Abstract
Summary
Associations of adiposity indices with bone mineral density (BMD) and bone turnover markers were evaluated in Chinese participants. Body mass index, fat mass, and lean mass are positively related to BMD in both genders. Subcutaneous fat area was proved to be negatively associated with BMD and positively correlated with osteocalcin in postmenopausal females.
Introduction
Obesity is highly associated with osteoporosis, but the effect of adipose tissue on bone is contradictory. Our study aimed to assess the associations of adiposity indices with bone mineral density (BMD) and bone turnover markers (BTMs) in the Chinese population.
Methods
Our study recruited 5215 participants from the Shanghai area, evaluated related anthropometric and biochemical traits in all participants, tested serum BTMs, calculated fat distribution using magnetic resonance imaging (MRI) images and image analysis software, and tested BMD with dual-energy X-ray absorptiometry.
Results
When controlled for age, all adiposity indices were positively correlated with BMD of all sites for both genders. As for the stepwise regression analysis, body mass index (BMI), fat mass, and lean mass were protective for BMD in both genders. However, subcutaneous fat area (SFA) was detrimental for BMD of the L1–4 and femoral neck (β ± SE −0.0742 ± 0.0174; p = 2.11E−05; β ± SE −0.0612 ± 0.0147; p = 3.07E−05). Adiposity indices showed a negative correlation with BTMs adjusting for age, especially with osteocalcin. In the stepwise regression analysis, fat mass was negatively correlated with osteocalcin (β ± SE −8.8712 ± 1.4902; p = 4.17E−09) and lean mass showed a negative correlation with N-terminal procollagen of type I collagen (PINP) for males (β ± SE −0.3169 ± 0.0917; p = 0.0006). In females, BMI and visceral fat area (VFA) were all negatively associated with osteocalcin (β ± SE −0.4423 ± 0.0663; p = 2.85E−11; β ± SE −7.1982 ± 1.1094; p = 9.95E−11), while SFA showed a positive correlation with osteocalcin (β ± SE: 5.5993 ± 1.1753; p = 1.98E−06).
Conclusion
BMI, fat mass, and lean mass are proved to be beneficial for BMD in both males and postmenopausal females. SFA is negatively associated with BMD and positively correlated with osteocalcin in postmenopausal females.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a systemic skeletal disorder characterized by reduced bone mass, deteriorated bone structure, increased bone fragility, and fracture risk. Due to global aging of the population, osteoporosis is becoming increasingly prevalent worldwide [1, 2], causing increased health and economic burdens. Many studies have attempted to explore the mechanism of osteoporosis to determine potent measures to retard its progression. Many factors [3,4,5], including race, age, gender, family history of fracture, hormonal factors, obesity, use of certain drugs (e.g., glucocorticoids), cigarette smoking, alcohol intake, physical activity, and serum vitamin D level, influent osteoporosis; among which, obesity plays an important role in bone metabolism [6] and interacts with other osteoporosis risk factors.
Although various studies have tried to evaluate the association between obesity and osteoporosis, the results were proved to be equivocal. Some results [7] indicated that body size was positively correlated with bone mineral density (BMD), while another study [8] found that fat mass might be detrimental for osteoporosis when the mechanical loading effect of body weight on bone mass was adjusted for. Besides, it has been revealed that visceral adiposity was linked to decreased bone mass [9]. Possible reasons might be the mechanical stress of body weight is beneficial for osteoporosis [10], but the pro-inflammatory cytokines of the adipose tissues are proved to be detrimental for bone metabolism [11]; it is hard to say which role of adiposity is more predominant in osteoporosis, therefore, the effect of obesity on osteoporosis remains complicated. Furthermore, recent studies [4, 9] also found that, besides load bearing of body weight, there are other factors such as adipocyte hormones in fat mass which might involve the relationship between obesity and osteoporosis. And it has been proved that fat mass in different parts of the body might play distinct roles in bone metabolism [12]. Although previous studies [13, 14] have tried to clarify the association between fat distribution and osteoporosis, the roles of fat mass in bone metabolism are still controversial due to different population, age, gender, or body size of the participants and so on.
