Abstract
Summary
In this meta-analysis, we evaluated the association between serum 25-hydroxyvitamin D (25(OH) vitamin D) level and the risk of total fractures and hip fractures. Low serum 25(OH) vitamin D level is associated with an increased risk of total and hip fractures.
Introduction
Data on the association between serum 25(OH) vitamin D level and the risk of fractures are conflicting. This study aimed to provide a summary of prospective cohort or nested case–control studies on the association between serum 25(OH) vitamin D level and the risk of total fractures and hip fractures.
Methods
We identified relevant studies by searching the PubMed, EMBASE, and OVID databases from their inception to June 1, 2016. We included published prospective cohort or nested case–control studies evaluating the associations of serum 25(OH) vitamin D level with the fracture risk. Two reviewers abstracted the data independently. Relative risks (RRs) with 95% confidence intervals (CIs) were derived throughout the whole analysis.
Results
Sixteen prospective cohort studies and three nested case–control studies were included. We found that low serum 25(OH) vitamin D level was significantly associated with the risk of total fractures (RR 1.25, 95% CI 1.06–1.43; I 2 = 31.3%, p for heterogeneity = 0.15) and hip fractures (RR 1.48, 95% CI 1.29–1.68; I 2 = 0%, p for heterogeneity = 0.51). The hip fracture risk was increased by 40% for each SD decrease in serum 25(OH) vitamin D level (RR 1.40, 95% CI 1.20–1.61; I 2 = 0%, p for heterogeneity = 0.51). The per SD decrease in serum 25(OH) vitamin D level was not associated with the increased risk of total fractures (RR 1.14, 95% CI 0.93–1.35; I 2 = 63.2%, p for heterogeneity = 0.04).
Conclusions
Our study suggests that low serum 25(OH) vitamin D level is associated with increased risks of total and hip fractures. In the analyzed studies, the per SD decrease in serum 25(OH) vitamin D level was associated with the hip fracture risk but not with the total fracture risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fracture is a major cause of disability, morbidity, and mortality, thus creating a considerable burden on the healthcare system annually [1–3]. Prevention of fractures by identifying and confirming the modifiable risk factors is important. Some factors such as age, smoking, bone mineral density (BMD), physical activity, and body mass index (BMI) are known to be involved in the risk of fracture [4–7].
Serum 25-hydroxyvitamin D (25(OH) vitamin D) level is associated with BMD, bone size (relative to body size), and bone strength [8, 9]. Several studies reported that low serum 25(OH) vitamin D level is a potentially modifiable risk factor for fractures. However, the findings were controversial [10–28]. On the basis of a literature search up to April 2009, only one meta-analysis—performed by Lai et al. [29], which included 17 case–control studies with 1903 fractures—has examined the association between serum 25(OH) vitamin D level and the risk of hip fracture. The results of that study showed a 33% lower serum 25(OH) vitamin D level in cases compared with controls, with significant heterogeneity. The authors did not include cohort studies in their meta-analysis, which may have reduced the strength of their conclusions and the level of evidence. After 2009, 15 cohort studies with a focus on this topic have been published. Moreover, Lai and colleagues only examined the hip fracture risk but did not assess the risk of total fractures.
We therefore performed a meta-analysis to evaluate the association between serum 25(OH) vitamin D level and the risk of total fractures and hip fractures, by using data from the published prospective cohort or nested case–control studies.
Materials and methods
We performed a meta-analysis of the available literature according to the PRISMA statement and the MOOSE guidelines [30, 31].
Search strategy and data sources
Two reviewers searched the PubMed, EMBASE, and OVID databases for published prospective cohort or nested case–control studies that investigated the association between serum 25(OH) vitamin D level and the risk of total fractures and hip fractures, from their inception to June 1, 2016, without restrictions. Our searches combined MeSH (medical subject) headings and free text, and the following search keywords were used: “vitamin D level” or “25-OH-D” or “25-hydroxyvitamin D” or “25(OH) vitamin D” and “fracture”. In addition, manual searches for the references of all relevant studies and the abstracts of meetings related to osteoporosis were performed to identify additional studies.
