Abstract
Summary
Alendronate therapy has been associated with serious side effects. Altering the alendronate concentration and combining with high-frequency loading as mechanical intervention was explored in this animal study as a treatment for osteoporosis. The bone anabolic potency of high-frequency loading was overruled by the different alendronate dosages applied in the present study. Further exploration of reduced hormonal therapy associated with mechanical interventions in osteoporosis treatment should be sought.
Introduction
The aim of the present study was to investigate the effect of alendronate (ALN) administration at two different dosages, associated or not with high-frequency (HF) loading, on the bone microstructural response.
Methods
Sixty-four female Wistar rats were used, of which 48 were ovariectomized (OVX) and 16 were sham-operated (shOVX). The OVX animals were divided into three groups: two groups were treated with alendronate, at a dosage of 2 mg/kg (ALN(2)) or at a reduced dosage of 1 mg/kg (ALN(1)) three times per week. A third OVX group did not receive pharmaceutical treatment. All four groups were mechanically stimulated via whole body vibration (WBV) at HF (up to 150 Hz) or left untreated (shWBV). ALN and HF were administered for 6 weeks, starting at 10-week post-(sh)OVX. Tibia bone structural parameters were analyzed using ex vivo microcomputed tomography.
Results
Trabecular bone loss and structural deterioration resulting from ovariectomy were partially restored by ALN administration, demonstrated by the improvement of trabecular patter factor (Tb.Pf), trabecular separation (Tb.Sp), and structure model index (SMI) of the ALN groups compared to that of the OVX group, regardless of the applied dosage [ALN(2) or ALN(1)] or mechanical loading regime (shWBV or WBV). However, a significant positive effect of the ALN(1) administration on trabecular (decrease of Tb.Sp and SMI) and cortical bone (increase of cortical thickness) microarchitecture compared to that of the OVX status group was observed for both loading regimes was not seen for ALN(2). Furthermore, HF loading resulted in cortical bone changes, with an increased trabeculary area and endocortical perimeter. Finally, the benefits of a combined therapy of ALN with HF loading could not be discerned in the present experimental conditions.
Conclusions
The bone anabolic potency of HF loading was overruled by the ALN dosages applied in the present study. Further altering the ALN dosage combined with robust mechanical stimuli needs to be considered in osteoporosis research and eventually therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a disease characterized by a systemic impairment of bone mass and bone microarchitecture [1]. In 2012, 22 million women and 5.5 million men were diagnosed as having osteoporosis in Europe [2]. The prevalence of osteoporosis in women over the age of 50 years is three to four times greater than in men [1]. This is explained by the estrogen deficiency at the menopausal age, deregulating the rates of osteoblastogenesis and osteoclastogenesis, which results in a mismatch between the formation and resorption of bone [3].
Pharmacological treatment with antiresorptive drugs has been routinely used for osteoporosis treatment. The bisphosphonate drug alendronate (ALN) is used primarily in the treatment of osteoporosis as a potent bone antiresorptive agent [4]. Furthermore, the suppression of bone remodeling also changes the bone material properties, thereby making the bone tissue more mineralized and as a consequence more brittle and susceptible to microdamage [5]. Consequently, ALN long-term therapy has been associated with the incidence of side effects such as osteonecrosis of jaw bone and atypical bone fractures [6].
Administration of a high dosage of ALN (7 mg/kg/week during 6 or 4 weeks in early or late osteoporosis treatment, respectively) has been reported as successful in osteoporosis treatment in rats, enhancing the bone microstructure and improving the bone quality [7]. However, as a downside, ALN concentrations of 10 μM (2,7 mg/ml) have been reported as cytotoxic for the human bone marrow mesenchymal stem cells [8]. Based on the above-mentioned, two dosages of ALN were proposed to be administered to osteoporotic rats in the present study, one used in previous studies (6 mg/kg/week) [9–11] and another relatively reduced dosage (“reduced” dosage 3 mg/kg/week).
In addition to the pharmacological treatment, the mechanical treatment of high-frequency (HF) loading via whole body vibration (WBV) has been related to promoting bone formation, increasing bone strength, and even recovering bone loss arising from disabling or osteoporotic conditions [9, 10, 12]. Hence, in order to achieve an efficient osteoporosis treatment with low levels of side effects and toxicity, moderate or reduced ALN concentrations combined with HF loading has been suggested as a potential “combi-treatment” to target an anabolic effect on bone [13]. The research hypothesis was that the alendronate dosage can be altered when combined with high-frequency mechanical loading in osteoporosis treatment. Therefore, the aim of the present study was to evaluate the impact of the combined treatment of mechanical (HF loading) and pharmaceutical (ALN) treatment at differing dosages on the trabecular and cortical bone microarchitecture of osteoporotic rats.
Material and methods
Animals and experimental design
The protocol of the animal experiment was approved by the ethical committee of KU Leuven (P050/2011), complied with ARRIVE guidelines for preclinical studies, and was performed according to the Belgian animal welfare regulations and guidelines.