Osteoporosis mainly depends on bone strength, which includes BMD and bone quality, and osteopenia occurs during the process of bone remodeling (bone resorption and bone formation) and ultimately reflects on BMD [15]. Therefore, evaluating the association of body composition with BMD and bone turnover markers (BTMs) [16] may help to clarify the role of body fat distribution in osteoporosis and develop new treatments for this disease.
To clarify the association between adiposity and bone, our study evaluated the association of adiposity indices with BMD and BTMs in males and postmenopausal females separately.
Materials and methods
Ethics statement
This study was approved by the institutional review board of the Shanghai Jiao Tong University Affiliated Sixth People’s Hospital and is in strict accordance with the principles of the Second Revision of the Declaration of Helsinki. Each participant has signed a written informed consent form.
Participants
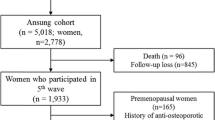
A total of 5404 participants aged from 26 to 81 years old were recruited from the Nicheng community of Shanghai from April 2013 to August 2014. All of them were unrelated subjects with eastern Han Chinese ancestry and resided in Shanghai. Participants with known cancer, hepatic disease, kidney disease, or other coexisting bone illnesses were excluded, and patients using medications (e.g., diphosphonate, glucocorticoids) that might influence bone metabolism were excluded prior to study. Premenopausal participants (n = 189) were also excluded. Finally, 5215 participants, including 949 males and 4266 postmenopausal females, were included for the analysis.
Clinical measurements
Anthropometric and biochemical traits were evaluated in all participants. Height (m) and weight (kg) were measured, and body mass index (BMI) was calculated as weight divided by height squared. Blood pressure (mmHg) was measured three times with 3-min intervals, and the average of the three measurements was separately retained for further analysis. Waist circumference was measured midway between the lowest rib and the iliac crest in the standing position. Serum triglycerides, total cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol levels were measured fasting using a type 7600-020 Automated Analyzer (Hitachi, Tokyo, Japan). HbA1c values were determined by high-performance liquid chromatography using a Bio-Rad Variant II hemoglobin testing system (Bio-Rad Laboratories, Hercules, CA, USA). Bone metabolism-associated factors, including β-cross-linked C-telopeptide of type l collagen (β-CTX), osteocalcin in the form of N-terminal osteocalcin molecules, N-terminal procollagen of type l collagen (PINP), 25-hydroxy vitamin D3 [25(OH)D3], and parathyroid hormone were measured fasting with an automated Roche electrochemiluminescence system (Roche Diagnostics Gmbh, Germany). The intra- and inter-assay coefficients of variation (CVs) were 5.6 and 8.0% for 25(OH)D3, 2.9 and 4.0% for osteocalcin, 2.3 and 2.8% for PINP, and 2.5 and 3.5% for β-CTX, respectively. Body fat percentage was determined with BC-420 Tanita Body Composition Analyzer (Tanita, Tokyo, Japan) with the subject standing with bare feet on the analyzer footpads, and the impedance between the two feet was measured with an alternating current passing through the lower body. Magnetic resonance imaging (MRI) images of all the subjects were obtained at the abdominal level between the fourth and fifth lumbar vertebrae in the supine position using 3.0-T MRI (Achieva; Philips, Best, The Netherlands), then abdominal visceral fat area (VFA), subcutaneous fat area (SFA), and lean mass were calculated by two trained observers who were unaware of the experimental design using image analysis software (SLICEOMATIC, version 4.2; TomoVision Inc., Montreal, QC, Canada). If the results of one subject differed by more than 10%, a third observer reanalyzed the image. Dual-energy X-ray absorptiometry (DEXA, Hologic QDR-2000, Hologic Corporation, Waltham, MA) was performed, and the BMD of the lumbar spine 1–4 (L1–4), femoral neck, and total hip was determined in each subject. The CV for the DXA measurements at L1–4, the total hip, and the femoral neck were 1.39, 0.7, and 2.22%, respectively. T-scores of L1–4, the femoral neck, and the total hip were separately calculated, based on the BMD at each site, using the formula: (Measured BMD − Young adult mean BMD)/Young adult population SD [17].