Study selection
The two reviewers evaluated the articles independently. Discrepancies were resolved by arbitration, and a consensus was reached on study inclusion and interpretation of data after a discussion. Studies were included in the present meta-analysis if they met the following inclusion criteria: (1) a prospective cohort or nested case–control design, (2) reported adult population, (3) reported serum 25(OH) vitamin D level as a risk factor and total fractures or hip fracture as the outcome, or (4) reported risk estimates such as relative risks (RRs), odds ratios (ORs), or hazard ratios (HRs) with 95% confidence intervals (CIs). Studies that did not meet the inclusion criteria were excluded. If different articles investigated the same cohort, we selected the most detailed study.
Data extraction
Two reviewers (Y.F. and G.C.) extracted the data independently by using a standardized data collection form for analysis. A third reviewer (B.C.) checked the reliability. The standard data extraction form included the first author’s last name, publication year, name of cohort, country where the study was performed, sex and age of the participants, recruitment time of the participants, years of follow-up, sample size, number of fractures, ascertainment of fracture, cutoff of 25(OH)vitamin D level, variables adjusted for analysis, and RR estimates with corresponding 95% CIs. We extracted the RRs and 95% CIs that reflected the greatest degree of control for potential confounders. The nine-star Newcastle–Ottawa Scale (NOS) [32] was used to assess study quality. The third reviewer (B.C.) was involved to resolve any disagreement concerning the abstracted data.
Statistical analyses
In the present analysis, we used RRs as the means of measuring the association across studies. Multivariable-adjusted HRs or ORs were transformed into RRs [33, 34]. One study [14] reported stratified risk estimates according to race, and we combined these estimates by using a random-effects model and then used pooled estimates for the meta-analysis [35–37]. For two studies [13, 28] that reported more than one cutoff of serum 25(OH) vitamin D level, we used 20 ng/mL (the definition of vitamin D deficiency in most studies [38, 39]) or the closest value to 20 ng/mL as the assigned cutoff [40, 41]. For studies that presented graded associations, we only used the estimates for the highest category [35, 37].
We used the Cochran Q test and I 2 statistics to estimate heterogeneity across studies [42]. If the p value for heterogeneity was <0.1, we considered I 2 values of <30% as low heterogeneity, 30–50% as moderate heterogeneity, and >75% as high heterogeneity, according to Higgins et al. [43]. We performed subgroup analyses for relevant study characteristics (i.e., geographical location, number of participants, length of follow-up, NOS scores, and adjustments). Publication bias was assessed by using the Begg and Egger regression asymmetry tests [44, 45]. To assess the possible effect of publication bias in our meta-analysis, we also performed the “trim and fill” process. This process is used to evaluate the possibility of hypothetical “missing” studies, imputing their RRs, and obtaining a pooled RR that includes the hypothetical missing studies as though they had actually been performed [44, 46]. Sensitivity analysis involved removing any one study and assessing whether the results would be markedly affected. All statistical tests were performed by using STATA software (version 12.0; StataCorp, College Station, TX, USA), and p <0.05 was considered statistically significant.
Results
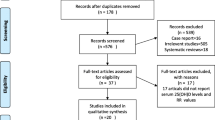
The procedure of the study selection is presented in Fig. 1. A total of 2679 studies were included from the initial database search. After title and abstract assessment, we excluded the duplicated studies and those that did not satisfy inclusion criteria, and 42 articles remained. Thereafter, we excluded some studies because of having a case–control design, a cross-sectional design, duplicate cohorts, or missing data. Finally, 19 studies were included in this meta-analysis [10–28].
Characteristics of the included studies
Table 1 shows the characteristics of the included prospective cohort (n = 15) or nested case–control (n = 4) studies. This meta-analysis included 47,341 participants, with 4762 total fractures and 3091 hip fractures. Eight studies included men and women, eight studies included only women, and three studies included only men. The included studies were performed in ten different countries (nine studies from the United States; two studies from Japan; and one study each from Sweden, Iceland, Australia, Norway, China, Netherlands, France, and Finland). All participants were ≥19 years old. The follow-up time of the included studies ranged from 4 to 16.9 years. The recruitment time of the included studies ranged from 1986 to 2007. The fractures were identified by using verified self-reports of fracture experience, radiological diagnosis, and medical records. The covariates most commonly taken into account were age, BMI, sex, race, smoking, alcohol use, and physical activity. Only six studies adjusted for BMD [16, 18, 22, 25–27].