A total of 64 female Wistar rats at 12 weeks of age were used in the present study. Forty-eight animals underwent ovariectomy surgery (OVX). The remaining 16 animals were subjected to sham-ovariectomy surgery (shOVX). Rats arrived at 5-day postsurgery, with a body weight ranging between 200 and 223 g. In an attempt to control the body weight changes, pair-feeding regimen was installed throughout the study. The animals were fed with standard laboratory diet (or chow) containing 0.7% phosphorus and 1% calcium (SSNIFF, Soest, Germany) and allowed tap water. In addition, no supplements with Vitamin D were provided to the animals. The average daily food consumption of the shOVX animals was determined and the quantified amount was then provided to all ovariectomized animals in an attempt to control the weight gain for all the groups throughout the study.
After ovariectomy, rats were left untreated for a period of 10 weeks. This time lapse was considered to be adequate for inducing significant bone changes in the rat long bones in response to ovariectomy [12, 14]. Thereafter, the OVX group was divided into three groups: one untreated group [OVX] (n = 16), one group treated with a previously used ALN dosage of 2 mg/kg (ALN(2)) [10] (n = 16), and one group treated with ALN at a reduced dosage of 1 mg/kg (ALN(1)) (n = 16). Alendronate sodium trihydrate (A4978-100MG, Sigma-Aldrich, Bornem, Belgium) was injected subcutaneously 3 days/week during 6 weeks. At the same time, saline (0.9% NaCl) was administrated to the rats of the OVX and shOVX groups. All injections were performed until the day of euthanasia.
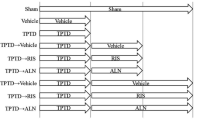
The HF WBV loading was administered via a custom-made vibration device during 6 weeks for 10 min/day according to a protocol that consisted of ten consecutive frequency steps (130, 135, 140, 145, 150, 130, 135, 140, 145, and 150 Hz), each of these applied for 1 min at an acceleration of 0.3 g. WBV was applied to the animals individually, taking into account the animal’s body weight. Intervals of 24 h between the loading sessions were respected [9, 10]. The animals of OVX, ALN(2), and ALN(1) groups were further divided into subgroups relative to the mechanical treatment by whole body vibration. The animals which did not receive the WBV were placed onto the same vibrating platform, for the same duration but without WBV (sham-WBV), resulting in six experimental groups [OVX-shWBV; OVX-WBV; ALN(2)-shWBV; ALN(2)-WBV; ALN(1)-shWBV; ALN(1)-WBV] as shown in Fig. 1.
As outlined above, the pharmacological treatment with ALN combined or not with the mechanical treatment via HF mechanical loading started at 10-week post-(sham) ovariectomy and lasted for a 6-week period. The seventh group, namely the shOVX group, did not receive mechanical stimulation and served as a negative control to illustrate normal bone characteristics over the course of the experiment. The OVX-shWBV group was pointed out as positive control to demonstrate the changes occurring during the development of an osteoporotic condition following ovariectomy.
Specimen preparation and micro-X-ray computed tomography analysis
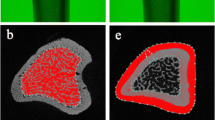
The specimen preparation and the micro-CT acquisition of rat tibiae were performed according to the protocol described previously by Hatori and coworkers [10]. Subsequently, the reconstructed 3D data sets were quantified using CTAn automated image analysis system (Bruker, Kontich, Belgium). To do so, the volume of interest (VOI) for trabecular and cortical analyses was defined in the axial direction by using the growth plate as reference. For trabecular bone analysis, the VOI started at a distance of 1.5 mm distally from the growth plate and extended towards the diaphysis over a distance of 2.4 mm (400 slices). For cortical bone analysis, the VOI started at a distance of 4.2 mm distally from the growth plate and extended towards the diaphysis over a distance of 1.5 mm (250 slices). On every tenth transverse slice of the VOI, the ROI was delineated manually matching with the area occupied by the trabecular bone. For the cortical bone, the ROI was defined automatically for the selected VOI (Fig. 2).
Representative μCT images of the rat tibia with the region of interest (ROI) started at a distance of 1.5 mm distally from the growth plate and extended towards the diaphysis over a distance of 2.4 mm (400 slices). For cortical bone analysis, the ROI started at a distance of 4.2 mm distally from the growth plate and extended towards the diaphysis over a distance of 1.5 mm (250 slices). Automatic delineating of volume of interest (VOI) for the cortical region and manual delineating of VOI for the trabecular region
For the trabecular region, the percent of bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), trabecular number (Tb.N), trabecular pattern factor (Tb.Pf), and structural model index (SMI) were calculated as measurements of bone mass and its distribution. For the cortical region, the cortical thickness (Ct.Th), porosity (Ct.Po), periosteal perimeter (Ps.Pm), trabeculary area (Ma.Ar), endocortical perimeter (Ec.Pm), mean polar moment inertia (MMI), and mean eccentricity (Ecc) were quantified.
