-DA) ROS activity assay kit (Cat. D6883) were purchased from Sigma-Aldrich, USA. The dosage of E2 was 1 mg/kg once daily by oral gavage. MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) reagent powder was purchased from Promega (Cat. G1111, USA). Alkaline phosphatase (ALP) assay kit and calcium/phosphate/creatinine colorimetric assay kits were purchased from Wako Pure Chemical Industries, Ltd., Japan. Oxidant/antioxidant detection kits were purchased from Keygen Biotech. Co., Ltd., China.
Rats and experimental design
Sixty-four Sprague-Dawley rats (6-month-old, virgin female, weighing 338 ± 5 g), supplied by Guangdong Provincial Medical Laboratory Animal Center, were randomly divided into eight groups (each with eight animals) based on their body weight. Six groups were OVX (n = 48) and the remaining two groups received sham operation (SHAM, n = 16). Both ovariectomy and sham operation followed the previous published procedures [22, 23], except that anaesthetization was applied with sodium pentobarbitone (100 mg/kg, i.p. injection, Cat. P3761 Sigma-Aldrich, USA), and wounds in rats were stitched by using surgical sutures (Chromic Catgut, Cat. VCP845G, ETHICON, USA) in the present study. After surgery, the rats were pair-fed with LFS diet based on the minimum intake amount among all of the groups each day. Four weeks after surgery, sham rats weighed 345 ± 7 g and OVX rats weighed 362 ± 6 g. Eight sham rats and 24 OVX rats were pair-fed with a LFS diet; the other eight sham rats and 24 OVX rats were pair-fed with a HFS; the feeding lasted 12 weeks. The total intake amount of LFS or HFS diet for each animal was tightly controlled by pair feeding and was based on the minimum intake amount among all of the groups each day which averaged ~ 14–16 g per day. OVX rats fed with either LFS or HFS diet were treated with SMA (600 mg/kg/day), or 17β-estradiol (1 mg/kg/day), or vehicle (1% ethanol, v/v) by oral gavage once daily for a total of 12 weeks. E2 was dissolved in 100% ethanol at the concentration of 10 mg/ml (as stock) and freshly diluted to 1 mg/ml by distilled water every day. SMA powder was dispersed into vehicle solution (1% ethanol, v/v) at the concentration of 600 mg/ml, and the solution was freshly prepared each day. OVX rats were administered 100 μl/100 g body weight of 1-mg/ml 17β-estradiol solution and 600-mg/ml SMA solution by oral gavage each day.
Animal experimental design and animal grouping are summarized in a flowchart (Fig. 1). All rats were housed in an environment with constant room temperature (20.5°), humidity (62%), and 12:12-h light–dark cycles. During the feeding study, rats were allowed free access to double distilled water. Body weight was recorded every week. All animal care and experiments were performed in accordance with the guidelines for the care and use of laboratory animals at The Hong Kong Polytechnic University. The experimental protocol was conducted under the animal license issued by the Department of Health, the Hong Kong SAR Government, and the Animal Subjects Ethics Sub-committee (ASESC No. 05 / 21) of The Hong Kong Polytechnic University.
Diet composition
The LFS control diet (TD.10592) and HFS diet (TD.10586) were purchased from Harlan Teklad. The HFS diet was modified from a common higher-fat diet (TD.88137, with 42% kcal from fat and 30% kcal from sucrose, and with 0.2% cholesterol) that matched the calcium (Ca) and phosphorus (P) levels of TD.98005 (0.6% Ca, 0.65% P) in our previous bone mineral metabolism studies [24]. A specific LFS control diet was generated, which contained 13% energy from fat, 15% energy from sucrose, and with some soybean oil added in order to provide sufficient essential fatty acids. The HFS diet contained 20% more energy than the LFS diet, but the micronutrient levels of the two diets were the same. It should be noted that LFS diet belongs to normal rat chowing diet, its fat and sucrose contents are very similar to those of the normal rat chowing diet AIN-93M. The composition of the LFS diet, the HFS diet, and normal rat chow (AIN-93M) diet is presented in Table 1.
Sample collection
The total treatment period lasted 12 weeks. Upon week 12, 2 days before sacrifice, animals were housed individually in metabolic cages for collection of urine and feces for 24 h. On the day of sacrifice, rats were euthanized by using pentobarbitone via i.p. injection (10-mg/100-g body weight). Blood was withdrawn from the abdominal aorta, and serum was prepared and stored at − 80 °C. Ovaries in rats were checked to see if they had been fully cut off during the OVX operation. Simultaneously, uteruses were examined on a rat-by-rat basis in order to check if they had atrophy in response to the OVX operation. Then uteruses were collected, and any adherent fatty tissue to them was separated and cleaned. The uteruses were dried in a drying oven (Universal Oven U, Memmert, Germany) at 50 °C overnight and weighed; their weight (sham rats vs. OVX rats without drug treatments) was an important parameter to determine how successful the OVX surgery was. The livers were immediately collected, rinsed, and stored at − 80 °C. The right tibias were collected for microcomputed tomography (μCT) analysis; they were wrapped in saline-soaked towels together with the muscle tissue and stored at − 20 °C for further analysis.