Serum 25(OH) vitamin D level and total fracture risk
Eleven studies were concerned with the association between serum 25(OH) vitamin D level and the risk of total fractures. The results of the meta-analysis are shown in Fig. 2a. The meta-analysis with a random-effects model showed a significant increase in the risk of total fractures in patients with low serum 25(OH) vitamin D level (RR 1.25, 95% CI 1.06–1.43), with moderate heterogeneity across studies (p = 0.149, I 2 = 31.3%). The Egger test revealed a publication bias (p < 0.01), and the Begg test showed no evidence of publication bias (p = 0.10). The trim-and-fill method confirmed that the six possibly missing studies could alter the pooled estimation of RR to 1.13 (95% CI 1.01–1.24). The sensitivity analysis showed that excluding any one study from the meta-analysis did not change the results substantially (Fig. 3a).
The subgroup analyses for the association between serum 25(OH) vitamin D level and the risk of total fractures are shown in Table 2. We assessed the geographical location; number of participants; NOS scores; and adjustments for BMI, physical activity, race, smoking status, alcohol intake, diabetes mellitus, and history of fracture in the subgroup analyses. The RRs were 1.15 (95% CI 1.03–1.28; p for heterogeneity = 0.09, I 2 = 47.2%) for studies conducted in the United States, 1.50 (95% CI 1.04–1.96; p for heterogeneity = 0.70, I 2 = 0%) for studies conducted in Asia, and 1.17 (95% CI 0.73–1.61; p for heterogeneity = 0.13, I 2 = 57.5%) for studies conducted in other geographical locations (p = 0.35). For the length of follow-up, the RRs were 1.02 (95% CI 0.83–1.20; p for heterogeneity = 0.32, I 2 = 15.1%) for <6 years and 1.28 (95% CI 1.13–1.43; p for heterogeneity = 0.40, I 2 = 2.8%) for >6 years (p = 0.02). For NOS scores, the RRs were 1.37 (95% CI 1.06–1.68; p for heterogeneity = 0.31, I 2 = 16.3%) for scores >7 and 1.18 (95% CI 0.95–1.41; p for heterogeneity = 0.17, I 2 = 33.8%) for scores ≤7 (p = 0.17). Concerning adjustment for BMI, the RRs were 1.24 (95% CI 1.09–1.39; p for heterogeneity = 0.53, I 2 = 0%) with adjustment for BMI and 1.09 (95% CI 0.90–1.27; p for heterogeneity = 0.05, I 2 = 62.1%) without adjustment for BMI (p = 0.22). Concerning adjustment for history of fracture, the RRs were 1.53 (95% CI 1.15–1.92, p for heterogeneity = 0.54, I 2 = 0%) with adjustment for history of fracture and 1.14 (95% CI 1.02–1.26; p for heterogeneity = 0.19, I 2 = 31.8%) without adjustment for history of fracture (p = 0.06).
Serum 25(OH) vitamin D level and hip fracture risk
Eleven studies were concerned about the association between serum 25(OH) vitamin D level and the risk of hip fractures. The results of the meta-analysis are shown in Fig. 2b. The meta-analysis with a random-effects model showed a significant increase in the risk of hip fractures among patients with low serum 25(OH) vitamin D level (RR 1.48, 95% CI 1.29–1.68), with no heterogeneity across studies (p = 0.514, I 2 = 0%). The Egger test revealed a publication bias (p = 0.03), and the Begg test showed no evidence of publication bias (p = 0.31). The trim-and-fill method confirmed that the seven possibly missing studies could alter the pooled estimation of RR to 1.36 (95% CI 1.18–1.54). The sensitivity analysis showed that excluding any one study from the meta-analysis did not change the results substantially (Fig. 3b).