Statistical analysis
Data were analyzed using SAS 9.2. Normality testing was performed, and variables with skewed distributions were log transformed for further analysis. Means ± SD or medians (interquartile range) were used for continuous variables. The differences in the clinical characteristics of patients were determined with the Kruskal-Wallis test for continuous variables and the χ 2 test for categorical variables. Pearson correlation was performed to evaluate the inter-correlations between adiposity indices. Multiple linear regression analysis and stepwise regression analysis were performed to evaluate the association of adiposity indices with BTMs and BMD. Considering multiple traits (six adiposity indices, three BMDs of different places, and three BTMs) were analyzed in two genders separately in the current study, Bonferroni correction was done for multiple tests. A p value <0.0007 [0.05 / (6 × 6 × 2)] was considered statistically significant, which was used in the multiple regression analysis, as well as the inclusion and exclusion criteria in the stepwise regression analysis.
For the influence degree of different adiposity indices on bone density, z-scores of the adiposity indices were calculated to eliminate the influence of unit, and associations of adiposity z-scores with BMD were evaluated by multiple linear regression.
Results
Descriptive statistics
The basic characteristics of the participants are summarized in Table 1. The BMI, blood lipids, body fat percentage, fat mass, and SFA were significantly lower in males than those in females; while waist circumference, lean mass, VFA, the BMD of L1–4, the femoral neck, and the total hip were significantly higher in males than those in females. As shown in Supplementary Table 1, all these adiposity indices were highly inter-correlated with each other.
Associations between adiposity indices and BMD
As shown in Table 2, BMI, body fat percentage, fat mass, lean mass, VFA, and SFA were all positively correlated with BMD of all sites when controlled for age in both genders. Then, we did stepwise regression analysis of the adiposity indices with BMD. For males, BMI was correlated with the BMD of the total hip (β ± SE 0.0163 ± 0.0014; p = 2.70E−28). Fat mass was significantly associated with the BMD of the L1–4 and femoral neck (β ± SE 0.3773 ± 0.0332; p = 1.41E−27; β ± SE 0.2212 ± 0.0310; p = 2.55E−12). Lean mass was correlated with the BMD of the femoral neck (β ± SE 0.2703 ± 0.0792; p = 6.78E−4). The stepwise regression analysis of adiposity indices with BMD for females is shown in Table 3. BMD of all sites decreased significantly with age, and fat mass and lean mass were positively correlated with BMD. However, SFA was negatively associated with BMD of the L1–4 and femoral neck. As shown in Supplementary Table 2, for the associations between adiposity z-scores and BMD, the influence of different adiposity indices on BMD was all significantly positive. BMI, fat mass, and lean mass were more influential than the other adiposity indices in males, while only fat mass was more influential than others in females.
Associations between adiposity indices and BTMs
The associations of adiposity indices with BMTs are shown in Table 4. In males, adiposity indices including BMI, body fat percentage, fat mass, VFA, and SFA were negatively correlated with BTMs, especially with osteocalcin adjusted for age, but in the stepwise regression analysis, only fat mass showed a negative correlation with osteocalcin (β ± SE −8.8712 ± 1.4902; p = 4.17E−09), and lean mass was negatively correlated with PINP (β ± SE −0.3169 ± 0.0917; p = 0.0006). Similar results were observed in females for body fat percentage, fat mass, SFA, and VFA, and lean mass was correlated with osteocalcin adjusting for age. In the stepwise regression analysis, BMI and VFA were all negatively correlated with osteocalcin (β ± SE −0.4423 ± 0.0663; p = 2.85E−11; β ± SE −7.1982 ± 1.1094; p = 9.95E−11), while SFA turned out to be positively correlated with osteocalcin (β ± SE 5.5993 ± 1.1753; p = 1.98E−06). While no significant association of adiposity indices with PINP and β-CTX for females was observed.