Biochemical analysis of serum and urine samples
Ca, P, and creatinine (Cr) concentrations in both serum and urine samples were measured using standard colorimetric methods with calcium/phosphate/creatinine colorimetric assay kits (Wako Pure Chemical Industries, Ltd., Japan). Urinary Ca and P excretion was expressed as ratios of urinary Ca or P to Cr level.
Lipid content and oxidant/antioxidant levels in the livers
Two grams of each liver sample was homogenized and filtered. One part of homogenized liver samples was centrifuged to obtain lipids, which were dried, weighed, and analyzed as previously reported [25, 26]. The liver lipid content was expressed as the weight of lipid in 1 g of liver. The levels of malondialdehyde (MDA) as well as the activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) were measured in duplicate using spectrophotometry-based commercial kits, including MDA/SOD/CAT/GPx Detection Kit (Keygen Biotech. Co., Ltd., Nanjing, PR China). MDA levels were expressed as nmol/mg protein, SOD, CAT, and GPx activities were expressed as U/mg protein.
Microcomputed tomography (μCT) analysis of rat tibia
Cone-beam X-ray μCT, vivaCT 40 (Scanco Medical, Brüttisellen, Switzerland), was used to take μCT images of proximal metaphysis of the tibia (PT) and tibial midshaft (MT) with a tube voltage of 70 kVp, tube content of 0.114 mA, slice thickness of 21 μm, and voxel size of 10.5 μm. The volume of interest was selected as a region that began 2.2 mm distal to the junction of the epiphyseal bony plate with the proximal boundary of the growth cartilage and extended distally 100 slices. The scanned bone contained both cortical and trabecular bone. We delineated the trabecular region from cortical bone by manually drawing a uniform region of interest, which was an irregular anatomic contour a few pixels away from the endocortical boundary. The scanned volume of the tibial midshaft was centered on the midpoint of the long bone. The region of interest of the cortical analysis was set at half the distance from the distal condyle to the junction of the epiphyseal bony plate with the proximal boundary of the growth cartilage and extended toward the distal diaphysis for 100 slices. μCT images were prepared, μCT reconstruction models were generated, and 3D bone parameters were calculated by a three-dimensional image analysis software (SCANCO Finite Element Software and MicroCT Analysis Software V6.6, Scanco Medical, Switzerland). The measured parameters for PT included trabecular bone mineral density, Tb.BMD; ratio of bone volume to total volume, BV/TV; trabecular number, Tb.N; trabecular thickness, Tb.Th; trabecular separation, Tb.Sp; connectivity density, Conn-Des; degree of anisotropy, DA; and structure model index, SMI. The measured parameters for MT included total bone mineral density, BMD; ratio of cortical bone volume to total volume, Ct.V/Tt.V; total cross-sectional area inside the periosteal envelope, Tt.Ar; cortical bone area, Ct.Ar; marrow area, Ma.Ar; and average cortical thickness, Ct.Th.
Human osteosarcoma MG63 cell culture
Human osteosarcoma MG63 cell line (Homo sapiens, ATCC® CRL-1427™) was purchased from ATCC and cultured in Minimal Essential Medium Eagle (MEM) with 10% fetal bovine serum (FBS) that was heat-inactivated and 100 mg/ml of streptomycin and 100-U/ml penicillin in a condition of 5% CO2 and 37 °C. Before drug treatment, the cells were changed into a low-serum (containing 1% FBS) medium for 24 h. N-acetyl-L-cysteine (L-NAC, 10−5 or 10−4 M) or SMA (1, 5, 10, 15 μg/ml) pre-treated MG63 cells for 24 h, followed by hydrogen peroxide (H2O2, 15 and 40 μM) treatment for another 24 h in cells. After drug treatment, the cells were collected for correspondent assays to determine cell viability, differentiation, and intracellular ROS levels.
Cell viability and differentiation and intracellular ROS levels
Standard MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay was used to measure cell viability. ALP activity was measured and normalized by cell protein concentration to reflect ALP activity. The intracellular ROS level was evaluated by DCFH-DA ROS activity assay. Cells were incubated with fluorescent solutions and the fluorescence intensity was recorded after normalization by cell viability. The experiments were repeated four times for each group.
Statistical analysis
Data from these experiments were reported as mean ± standard error of mean (SEM) for each group. Intergroup differences between different treatments in either LFS-fed rats (including groups of SLV, OLV, OLE, and OLD) or HFS-fed rats (including groups of SHV, OHV, OHE, and OHD) were separately analyzed by using one-way analysis of variance (ANOVA) (Prism 5.0 for Windows, GraphPad, CA, USA), and statistical significance between individual groups was determined using the Bonferroni method. Differences in P value of less than 0.05 were considered statistically significant. Analysis of the effects of HFS diet and SMA treatment and their interactions on different parameters was performed on OVX rats by using two-way ANOVA analysis. The groups of OLV, OLD, OHV, and OHD were included in two-way ANOVA analysis. Statistical significance between individual groups was determined using the Bonferroni method. Differences in P value of less than 0.05 were considered statistically significant. Statistical analysis of the effects of different treatment in MG-63 cells and comparison between the effects of LFS and those of HFS diet on the same treatment groups was carried out by using Student’s T-test. Differences in P value of less than 0.05 were considered statistically significant.