The subgroup analyses for the association between serum 25(OH) vitamin D level and the risk of hip fractures are shown in Table 3. We assessed the geographical location; number of participants; NOS scores; and adjustments for BMI, physical activity, race, smoking status, alcohol intake, diabetes mellitus, and history of fracture in the subgroup analyses. The RRs were 1.47 (95% CI 1.21–1.73; p for heterogeneity = 0.57, I 2 = 0%) for studies conducted in the United States, 2.12 (95% CI 0.57–3.67; p for heterogeneity = 0.87, I 2 = 0%) for studies conducted in Asia, and 1.48 (95% CI 1.18–1.77; p for heterogeneity = 0.05, I 2 = 73.0%) for studies conducted in Europe (p = 0.72). For the length of follow-up, the RRs were 1.61 (95% CI 1.18–2.04; p for heterogeneity = 0.29, I 2 = 20.2%) for <6 years and 1.45 (95% CI 1.24–1.67; p for heterogeneity = 0.54, I 2 = 0%) for >6 years (p = 0.53). For NOS scores, the RRs were 1.90 (95% CI 1.50–2.30; p for heterogeneity = 0.66; I 2 = 0%) for scores >7 and 11.36 (95% CI 1.14–1.58; p for heterogeneity = 0.93; I 2 = 0%) for scores ≤7 (p = 0.02). Concerning adjustment for BMI, the RRs were 1.48 (95% CI 1.27–1.68; p for heterogeneity = 0.58, I 2 = 0%) with adjustment for BMI and 1.54 (95% CI 1.04–2.04; p for heterogeneity = 0.22, I 2 = 32.1%) without adjustment for BMI (p = 0.82). Concerning adjustment for history of fracture, the RRs were 1.18 (95% CI 1.03–2.58; p for heterogeneity = 0.63, I 2 = 0%) with adjustment for history of fracture and 1.46 (95% CI 1.27–1.66; p for heterogeneity = 0.41, I 2 = 3.1%) without adjustment for history of fracture (p = 0.40).
Per SD decrease of serum 25(OH) vitamin D level and the risk of total fractures and hip fractures
Four studies [11, 21, 26, 27] were concerned with the association between the per SD decrease of serum 25(OH) vitamin D level and the risk of total fractures, and three studies [12, 20, 27] were concerned with the association between the per SD decrease of serum 25(OH) vitamin D level and the risk of hip fractures. The covariates most commonly taken into account were age, BMI, and physical activity. Only two studies adjusted for BMD [26, 27]. The hip fracture risk was increased by 40% for each SD decrease in serum 25(OH) vitamin D level (RR 1.40, 95% CI 1.20–1.61; I 2 = 0%, p for heterogeneity = 0.51). The per SD decrease in serum 25(OH) vitamin D level was not associated with the risk of total fractures (RR 1.14, 95% CI 0.93–1.35; I 2 = 63.2%, p for heterogeneity = 0.04).
Discussion
The present meta-analysis indicated that there was an increased risk of total fractures and hip fractures in patients with lower serum 25(OH) vitamin D levels. The hip fracture risk was increased by 40% for each SD decrease in serum 25(OH) vitamin D level. However, the per SD decrease in serum 25(OH) vitamin D level was not associated with the risk of total fractures.
Several plausible mechanisms have been proposed for the associations between serum 25(OH) vitamin D level and the risk of fractures. A low serum 25(OH) vitamin D level usually indicates vitamin D deficiency. First, vitamin D increases the serum calcium concentrations and stimulates osteoblasts to produce RANKL, a protein that stimulates osteoclastogenesis [8]. Low serum 25(OH) vitamin D level may induce an increase in parathyroid hormone (PTH) level, which may result in bone loss [47]. Calcium mobilization from the bone is affected by both vitamin D and PTH. Second, vitamin D deficiency has also been shown to be related to low muscle mass and muscle weakness [9, 48]. Low serum 25(OH) vitamin D level may also be a cofounder in idiopathic inflammatory myopathies [49], alcoholic skeletal muscle myopathy [50], and diffuse musculoskeletal pain [9]. Koeckhoven et al. used the data of the Amsterdam osteoarthritis cohort and found that serum 25(OH) vitamin D level was significantly associated with muscle strength [51]. Moreover, another study performed by Orces et al. [52] showed coincident results: compared with subjects with normal muscle strength, the prevalence rates of 25(OH) vitamin D deficiency were 31 and 43% higher among men and women with muscle weakness. Third, numerous studies suggested an association between vitamin D insufficiency and falls. Snijder et al. reported that low serum 25(OH) vitamin D level was significantly associated with increased falls in elderly persons [53]. Rothenbacher et al. [54] performed a prospective population-based cohort study and showed an association between serum 25(OH) vitamin D level and the risk of first fall [HRR = 1.93 (95% CI 1.10–3.37) for serum 25(OH) vitamin D level < 20 mg/mL]. Fourth, the association between serum 25(OH) vitamin D level and BMD was investigated in several studies [24, 26, 55]. The cohort study performed by Swanson et al. [27] showed that higher levels of serum 25(OH) vitamin D were associated with higher baseline BMD and slower bone loss at the hip. Steingrimsdottir et al. [24] performed a prospective study of 5764 men and women, and showed that compared with reference values (50–75 nmol/l), values <30 nmol/l were associated with significantly lower BMD of the femoral neck.