Discussion
Our study involved 5215 participants (949 males and 4266 postmenopausal females) from the Shanghai area and mainly evaluated the association of adiposity indices with BMD and BTMs. When controlled for age, adiposity indices including body fat percentage, fat mass, lean mass, VFA, and SFA were all positively correlated with BMD of all sites. In the stepwise regression analysis, BMI, fat mass, and lean mass were proved to be beneficial for BMD in both genders. However, SFA was detrimental for BMD of the L1–4 and femoral neck in postmenopausal females. Adiposity indices showed negative correlation with BTMs, especially with osteocalcin when adjusted for age. In the stepwise regression analysis, fat mass was negatively associated with osteocalcin, and lean mass was negatively correlated with PINP for males. As to the stepwise regression analysis in females, BMI and VFA were all negatively correlated with osteocalcin, while SFA was significantly correlated with osteocalcin, and no significant association of adiposity indices with PINP and β-CTX was observed.
Proper load bearing is traditionally viewed to be beneficial for bone strength due to the positive mechanical loading effect on bone formation conferred by body weight [10]. In this study, body fat percentage, fat mass, lean mass, VFA, and SFA were positively correlated with BMD and negatively correlated with osteocalcin controlled for age in both genders, suggesting that mechanical loading effect is beneficial for bone. For stepwise regression analysis, BMI, fat mass, and lean mass were shown to be positively correlated with BMD for both genders. As to the associations between adiposity z-scores and BMD, after eliminating the influence of unit, BMI, fat mass, and lean mass were more influential on BMD than the other adiposity indices in males, due to their mechanical loading effect on bone [14, 18]. However, only fat mass was more predictive than others in females. We also observed females had higher fat mass and lower lean mass compared with males, which might induce a more predominant effect of fat mass on BMD in females. These results are consistent with those of previous researches [10, 14, 18] and confirmed the positive mechanical loading effect of body weight (BMI, fat mass, and lean mass) on bone strength.
For stepwise regression analysis in females, SFA was inversely correlated with the BMD of the L1–4 and the femoral neck. Besides, SFA was significantly correlated with osteocalcin, suggesting that SFA was related to a high bone turnover rate. Because bone loss was caused by the imbalance between bone formation and resorption, which occurs during the bone turnover process and results in a decrease in BMD; therefore, the results that SFA was inversely correlated with BMD and positively correlated with osteocalcin are logical in suggesting that SFA has a negative effect on bone metabolism. Although SFA has been considered to be protective or neutral in bone health [12, 19], the role of SFA is still ambiguous. In this study, the SFA in females was significantly higher than that in males, as estrogen differentially augments the sympathetic tone to adipose tissue between genders, which prompts lipid accumulation in the subcutaneous area in women and visceral fat deposition in men [20]. Furthermore, the total estrogen produced postmenopause is far less than that during pre-menopausal period, and it has been well-documented that estrogen deficiency can cause accelerated bone loss [21, 22]. Therefore, only in postmenopausal females, SFA was associated with a higher bone turnover rate and had an adverse effect on bone metabolism due to attenuation of estrogen protection.
VFA showed negative association with osteocalcin and positive correlation with BMD in both genders adjusting for age. While in the stepwise regression model, VFA was only negatively associated with osteocalcin in postmenopausal females, which was protective against bone loss, although no significant association of VFA with BMD was observed. This phenomenon is inconsistent with that of some previous studies for the following reasons. First, the bone mechanism characteristics differ depending on age, gender, or body size [23,24,25], and our study focused on postmenopausal females and older males (67.2 ± 2.1 years), while most previous research [12, 26] has focused on young participants with a limited sample size. Second, although VFA was negatively associated with osteocalcin and positively correlated with BMD adjusting for age, in the stepwise regression analysis, this association disappeared and VFA was only negatively correlated with osteocalcin in postmenopausal females, prompting that the effect of VFA on bone metabolism might be weaker than the mechanical loading effect of other adiposity indices. Additional research is needed to validate the function of VFA in bone metabolism.