Results
Body and uterus weight
As shown in Table 2, no significant differences were found in body weight at week 0 before treatment among the different groups in either LFS- or HFS-fed rats. At week 12, ovariectomy resulted in a significantly greater (threefold) weight gain in LFS-fed rats (P < 0.001 LFS-fed OVX-vehicle vs. sham-vehicle rats). The weight gain in LFS-fed OVX rats was significantly lower with 17β-estradiol treatment (P < 0.01 vs. LFS-fed OVX-vehicle rats) and also with SMA treatment (P < 0.05 vs. LFS-fed OVX-vehicle rats, Table 2). HFS-fed rats had higher weight gain than LFS-fed rats in sham (P < 0.01) and SMA (P < 0.001)-treated groups. However, OVX was not associated with greater weight gain in rats fed with HFS diet (HFS-fed OVX-vehicle vs. sham-vehicle rats) at week 12. Neither 17β-estradiol nor SMA treatment reduced the weight gain of the HFS-fed OVX rats (vs. HFS-fed OVX-vehicle rats).
Uterus weight in rats was significantly reduced by ovariectomy in either LFS- or HFS-fed rats (P < 0.001 vs. LFS or HFS-fed sham-vehicle rats). E2, but not SMA, increased the uterine weight in both OVX rats fed with LFS diet and those fed with HFS diet (P < 0.05 or P < 0.001 vs. LFS or HFS-fed OVX-vehicle rats, Table 2).
Two-way ANOVA analysis showed no interactive effects between HFS diet and SMA treatment on alterations of body weight and weight gain and uterine weight in OVX rats (Table 3). However, two-way ANOVA analysis showed a borderline (~ 20% higher) effect of SMA on uterine weight (P = 0.0587, Table 3), suggesting that SMA was marginally estrogenic at this dosage.
Serum and urine chemistries
Serum Ca levels were not altered in rats in response to different treatments in LFS- or HFS-fed rats (Table 2). Serum P was only shown to be significantly decreased in HFS-fed rats by ovariectomy (P < 0.05 vs. HFS-fed sham-vehicle rats, Table 2). Urinary Ca excretion was significantly reduced in LFS-fed OVX rats with 17β-estradiol treatment (P < 0.01 vs. LFS-fed OVX-vehicle rats). SMA treatment significantly suppressed urinary Ca excretion in both LFS- and HFS-fed OVX rats (P < 0.05 or P < 0.01 vs. LFS or HFS-fed OVX-vehicle rats, Table 2). Specifically, urinary Ca excretion was much lower in HFS-fed rats than in LFS-fed rats in the SMA-treated groups (P < 0.05). In contrast, urinary P excretion was not altered in rats in response to different treatments in LFS- or HFS-fed rats (Table 2). Two-way ANOVA analysis showed no interactive effects between HFS diet and SMA treatment on the alterations of those serum and urine chemistries parameters in OVX rats (Table 3).
Lipid content in the liver
Neither E2 nor SMA altered the lipid depositions in the liver of LFS-fed OVX rats (OLE vs. OLV; OLD vs. OLV) (Fig. 2). HFS diet prominently increased lipid depositions in both sham and OVX rats (P < 0.05, SHV vs. SLV; P < 0.01, OHV vs. OLV). Most importantly, both E2 and SMA treatment prevented HFS-induced lipid depositions in OVX rats. Figure 2 shows that the liver lipid contents were not altered in response to treatments in LFS-fed rats but they were significantly reduced by E2 (OHE) and SMA (OHD) treatment in the HFS-fed OVX rats (P < 0.01 vs. OHV rats, Fig. 2). Two-way ANOVA analysis showed no interactive effects between HFS diet and SMA treatment on liver lipid contents in OVX rats (Table 4).
Total lipid contents (g of lipid per g of liver) in the livers from OVX and sham-operated 6-month-old female rats fed with HFS or LFS diet in response to different treatments for 12 weeks. Values are expressed as mean ± SEM, n = 8. ## P < 0.01 vs. OHV rats. ^ P < 0.05, ^^ P < 0.01 vs. LFS-fed rats under same treatment
Oxidant/antioxidant levels in the liver
Malondialdehyde (MDA) levels in rat livers were significantly increased in response to ovariectomy in rats fed with HFS-diet (OHV), but not those with LFS-diet (OLV) (P < 0.05 vs. SHV, Fig. 3a). Treatment of HFS-fed OVX rats with E2 (OHE) and SMA (OHD) significantly suppressed OVX-induced increase in MDA levels (P < 0.001 vs. OHV, Fig. 3a). HFS-fed rats had higher MDA levels and lower CAT activity (Fig. 3c) than LFS-fed rats in the OVX-vehicle-treated groups (P < 0.05). The levels of antioxidative enzymes were not altered in rats fed with LFS-diet in response to different treatment, except for the significant inductive effects of SMA on SOD activities in OVX rats (P < 0.05, OLD vs. OLV, Fig. 3b). In contrast, treatment of OVX rats with E2 (OHE) significantly increased SOD activities in rats fed with HFS-diet (P < 0.05 vs. OHV rats, Fig. 3b), while SMA (OHD) treatment significantly increased SOD (P < 0.05 vs. OHV, Fig. 3b) as well as GPx (P < 0.01 vs. OHV, Fig. 3d) activities in the liver of OVX rats fed with HFS-diet. Two-way ANOVA analysis indicated that HFS diet and SMA treatment interactively acted on the levels of MDA (P < 0.01) in OVX rats, but they showed no interactive effects on the alterations of the other three antioxidant enzyme activities in OVX rats (Table 4).