Furthermore, many previous meta-analyses investigated the associations between oral vitamin D supplementation and the risk of fractures. The meta-analysis conducted by Bischoff-Ferrari et al. found that, in elderly persons, oral vitamin D supplementation of between 700 and 800 IU/day may reduce the risk of non-vertebral fractures and hip fractures [56]. Furthermore, the same team reported that prevention of non-vertebral fractures with vitamin D supplementation was dose dependent [57]. Moreover, another meta-analysis showed that oral calcium plus vitamin D supplements may reduce the fracture risk in both community-dwelling and institutionalized elderly adults [58].
Only one meta-analysis [29] examined the association between serum 25(OH) vitamin D level and the risk of hip fractures (from the included publications up to April 2009—a total 17 case–control studies), and the results showed 33% lower serum 25(OH) vitamin D level in cases compared with controls, based on 1903 cases, with significant heterogeneity existing among studies. The authors, however, did not include cohort studies in their meta-analysis, which may have reduced the strength of their conclusions. Moreover, Lai and colleagues only examined hip fracture risk but did not assess the risk of total fractures. In comparison with this previous meta-analysis, our meta-analysis included 15 prospective cohort studies and 4 nested case–control studies with a total of 47,341 participants, and focused on total fractures and hip fractures. The present study is the most comprehensive research in terms of the amount of data contributing to the summary estimates.
Our study has some advantages. First, through a wide-ranging search of the literature, we included a substantial number of participants and fracture cases (up to 47,341 participants with 4762 total fractures cases and 3091 hip fractures cases), which significantly increased the statistical power of our analysis. Second, the quantitative assessment of the analysis was based on prospective cohort studies, which may have minimized the selection or recall bias. Third, all the included studies in the present meta-analysis had a long duration of follow-up, which may have increased the statistical power of the results. Fourth, the fractures in most of the included studies were verified.
Despite these strengths, our meta-analysis has some limitations. First, as a meta-analysis, there could be inherent confounding factors in the included studies, which may have underestimated or exaggerated the risk estimates. Nevertheless, most of the prospective studies adjusted for major potential confounders, including age, BMI, sex, race, smoking, alcohol use, and physical activity. Moreover, the moderate heterogeneity in the present study may have been due to the methodological differences among the studies. Although many of the I 2 values we estimated were assessed as moderate to high in the subgroup analysis, we investigated the length of follow-up for potential sources of heterogeneity in the analysis of the total fracture risk; only NOS scores were considered for the analysis of hip fracture risk. The heterogeneity in the present study may have reduced the power of our conclusions.
Second, another concern could be the potential publication bias because smaller studies or studies reporting null results are difficult to publish, as we have established in the Egger test in this meta-analysis. However, the Begg test showed no evidence of publication bias, and further trim-and-fill analysis showed that after adding possibly missing studies, the pooled estimation RRs were 1.13 (95% CI 1.01–1.24) for serum 25(OH) vitamin D level and total fracture risk and 1.36 (95% CI 1.18–1.54) for serum 25(OH) vitamin D level and hip fracture risk. Moreover, our sensitivity test showed that the findings were robust.
Third, only four studies on the association between the per SD decrease of serum 25(OH) vitamin D level and the risk of total fractures and three studies on the association between the per SD decrease of serum 25(OH) vitamin D level and the risk of hip fractures were included in our meta-analysis, which made the results less meaningful.
Fourth, the included studies used different serum 25(OH) vitamin D assays, and the 25(OH) vitamin D levels have not been standardized. Therefore, the results need to be interpreted with caution. However, we performed further meta-analyses by using different cutoffs of serum 25(OH) vitamin D and recalculated the pooled RR. The results showed that the pooled RR for the association between serum 25(OH) vitamin D level and the risk of total fractures did not change substantially [the pooled RR decreased from 1.34 (95% CI 1.10–1.58) to 1.23 (95% CI 1.03–1.43)]. In addition, in this meta-analysis, three included studies [15, 18, 27] reported non-vertebral fracture risk, and we considered these non-vertebral fractures as total fractures according to previous meta-analyses [2, 59]. However, we performed further meta-analysis after excluding these studies, and the results showed that the pooled RR for the association between serum 25(OH) vitamin D level and the risk of total fractures did not change substantially (RR 1.33, 95% CI 1.11–1.55; I 2 = 17.7%, p for heterogeneity = 0.29). Moreover, the definition of fractures differed across studies (osteoporotic fractures, low-trauma fractures, low-energy fractures, or exclusion of pathological fractures, fractures of unknown etiology, traumatic fractures, etc.). Nevertheless, it is difficult to determine whether the differences of definition of fractures were responsible for the observed outcomes. Further individual patient data meta-analysis may offer an additional insight into this issue.