Although we took gender, age, and menopausal status in consideration and measured the BMD of different sites, there are several limitations of our study. First, our study was a cross-sectional observational study, and we did not test the serum adipocyte cytokines which might play a key role in the link between adiposity and bone metabolism. The impact of adipose tissue on bone metabolism is only a phenomenon based on our results, and further prospective studies containing adipocyte cytokines measurement are needed to clarify the role of adipose tissue in bone metabolism. Second, although we set a more strict criteria (p < 0.0007) in the stepwise regression analysis, our study includes many tests and thus there might be a risk of spurious associations; therefore, further studies with larger samples are needed to certify the association of body composition with bone metabolism. Third, as the fat-related variables (body fat percentage, fat mass, lean mass, VFA, and SFA) evaluate adiposity from different aspects and they were closely interrelated with each other, we used stepwise regression analysis for the associations of adiposity indices with BMD and BTMs, trying to find the more predictive adiposity indices for bone density. However, it turned out to be hard to tell which one of them was significantly more predictive than others for bone density in the present study. Further researches are needed to figure out which adiposity index is the main determinant of bone density.
In summary, BMI, fat mass, and lean mass are positively correlated with BMD in both males and postmenopausal females. SFA is negatively associated with BMD and positively correlated with osteocalcin in postmenopausal females.
References
Svedbom A, Hernlund E, Ivergard M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jonsson B, Kanis JA (2013) Osteoporosis in the European Union: a compendium of country-specific reports. Arch Osteoporos 8:137. doi:10.1007/s11657-013-0137-0
Qu B, Ma Y, Yan M, Wu HH, Fan L, Liao DF, Pan XM, Hong Z (2014) The economic burden of fracture patients with osteoporosis in western China. Osteoporos Int 25(7):1853–1860. doi:10.1007/s00198-014-2699-0
Hernandez-Rauda R, Martinez-Garcia S (2004) Osteoporosis-related life habits and knowledge about osteoporosis among women in El Salvador: a cross-sectional study. BMC Musculoskelet Disord 5:29. doi:10.1186/1471-2474-5-29
Reid IR (2008) Relationships between fat and bone. Osteoporos Int 19(5):595–606. doi:10.1007/s00198-007-0492-z
Lloyd JT, Alley DE, Hochberg MC, Waldstein SR, Harris TB, Kritchevsky SB, Schwartz AV, Strotmeyer ES, Womack C, Orwig DL (2016) Changes in bone mineral density over time by body mass index in the health ABC study. Osteoporos Int 27(6):2109–2116. doi:10.1007/s00198-016-3506-x
Reid IR (2010) Fat and bone. Arch Biochem Biophys 503(1):20–27. doi:10.1016/j.abb.2010.06.027
Douchi T, Oki T, Nakamura S, Ijuin H, Yamamoto S, Nagata Y (1997) The effect of body composition on bone density in pre- and postmenopausal women. Maturitas 27(1):55–60
Zhao LJ, Liu YJ, Liu PY, Hamilton J, Recker RR, Deng HW (2007) Relationship of obesity with osteoporosis. J Clin Endocrinol Metab 92(5):1640–1646. doi:10.1210/jc.2006-0572
Jankowska EA, Rogucka E, Medras M (2001) Are general obesity and visceral adiposity in men linked to reduced bone mineral content resulting from normal ageing? A population-based study. Andrologia 33(6):384–389
Reid IR (2002) Relationships among body mass, its components, and bone. Bone 31(5):547–555
Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF Jr, Meigs JB, Lipinska I, Kathiresan S, Murabito JM, O’Donnell CJ, Benjamin EJ, Fox CS (2007) Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation 116(11):1234–1241
Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD (2009) Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab 94(9):3387–3393. doi:10.1210/jc.2008-2422