The levels of MDA (a), SOD (b), CAT (c), and GPx (d) in the livers of OVX and sham-operated 6-month-old female rats fed with HFS or LFS diet in response to different treatments for 12 weeks. MDA, malondialdehyde; SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase. Values are expressed as mean ± SEM, n = 8. *P < 0.05 vs. OLV rats; # P < 0.05, ## P < 0.01, ### P < 0.001 vs. OHV rats. ^ P < 0.05 vs. LFS-fed rats under the same treatment
Bone properties evaluated by μCT
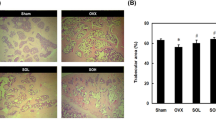
Figure 4a shows the 3D images of proximal metaphysis of the tibia (PT). OVX rats had significantly lower values of Tb.BMD (Fig. 4b), BV/TV, Tb.N, Conn-Des, and higher values of Tb.Sp and SMI at PT (vs. LFS or HFS fed sham-vehicle rats, Table 5). E2-treated OVX rats (LFS-fed) had significantly higher values of Tb.BMD (vs. OLV, Fig. 4b) and microarchitectural parameters such as BV/TV, Tb.N, Conn-Des, and lower values of Tb.Sp at PT (vs. LFS-fed vehicle-OVX rats, Table 5). The rats fed with HFS diet had lower values of Tb.BMD (Fig. 4b) and higher values of Tb.Sp (Table 5) than LFS-fed rats in vehicle or SMA-treated groups. The protective effects of E2 on bone were prominent in HFS-fed OVX rats as revealed by the significantly higher values of Tb.BMD (vs. OHV, Fig. 4b), BV/TV, Tb.N, Conn-Des, and lower values of Tb.Sp at PT (vs. HFS-fed OVX-vehicle rats, Table 5). In LFS-fed rats, SMA-treated OVX rats did not change significantly in values of Tb.BMD and values of bone microarchitectural parameters at PT (vs. LFS-fed OVX-vehicle rats, Fig. 4b and Table 5). In HFS-fed rats, SMA-treated OVX rats had significantly higher values of Tb.BMD (Fig. 4b), BV/TV (Table 5), and lower values of Tb.Sp (Table 5) at PT (vs. HFS-fed OVX-vehicle rats). Two-way ANOVA analysis indicated that significant interaction existed between HFS diet and SMA treatment on Tb.BMD, BV/TV, Conn-Des, and SMI at PT in OVX rats (Table 6).
Cone-beam X-ray microcomputed tomography (μCT) images (a) and bone mineral density (BMD) (b) at proximal metaphysis of the tibia (PT) of OVX and sham-operated 6-month-old female rats fed with HFS or LFS diet in response to different treatments for 12 weeks. In μCT images (a), the first row of images are from LFS-fed rats and the second row of images are from HFS-fed rats. Values in b are expressed as mean ± SEM, n = 8. **P < 0.01, ***P < 0.001 vs. OLV rats; # P < 0.05, ## P < 0.01, ### P < 0.001 vs. OHV rats. ^ P < 0.05 vs. LFS-fed rats under same treatment
Meanwhile, HFS diet consumption did not significantly result in bone mass changes at tibial midshaft (MT) of sham-operated and OVX rats, compared with LFS consumption (Table 7). OVX rats had significantly lower values of BMD, Ct.V/Tt.V, and Ma.Ar (vs. LFS-fed sham-vehicle rats, Table 7); E2-treated OVX rats had higher values of BMD, Ct.V/Tt.V, and Ma.Ar (vs. LFS-fed OVX-vehicle rats, Table 7) at MT of LFS-fed rats. SMA treatment failed to induce any changes in measured parameters at MT in either LFS-fed or HFS-fed OVX rats (Table 7). There were no significant interactions between HFS diet and SMA treatment on cortical bone parameters, as demonstrated by two-way ANOVA analysis (Table 8).