Fifth, owing to a lack of related studies, we did not conduct a non-linear dose–response analysis for the association between serum 25(OH) vitamin D level and the risk of total fractures. Especially, our meta-analysis showed a significant increase in the risk of total fractures in patients with low serum 25(OH) vitamin D level. However, the per SD decrease in serum 25(OH) vitamin D level was not associated with an increased risk of total fractures. Therefore, a non-linear dose–response analysis may offer potential insights to this issue. Bleicher et al. [22] reported that the association between serum 25(OH) vitamin D level and the risk of total fractures was U-shaped, and an increased risk of total fractures was observed with either high or low serum 25(OH) vitamin D levels. Further studies should be conducted to quantitatively assess the dose–response association between serum 25(OH) vitamin D level and the risk of total fractures.
Conclusion
This study found an increased risk of total fractures and hip fractures in patients with low serum 25(OH) vitamin D levels. The hip fracture risk was increased by 40% for each SD decrease in serum 25(OH) vitamin D level. However, the per SD decrease in serum 25(OH) vitamin D level was not associated with the risk of total fractures. Further well-designed and stratified cohort studies should be conducted to quantitatively assess the non-linear dose–response association between serum 25(OH) vitamin D level and the risk of total fractures.
References
Wu ZJ, He JL, Wei RQ, Liu B, Lin X, Guan J, Lan YB (2015) C-reactive protein and risk of fracture: a systematic review and dose-response meta-analysis of prospective cohort studies. Osteoporos Int 26:49–57
Xiao F, Qu X, Zhai Z, Jiang C, Li H, Liu X, Ouyang Z, Gu D (2015) Association between loop diuretic use and fracture risk. Osteoporos Int 26:775–784
Zhang X, Yu Z, Yu M, Qu X (2015) Alcohol consumption and hip fracture risk. Osteoporos Int 26:531–542
Thorin MH, Wihlborg A, Akesson K, Gerdhem P (2015) Smoking, smoking cessation, and fracture risk in elderly women followed for 10 years. Osteoporos Int
Mazziotti G, Biagioli E, Maffezzoni F, Spinello M, Serra V, Maroldi R, Floriani I, Giustina A (2015) Bone turnover, bone mineral density, and fracture risk in acromegaly: a meta-analysis. J Clin Endocrinol Metab 100:384–394
Johansson H, Kanis JA, Oden A et al (2014) A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res 29:223–233
Qu X, Zhang X, Zhai Z, Li H, Liu X, Li H, Liu G, Zhu Z, Hao Y, Dai K (2014) Association between physical activity and risk of fracture. J Bone Miner Res 29:202–211
Iolascon G, Di Pietro G, Gimigliano F (2009) Vitamin D supplementation in fractured patient: how, when and why. Clin Cases Miner Bone Metab 6:120–124
Tanner SB, Harwell SA (2015) More than healthy bones: a review of vitamin D in muscle health. Ther Adv Musculoskelet Dis 7:152–159
Cummings SR, Browner WS, Bauer D, Stone K, Ensrud K, Jamal S, Ettinger B (1998) Endogenous hormones and the risk of hip and vertebral fractures among older women. Study of Osteoporotic Fractures Research Group. N Engl J Med 339:733–738
Garnero P, Munoz F, Sornay-Rendu E, Delmas PD (2007) Associations of vitamin D status with bone mineral density, bone turnover, bone loss and fracture risk in healthy postmenopausal women. The OFELY study. Bone 40:716–722
Cauley JA, Lacroix AZ, Wu L et al (2008) Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med 149:242–250
van Schoor NM, Visser M, Pluijm SM, Kuchuk N, Smit JH, Lips P (2008) Vitamin D deficiency as a risk factor for osteoporotic fractures. Bone 42:260–266
Cauley JA, Danielson ME, Boudreau R et al (2011) Serum 25-hydroxyvitamin D and clinical fracture risk in a multiethnic cohort of women: the Women’s Health Initiative (WHI). J Bone Miner Res 26:2378–2388
Chan R, Chan CC, Woo J, Ohlsson C, Mellstrom D, Kwok T, Leung PC (2011) Serum 25-hydroxyvitamin D, bone mineral density, and non-vertebral fracture risk in community-dwelling older men: results from Mr. Os, Hong Kong. Arch Osteoporos 6:21–30