Cui LH, Shin MH, Kweon SS, Park KS, Lee YH, Chung EK, Nam HS, Choi JS (2007) Relative contribution of body composition to bone mineral density at different sites in men and women of South Korea. J Bone Miner Metab 25(3):165–171. doi:10.1007/s00774-006-0747-3
Lekamwasam S, Weerarathna T, Rodrigo M, Arachchi WK, Munidasa D (2009) Association between bone mineral density, lean mass, and fat mass among healthy middle-aged premenopausal women: a cross-sectional study in southern Sri Lanka. J Bone Miner Metab 27(1):83–88. doi:10.1007/s00774-008-0006-x
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377(9773):1276–1287. doi:10.1016/S0140-6736(10)62349-5
Garnero P (2008) Biomarkers for osteoporosis management: utility in diagnosis, fracture risk prediction and therapy monitoring. Mol Diagn Ther 12(3):157–170
Blake GM, Fogelman I (2007) The role of DXA bone density scans in the diagnosis and treatment of osteoporosis. Postgrad Med J 83(982):509–517. doi:10.1136/pgmj.2007.057505
Lloyd JT, Alley DE, Hawkes WG, Hochberg MC, Waldstein SR, Orwig DL (2014) Body mass index is positively associated with bone mineral density in US older adults. Arch Osteoporos 9:175. doi:10.1007/s11657-014-0175-2
Porter SA, Massaro JM, Hoffmann U, Vasan RS, O’Donnel CJ, Fox CS (2009) Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care 32(6):1068–1075. doi:10.2337/dc08-2280
Palmer BF, Clegg DJ (2015) The sexual dimorphism of obesity. Mol Cell Endocrinol 402:113–119. doi:10.1016/j.mce.2014.11.029
Finkelstein JS, Brockwell SE, Mehta V, Greendale GA, Sowers MR, Ettinger B, Lo JC, Johnston JM, Cauley JA, Danielson ME, Neer RM (2008) Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab 93(3):861–868. doi:10.1210/jc.2007-1876
Khosla S, Oursler MJ, Monroe DG (2012) Estrogen and the skeleton. Trends in endocrinology and metabolism: TEM 23(11):576–581. doi:10.1016/j.tem.2012.03.008
Manolagas SC (2000) Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21(2):115–137. doi:10.1210/edrv.21.2.0395
Yoo HJ, Park MS, Yang SJ, Kim TN, Lim KI, Kang HJ, Song W, Baik SH, Choi DS, Choi KM (2012) The differential relationship between fat mass and bone mineral density by gender and menopausal status. J Bone Miner Metab 30(1):47–53. doi:10.1007/s00774-011-0283-7
Kang DH, Guo LF, Guo T, Wang Y, Liu T, Feng XY, Che XQ (2015) Association of body composition with bone mineral density in northern Chinese men by different criteria for obesity. J Endocrinol Investig 38(3):323–331. doi:10.1007/s40618-014-0167-5
Bredella MA, Lin E, Gerweck AV, Landa MG, Thomas BJ, Torriani M, Bouxsein ML, Miller KK (2012) Determinants of bone microarchitecture and mechanical properties in obese men. J Clin Endocrinol Metab 97(11):4115–4122. doi:10.1210/jc.2012-2246
Acknowledgments
This work was supported by grants from the National 863 Program (2015AA020110), National Key Research and Development Project (2016YFC1304902), Drug Innovation Program of the National Science and Technology Project (2011ZX09307-001-02), Shanghai Young Doctor Training and Funding Program, Shanghai Jiao Tong Medical/Engineering Foundation (YG2014MS18), Shanghai Municipal Education Commission–Gaofeng Clinical Medicine Grant Support (20152527), National Program for Support of Top-notch Young Professional, Shanghai Health and Family Planning Commission (2013ZYJB1001), Innovation Foundation of Translational Medicine of Shanghai Jiao Tong University School of Medicine (15ZH4006), Shanghai SJTUSM Biobank, and Shanghai Hospital Development Center (SHDC12013115). We acknowledge the assistance of the nursing and medical staff at the Shanghai Clinical Center for Diabetes. We gratefully appreciate all of the participants in this research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None.
Electronic supplementary material
ESM 1
(DOC 85 kb)
Rights and permissions
About this article
Cite this article
Wang, J., Yan, D., Hou, X. et al. Association of adiposity indices with bone density and bone turnover in the Chinese population. Osteoporos Int 28, 2645–2652 (2017). https://doi.org/10.1007/s00198-017-4081-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-4081-5