Osteoblast cell proliferation and differentiation
As shown in Fig. 5, 15-μM H2O2 significantly decreased MG63 cell viability (P < 0.01 vs. control, Fig. 5a) and 40-μM H2O2 significantly reduced MG63 cell differentiation (P < 0.05 vs. control, Fig. 5b). SMA treatment at 1–10 μg/ml alone did not alter MG63 cell proliferation (Fig. 5a) or differentiation (Fig. 5b). However, pretreatment with either N-acetyl-L-cysteine (L-NAC, 10−5 and 10−4 M) or SMA at 5 and 10 μg/ml significantly increased cell viability (Fig. 5a) and ALP activities (Fig. 5b) in H2O2-treated MG63 cells (P < 0.05 or P < 0.01 vs. H2O2-induced control), suggesting that these treatments protected against ROS-induced cytotoxicity in MG63 cells (Table 8).
Effects of aqueous extract of Salvia miltiorrhiza Bunge (SMA) on cell viability (a), ALP activity (b), and intracellular ROS levels (c) in preosteoblast MG63 cells with/without H2O2 induction. ALP, alkaline phosphatase; ROS, reactive oxygen species. Values are expressed as mean ± SEM, n = 4. *P < 0.05, **P < 0.01 vs. negative control without H2O2 induction. # P < 0.05, ## P < 0.01, ### P < 0.001 vs. negative control with H2O2 induction
Intracellular ROS levels
As shown in Fig. 5c, intracellular ROS activities in MG63 cells increased by 38% in response to treatment with H2O2 (25 μM) administration (P < 0.001 vs. control, Fig. 5c). Pretreatment with L-NAC at 10−5 M and SMA at 5–15 μg/ml significantly attenuated the H2O2-induced increase in ROS release in MG63 cells (P < 0.01 or P < 0.001 vs. H2O2-induced control, Fig. 5c).
Discussion
Our study showed that SMA treatment for 12 weeks could partially protect against bone loss in OVX rats only during the feeding of an HFS diet. Moreover, such bone protective effects of SMA in HFS-fed OVX rats were accompanied by reduction in the levels of lipid deposition and ROS in the rat livers. Furthermore, in vitro study showed that SMA exerted direct protective effects in MG63 cells against H2O2-induced cytotoxicity. These results suggested the possibility that the osteoprotective effects of SMA in HFS-fed OVX rats were associated with the antioxidative properties of SMA. The inability of SMA to exert significant effects on bone properties in LFS-fed OVX rats suggested that SMA was not effective in improving estrogen-deficiency-induced bone loss. It appeared that the effects of SMA on bone were influenced by levels of dietary fat and sucrose consumption in rats.
In the present study, a combination of HFS diet and estrogen deficiency in female rats resulted in high lipid deposition and elevation of OS in the liver. Such results are consistent with those as shown in our previous study [17]. E2 treatment helped to downregulate MDA levels and upregulate SOD activities in the livers of HFS-fed OVX rats. SMA treatment for 12 weeks greatly reduced lipid deposition in the livers of HFS-fed, but not LFS-fed OVX rats. In addition, SMA treatment reduced MDA levels and improved SOD, CAT, and GPx activities in the livers of HFS-fed OVX rats. These in vivo results suggested that SMA could play a significant role, as estrogen does, in reducing increased lipid deposition and downregulating ROS levels in tissues of HFS-fed OVX rats.
Our study showed that SMA treatment (600 mg/kg/day) for 12 weeks could partially protect against bone loss in OVX rats only during their feeding of an HFS diet. The bone efficacy of SM extracts or its components have been demonstrated repeatedly by others in experimental animals [27,28,29,30,31,32]. Administration of SM ethanolic extract at 30 mg/kg/day for 8 weeks significantly increased BMD and BMC and attenuated the deterioration of the trabecular bone structure in 20-week-old OVX rats [27]. Administration of SMA at 5 g/kg/day for 8 weeks improved BMD and increased serum osteocalcin and ALP levels in alloxan-induced diabetic osteoporotic rats [28]. Chemical constituents in SM are found to exert osteoprotective effects via different drug targets. For instance, tanshinone and its derivatives in SM are potent cathepsin K inhibitors that inhibit osteoclast activity [33]; salvianolic acids in SM appeared to be pro-osteogenic in vivo in glucocorticoid-treated rats [30]. In our present study, SMA did not improve bone properties in LFS-fed OVX rats, but it significantly enhanced Tb. BMD and BV/TV and decreased Tb.Sp in HFS-fed OVX rats. Such effect is different from that of estradiol, whose effects are independent of animal diet fed in OVX rats. Furthermore, the two-way ANOVA analysis performed in OVX rats demonstrated the significant interaction between HFS diet and SMA treatment on regulation of bone parameters at PT and MDA levels in the livers of OVX rats. These results suggested that levels of dietary fat and sucrose consumption in rats influenced the effects of SMA on bone and mineral metabolism; thereby, reduction of high lipid deposition and OS levels as a result of SMA treatment might be responsible for its osteoprotective effects in HFS-fed OVX rats.