Nakamura K, Saito T, Oyama M, Oshiki R, Kobayashi R, Nishiwaki T, Nashimoto M, Tsuchiya Y (2011) Vitamin D sufficiency is associated with low incidence of limb and vertebral fractures in community-dwelling elderly Japanese women: the Muramatsu Study. Osteoporos Int 22:97–103
Robinson-Cohen C, Katz R, Hoofnagle AN, Cauley JA, Furberg CD, Robbins JA, Chen Z, Siscovick DS, de Boer IH, Kestenbaum B (2011) Mineral metabolism markers and the long-term risk of hip fracture: the cardiovascular health study. J Clin Endocrinol Metab 96:2186–2193
Barbour KE, Houston DK, Cummings SR et al (2012) Calciotropic hormones and the risk of hip and nonspine fractures in older adults: the Health ABC Study. J Bone Miner Res 27:1177–1185
Holvik K, Ahmed LA, Forsmo S, Gjesdal CG, Grimnes G, Samuelsen SO, Schei B, Blomhoff R, Tell GS, Meyer HE (2013) Low serum levels of 25-hydroxyvitamin D predict hip fracture in the elderly: a NOREPOS study. J Clin Endocrinol Metab 98:3341–3350
Kauppi M, Impivaara O, Maki J, Heliovaara M, Jula A (2013) Quantitative ultrasound measurements and vitamin D status in the assessment of hip fracture risk in a nationally representative population sample. Osteoporos Int 24:2611–2618
Looker AC (2013) Serum 25-hydroxyvitamin D and risk of major osteoporotic fractures in older U.S. adults. J Bone Miner Res 28:997–1006
Bleicher K, Cumming RG, Naganathan V, Blyth FM, Le Couteur DG, Handelsman DJ, Waite LM, Seibel MJ (2014) U-shaped association between serum 25-hydroxyvitamin D and fracture risk in older men: results from the prospective population-based CHAMP study. J Bone Miner Res 29:2024–2031
Buchebner D, McGuigan F, Gerdhem P, Malm J, Ridderstrale M, Akesson K (2014) Vitamin D insufficiency over 5 years is associated with increased fracture risk—an observational cohort study of elderly women. Osteoporos Int 25:2767–2775
Steingrimsdottir L, Halldorsson TI, Siggeirsdottir K et al (2014) Hip fractures and bone mineral density in the elderly—importance of serum 25-hydroxyvitamin D. PLoS One 9:e91122
Tanaka S, Kuroda T, Yamazaki Y, Shiraki Y, Yoshimura N, Shiraki M (2014) Serum 25-hydroxyvitamin D below 25 ng/mL is a risk factor for long bone fracture comparable to bone mineral density in Japanese postmenopausal women. J Bone Miner Metab 32:514–523
Cauley JA, Greendale GA, Ruppert K, Lian Y, Randolph JF Jr, Lo JC, Burnett-Bowie SA, Finkelstein JS (2015) Serum 25 hydroxyvitamin D, bone mineral density and fracture risk across the menopause. J Clin Endocrinol Metab 100:2046–2054
Swanson CM, Srikanth P, Lee CG et al (2015) Associations of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D with bone mineral density, bone mineral density change, and incident nonvertebral fracture. J Bone Miner Res 30:1403–1413
Takiar R, Lutsey PL, Zhao D, Guallar E, Schneider AL, Grams ME, Appel LJ, Selvin E, Michos ED (2015) The associations of 25-hydroxyvitamin D levels, vitamin D binding protein gene polymorphisms, and race with risk of incident fracture-related hospitalization: twenty-year follow-up in a bi-ethnic cohort (the ARIC Study). Bone 78:94–101
Lai JK, Lucas RM, Clements MS, Roddam AW, Banks E (2010) Hip fracture risk in relation to vitamin D supplementation and serum 25-hydroxyvitamin D levels: a systematic review and meta-analysis of randomised controlled trials and observational studies. BMC Public Health 10:331
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605
Zhang J, Yu KF (1998) What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 280:1690–1691
McNutt LA, Wu C, Xue X, Hafner JP (2003) Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol 157:940–943
Dong JY, Zhang YH, Tong J, Qin LQ (2012) Depression and risk of stroke: a meta-analysis of prospective studies. Stroke 43:32–37
Bonequi P, Meneses-Gonzalez F, Correa P, Rabkin CS, Camargo MC (2013) Risk factors for gastric cancer in Latin America: a meta-analysis. Cancer Causes Control 24:217–231