SMA simultaneously reduced lipid deposition in the livers and improved bone properties in HFS-fed OVX rats. Indeed, our previous study demonstrated that HFS-induced changes in rat liver lipid content were associated with changes in bone properties [17]. Recent case–control studies reported lower bone mineral density at all bone sites in subjects with non-alcoholic fatty liver disease [34]. A reciprocal relationship between adipogenesis and osteogenesis was suggested [35]. Salvianolic acid A, a major component from SM, was reported to promote osteogenesis and simultaneously suppress adipogenesis in prednisone-treated rats [36]. Thus, our results suggested that the bone protective actions of SMA in HFS-fed OVX rats might be mediated via its ability to decrease lipid content of liver.
High dosages of H2O2 were used in the present study to induce MG63 cells, a human osteosaroma osteoblastic cell line [37], to mimic the conditions of osteoblasts in HFS-fed OVX rats with high OS status. H2O2-induced MG63 cells are commonly used as a platform to study the osteoprotective effects of potential agents with antioxidant properties that can prevent ROS-induced bone loss [37]. Our study showed that SMA could protect cell proliferation and differentiation in H2O2-induced osteoblast cell model by attenuating cellular ROS levels. The effective dosages of SMA in MG63 cells were shown to be around 10–15 μg/ml in the present study. Based on the reported content of danshensu (0.19%) and salvianolic acid B (3.93%) in the SMA extract used in our study [21], the effective concentrations of danshensu and salvianolic acid B present in SMA extract were equivalent to 10−13 to 10−12 M in vitro. Such working concentration of danshensu would likely be achieved in vivo by feeding rats with 0.8–8-mg/kg danshensu according to reported pharmacokinetic studies of SM extract [38]. Indeed, the concentration of danshensu administrated daily in our in vivo study from 600 mg/kg of SMA extract was estimated to be 1.14 mg/kg. Our calculation herein suggested that the in vitro working concentrations of SMA extract were comparable to the dosages used in the in vivo study. Thus, the observed in vitro antioxidative and bone protective effects of SMA might also occur in vivo and provide further support that the in vivo bone protective effects of SMA were mediated by its antioxidative effects in bone cells, especially in conditions with high OS challenge.
SM components interact with multiple pathways that are targeted in single-drug approaches for OP therapy. For instance, tanshinone and its derivatives in SM could inhibit osteoclast activity and are potent cathepsin K inhibitors [33]; salvianolic acids in SM seemed to be pro-osteogenic [30] . In addition, SM was found to exert estrogenic effects on mice by stimulating biosynthesis of estrogen and increasing estrogen receptors in target tissues without side effects on reproductive tissues [39]. The present results also showed that SMA is marginally estrogenic at the present applied dosage. Hence, the scope of the present study was limited to characterizing the osteoprotective effects of SM in estrogen-deficient animals under different dietary patterns (LFS and HFS), and it did not fully delineate the mechanisms of SM and its potentially active components on bone metabolism. Furthermore, a single dose of SMA applied in the present study might not provide overall understanding of its efficacy in vivo. SMA treatment in the present OVX rat study was started at 4 weeks post-OVX, a time when some OVX-induced bone loss has already occurred in long bone metaphyses. However, OVX-induced bone loss lasts from 4 to 6 months in long bone metaphyses and even longer in other sites, such as the vertebral body. Thus, our experiment was mainly a prevention study in which the positive findings for SMA on bone mass and microarchitecture in HFS diet OVX rats can easily be attributed to prevention of bone loss. Additional experiments, which begin SMA treatment at 16 weeks post-OVX when most OVX-induced bone loss has ceased, should be carried out. In such case, restoration of bone mass through potential osteogenic effects of SMA in the presence of a HFS diet through dynamic histomorphometric analysis can be more clearly measured.
In summary, SMA could protect OVX rats fed with HFS diet from bone loss. The mechanism was associated with the protective effects of SMA against high lipid deposition and OS levels in tissues of HFS-fed OVX rats. The direct actions of SMA on promoting preosteoblast cell proliferation and differentiation in H2O2-induced MG63 cells through reducing cellular ROS levels further demonstrated that the antioxidant properties of SM may play an important role in bone protection. SM might be an alternative regimen for combating OS for women who regularly consume high-fat and high-sucrose diets. Further studies are required to characterize the active ingredients in SMA that account for its bone protective effects and delineate the mechanism by which dietary fat and sugars might alter the osteoprotective effects of SMA in rats.
Abbreviations
- ANOVA:
-
analysis of variance
- ALP:
-
alkaline phosphatase
- BMC:
-
bone mineral content
- BMD:
-
bone mineral density
- BV/TV:
-
ratio of bone volume to total volume
- Ca:
-
calcium
- CAT:
-
catalase
- Conn-Des:
-
connectivity density
- Cr:
-
creatinine
- Ct.Th:
-
average cortical thickness
- Ct.V/Tt.V:
-
ratio of cortical bone volume to total volume
- DA:
-
degree of anisotropy
- DCFH-DA:
-
2′,7′-dichlorodihydrofluorescin diacetate
- FBS:
-
fetal bovine serum
- GPx:
-
glutathione peroxidase
- HFS:
-
high fat-sucrose
- H2O2 :
-
hydrogen peroxide
- LFS:
-
low fat-sucrose
- Ma.Ar:
-
marrow area
- MDA:
-
malondialdehyde
- MEM:
-
minimal essential medium eagle
- μCT:
-
microcomputed tomography
- MT:
-
tibia midshaft
- MTS:
-
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- OS:
-
oxidative stress
- OVX:
-
ovariectomized
- P:
-
phosphorus
- PMS:
-
phenazine methosulfate
- PT:
-
proximal metaphysis of the tibia
- ROS:
-
reactive oxygen species
- SEM:
-
standard error of mean
- SHAM:
-
sham-operation
- SM:
-
Salvia miltiorrhiza
- SMA:
-
Salvia miltiorrhiza aqueous extract
- SMI:
-
structure model index
- SOD:
-
superoxide dismutase
- Tb. BMD:
-
trabecular bone mineral density
- Tb.N:
-
trabecular number
- Tb.Sp:
-
trabecular separation
- Tb.Th:
-
trabecular thickness
- Tt.Ar:
-
total cross-sectional area inside the periosteal envelope
References
Seeman E (2003) Reduced bone formation and increased bone resorption: rational targets for the treatment of osteoporosis. Osteoporos Int 14(Suppl 3):S2–S8
Zhou Q, Zhu L, Zhang D et al (2016) Oxidative stress-related biomarkers in postmenopausal osteoporosis: a systematic review and meta-analyses. Dis Markers 2016:7067984
Callaway DA, Jiang JX (2015) Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J Bone Miner Metab 33(4):359–370
Lee YJ, Hong JY, Kim SC et al (2015) The association between oxidative stress and bone mineral density according to menopausal status of Korean women. Obstet Gynecol Sci 58(1):46–52
Basu S, Michaelsson K, Olofsson H et al (2001) Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun 288(1):275–279
Sharma T, Islam N, Ahmad J et al (2015) Correlation between bone mineral density and oxidative stress in postmenopausal women. Indian J Endocrinol Metab 19(4):491–497
Sanchez-Rodriguez MA, Ruiz-Ramos M, Correa-Munoz E et al (2007) Oxidative stress as a risk factor for osteoporosis in elderly Mexicans as characterized by antioxidant enzymes. BMC Musculoskelet Disord 8:124
Almeida M, O’Brien CA (2013) Basic biology of skeletal aging: role of stress response pathways. J Gerontol A Biol Sci Med Sci 68(10):1197–1208
Finck H, Hart AR, Lentjes MA et al (2015) Cross-sectional and prospective associations between dietary and plasma vitamin C, heel bone ultrasound, and fracture risk in men and women in the European Prospective Investigation into Cancer in Norfolk cohort. J Am J Clin Nutr 102(6):1416–1424
Talaulikar VS, Chambers T, Manyonda I (2012) Exploiting the antioxidant potential of a common vitamin: could vitamin C prevent postmenopausal osteoporosis? J Obstet Gynaecol Res 38(1):253–257
Sahni S, Hannan MT, Gagnon D et al (2009) Protective effect of total and supplemental vitamin C intake on the risk of hip fracture—a 17-year follow-up from the Framingham Osteoporosis Study. Osteoporos Int 20(11):1853–1861
Kreijkamp-Kaspers S, Kok L, Grobbee DE et al (2004) Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. JAMA 292(1):65–74
Alekel DL, Germain AS, Peterson CT et al (2000) Isoflavone-rich soy protein isolate attenuates bone loss in the lumbar spine of perimenopausal women. Am J Clin Nutr 72(3):844–852
Liu Y, Xu J, Guo Y et al (2015) Ameliorative effect of vanadyl(IV)-ascorbate complex on high-fat high-sucrose diet-induced hyperglycemia, insulin resistance, and oxidative stress in mice. J Trace Elem Med Biol 32:155–161
Tintut Y, Morony S, Demer LL (2004) Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol 24(2):e6–10
Brodeur MR, Brissette L, Falstrault L et al (2008) Influence of oxidized low-density lipoproteins (LDL) on the viability of osteoblastic cells. Free Radic Biol Med 44(4):506–517
Dong XL, Li CM, Cao SS et al (2016) A high-saturated-fat, high-sucrose diet aggravates bone loss in ovariectomized female rats. J Nutr 146(6):1172–1179
Li MH, Chen JM, Peng Y et al (2008) Investigation of Danshen and related medicinal plants in China. J Ethnopharmacol 120(3):419–426
Liu Y, Liu JP, Xia Y (2014) Chinese herbal medicines for treating osteoporosis. Cochrane Database Syst Rev 3:1–127
Su CY, Ming QL, Rahman K et al (2015) Salvia miltiorrhiza: traditional medicinal uses, chemistry, and pharmacology. Chin J Nat Med 13(3):163–182