Dong JY, Zhang YH, Qin LQ (2011) Erectile dysfunction and risk of cardiovascular disease: meta-analysis of prospective cohort studies. J Am Coll Cardiol 58:1378–1385
Holick MF (2009) Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol 19:73–78
Holick MF (2006) High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 81:353–373
Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H (2009) Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther 30:113–125
Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H (2010) Meta-analysis: serum vitamin D and breast cancer risk. Eur J Cancer 46:2196–2205
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Begg CB (2002) A comparison of methods to detect publication bias in meta-analysis by P. Macaskill, S. D. Walter and L. Irwig, Statistics in Medicine, 2001; 20:641–654. Stat Med 21:1803; author reply 1804
Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR (2000) Empirical assessment of effect of publication bias on meta-analyses. BMJ 320:1574–1577
Sakuma M, Endo N, Oinuma T (2007) Serum 25-OHD insufficiency as a risk factor for hip fracture. J Bone Miner Metab 25:147–150
Ghose RR (2005) Vitamin D deficiency and muscle weakness in the elderly. N Z Med J 118:U1582
Azali P, Barbasso Helmers S, Kockum I, Olsson T, Alfredsson L, Charles PJ, Piehl Aulin K, Lundberg IE (2013) Low serum levels of vitamin D in idiopathic inflammatory myopathies. Ann Rheum Dis 72:512–516
Wijnia JW, Wielders JP, Lips P, van de Wiel A, Mulder CL, Nieuwenhuis KG (2013) Is vitamin D deficiency a confounder in alcoholic skeletal muscle myopathy? Alcohol Clin Exp Res 37(Suppl 1):E209–E215
Koeckhoven E, van der Leeden M, Roorda LD, van Schoor NM, Lips P, de Zwart A, Dekker J, van der Esch M, Lems WF (2016) The association between serum 25-hydroxy vitamin D level and upper leg strength in patients with knee osteoarthritis: results of the Amsterdam osteoarthritis cohort. J Rheumatol 43:1400–1405
Orces CH (2016) Prevalence of clinically relevant muscle weakness and its association with vitamin D status among older adults in Ecuador. Aging Clin Exp Res
Snijder MB, van Schoor NM, Pluijm SM, van Dam RM, Visser M, Lips P (2006) Vitamin D status in relation to one-year risk of recurrent falling in older men and women. J Clin Endocrinol Metab 91:2980–2985
Rothenbacher D, Klenk J, Denkinger MD et al (2014) Prospective evaluation of renal function, serum vitamin D level, and risk of fall and fracture in community-dwelling elderly subjects. Osteoporos Int 25:923–932
Olmos JM, Hernandez JL, Garcia-Velasco P, Martinez J, Llorca J, Gonzalez-Macias J (2016) Serum 25-hydroxyvitamin D, parathyroid hormone, calcium intake, and bone mineral density in Spanish adults. Osteoporos Int 27:105–113
Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B (2005) Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 293:2257–2264
Bischoff-Ferrari HA, Willett WC, Wong JB, Stuck AE, Staehelin HB, Orav EJ, Thoma A, Kiel DP, Henschkowski J (2009) Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med 169:551–561
Weaver CM, Alexander DD, Boushey CJ, Dawson-Hughes B, Lappe JM, LeBoff MS, Liu S, Looker AC, Wallace TC, Wang DD (2016) Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int 27:367–376
Eom CS, Lee HK, Ye S, Park SM, Cho KH (2012) Use of selective serotonin reuptake inhibitors and risk of fracture: a systematic review and meta-analysis. J Bone Miner Res 27:1186–1195
Acknowledgements
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Electronic supplementary material
Table S1
Exclusion articles during full-text articles assessment process. (DOC 62 kb)
Rights and permissions
About this article
Cite this article
Feng, Y., Cheng, G., Wang, H. et al. The associations between serum 25-hydroxyvitamin D level and the risk of total fracture and hip fracture. Osteoporos Int 28, 1641–1652 (2017). https://doi.org/10.1007/s00198-017-3955-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-3955-x