Li CM, Dong XL, Fan XD et al (2013) Aqueous extract of Danshen (Salvia miltiorrhiza Bunge) protects ovariectomized rats fed with high-fat diet from endothelial dysfunction. Menopause 20(1):100–109
Strom JO, Theodorsson A, Ingberg E et al (2012) Ovariectomy and 17beta-estradiol replacement in rats and mice: a visual demonstration. J Vis Exp 64:4013–4016
Idris AI (2012) Ovariectomy/orchidectomy in rodents. Methods Mol Biol 816:545–551
Zhang Y, Lai WP, Leung PC et al (2008) Improvement of Ca balance by Fructus Ligustri Lucidi extract in aged female rats. Osteoporos Int 19(2):235–242
Li CM, Guo YQ, Dong XL et al (2014) Ethanolic extract of rhizome of Ligusticum chuanxiong Hort. (chuanxiong) enhances endothelium-dependent vascular reactivity in ovariectomized rats fed with high-fat diet. Food Funct 5(10):2475–2485
Li CM, Wu JH, Yang RF et al (2013) Ligusticum chuanxiong prevents ovariectomy-induced liver and vascular damage in rats. Am J Chin Med 41(4):831–848
Cui Y, Bhandary B, Marahatta A et al (2011) Characterization of Salvia Miltiorrhiza ethanol extract as an anti-osteoporotic agent. BMC Complement Altern Med 11(1):120
Miao B, Wang J, Zhu Y et al (2012) Experimental study on effect of Salvia miltiorrhiza on alveolar bone metabolism and variation in bone mass in diabetic rats. Zhongguo Zhong Yao Za Zhi 37(11):1659–1662
Wang Y, Wang XX, Zhang LN et al (2012) Effects of traditional Chinese medicine on bone remodeling during orthodontic tooth movement. J Ethnopharmacol 141(2):642–646
Cui L, Li T, Liu Y et al (2012) Salvianolic acid B prevents bone loss in prednisone-treated rats through stimulation of osteogenesis and bone marrow angiogenesis. PLoS One 7(4):e34647
Wu X, Li Z, Yang Z et al (2012) Caffeic acid 3,4-dihydroxy-phenethyl ester suppresses receptor activator of NF-kappaB ligand-induced osteoclastogenesis and prevents ovariectomy-induced bone loss through inhibition of mitogen-activated protein kinase/activator protein 1 and Ca2+-nuclear factor of activated T-cells cytoplasmic 1 signaling pathways. J Bone Miner Res 27(6):1298–1308
Zhang ZP, You TT, Zou LY et al (2008) Effect of Danshen root compound on blood lipid and bone biomechanics in mice with hyperlipemia-induced osteoporosis. Nan Fang Yi Ke Da Xue Xue Bao 28(9):1550–1553
Cui L, Wu T, Liu YY et al (2004) Tanshinone prevents cancellous bone loss induced by ovariectomy in rats. Acta Pharmacol Sin 25(5):678–684
Xia MF, Lin HD, Yan HM et al (2016) The association of liver fat content and serum alanine aminotransferase with bone mineral density in middle-aged and elderly Chinese men and postmenopausal women. J Transl Med 14:11
Verma S, Rajaratnam JH, Denton J et al (2002) Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol 55(9):693–698
Cui L, Liu YY, Wu T et al (2009) Osteogenic effects of D+beta-3,4-dihydroxyphenyl lactic acid (salvianic acid A, SAA) on osteoblasts and bone marrow stromal cells of intact and prednisone-treated rats. Acta Harmacol Sin 30(3):321–332
Cao L, Bu R, Oakley JI et al (2003) Estrogen receptor-beta modulates synthesis of bone matrix proteins in human osteoblast-like MG63 cells. J Cell Biochem 89(1):152–164
Zhang YJ, Wu L, Zhang QL et al (2011) Pharmacokinetics of phenolic compounds of Danshen extract in rat blood and brain by microdialysis sampling. J Ethnopharmacol 136(1):129–136
Xu Y, Chen T, Li X et al (2016) Salvia miltiorrhiza bunge increases estrogen level without side effects on reproductive tissues in immature/ovariectomized mice. Aging 9(1):156–172
Acknowledgements
We thank the Shenzhen Key Laboratory of Food Biological Safety and the State Key Laboratory of Chinese Medicine and Molecular Pharmacology (Incubation) for their support.
Funding
This work was supported by the Shenzhen Basic Research Program (grant number JCYJ20140819153305696), the Shenzhen Basic Research Program (grant number JCY201506301152579000), the National Natural Science Foundation of China (grant number 81528024), and the National Natural Science Foundation of China (grant number 81601110).
Author information
Authors and Affiliations
Contributions
Xiao Li Dong and Man Sau Wong designed the experiment; Xiao Li Dong, Wen Xuan Yu, and Chun Mei Li conducted most of the experiments and analyzed the data; Shan He did cell culture experiments; Li Ping Zhou detected the oxidant/antioxidant levels in the liver of the experiment; Chui Wa Poon analyzed the microCT data; Man Sau Wong had primary responsibility for the final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Dong, X.L., Yu, W.X., Li, C.M. et al. Danshen (Salvia miltiorrhiza) protects ovariectomized rats fed with high-saturated fat-sucrose diet from bone loss. Osteoporos Int 29, 223–235 (2018). https://doi.org/10.1007/s00198-017-4254-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-4254-2