Statistical analysis
Results of the bone microstructural parameters were expressed as means ± standard deviation (SD). The software SPSS (SPSS ver.13.0, Chicago, IL, USA) was used to perform the statistical analysis. The data were analyzed by two-way analysis of variance (ANOVA) and pairwise comparison tests in order to assess the effect of the hormonal status and of the mechanical status (two independent variables) and their interactions on the trabecular and cortical bone microarchitectural changes. Pairwise comparison was performed on cases when a positive relationship was found with Bonferroni adjustment and differences were considered significant at p < 0.05.
Results
Interaction mechanical and hormonal status
Two-way ANOVA was performed and the interaction between the mechanical status and the hormonal status on the trabecular region analysis was positive for the parameter Tb.Sp. For the other trabecular parameters and for all the parameters of the cortical bone, an interaction was not found.
Mechanical status
For the trabecular bone region, HF loading did not exert an effect on the bone microarchitecture, proven by the absence of statistical differences comparing WBV with shWBV for all quantified parameters (Fig. 3). In contrast, HF loading did have an effect on the cortical bone microarchitecture, in particular on Ma.Ar and Ec.Pm with an increase of 8.56% ± 16.66 and 9.65% ± 21.96, respectively (Fig. 4).
Micro-CT results of trabecular bone structural parameters according to the hormonal and mechanical status, for the percent of bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), trabecular number (Tb.N), trabecular pattern factor (Tb.Pf), and structural model index (SMI). Means and standard deviations are shown. Connected bars with one asterisk denote statistically significant difference relative to the hormonal groups shOVX, OVX, ALN(2), and ALN(1). The mechanical groups (shWBV versus WBV) did not show statistically significant differences for the trabecular bone parameters
Micro-CT results of cortical bone structural parameters according to the hormonal and mechanical status, for the cortical thickness (Ct.Th), porosity (Ct.Po), periosteal perimeter (Ps.Pm), medullary area (Ma.Ar), endocortical perimeter (Ec.Pm), mean polar moment inertia (MMI), and mean eccentricity (Ecc). Means and standard deviations are shown. Connected bars with one asterisk denote a statistically significant difference relative to the hormonal groups shOVX, OVX, ALN(2), and ALN(1). Connected bars with two asterisks denote a statistically significant difference relative to the mechanical groups shWBV and WBV
Hormonal status
Marked deterioration of the trabecular bone mass and microstructure was found as a result of ovariectomy (OVX versus shOVX), manifested as a significant decrease in BV/TV (−59.02% ± 10.61) and Tb.N (−68.28% ± 9.76) and an increase in Tb.Sp (197.73% ± 116.12), Tb.Pf (103.54% ± 25.88), and SMI (168.64% ± 49.52). Simultaneously, as a compensatory mechanism after ovariectomy, an increase in Tb.Th (30.7% ± 20.12) was noticed (Fig. 3).
The ALN treatment exerted a positive impact on the trabecular bone microarchitecture after ovariectomy, with some microarchitectural parameters of the trabecular bone in the same range as the one in the normal bone condition (shOVX). This can be observed in the Tb.Th parameter where the value of ALN(2) and ALN(1) were statistically equal to those presented by the shOVX. In addition, partial recovering of the bone loss caused by OVX through ALN administration was observed, demonstrated by the improvement of specific parameters such as Tb.Pf for both dosages [ALN(1) or ALN(2)] and Tb.Sp and SMI for the reduced dosage [ALN(1)] compared to that of the OVX group, as shown in the Fig. 3. In general, the quantified trabecular bone parameters were similar for both dosages of ALN [ALN(2) versus ALN(1)], without a statistically significant difference between these dosages (Fig. 3).
OVX did not cause marked cortical bone deterioration (OVX versus shOVX), statistical difference was found only for Ct.Po with a significant decrease for Ct.Po for the OVX cortical bone compared to that for the shOVX bone (−34.01% ± 39.26) as shown in Fig. 4. The ALN treatment at both concentrations was able to restore the decreased cortical bone porosity (Ct.Po) caused by ovariectomy, resulting in values for Ct.Po no longer differing from the shOVX values. Furthermore, the thickness of the cortical bone increased when administrating ALN(1), differing significantly from the OVX Ct.Th values, although without a difference from the Ct.Th values of the shOVX group. In addition, the dosage of ALN [ALN(2) versus ALN(1)] did not result in a significant difference related to the quantified cortical bone parameters, in line with the trabecular region results (Fig. 4).
Discussion
Osteoporosis is caused primarily by the estrogen deficiency in elderly women [15]. The ovariectomized rat is an appropriate model to simulate postmenopausal osteoporosis [14]. The withdrawal of estrogen following ovariectomy surgery induces a rapid increase in bone turnover associated with a substantial bone loss [14, 16–18]. Indeed, substantial trabecular bone deterioration was observed in the OVX group, demonstrated in the present study at the trabecular bone level by the decrease in BV/TV, Tb.N, and the increase in Tb.Sp, Tb.Pf, Tb.Th, and SMI, in line with the literature on the effects of OVX in rat tibia [14, 18, 19].
Concerning Tb.Th, opposite results have been reported in literature. Some authors observed a decrease of Tb.Th after OVX [20], whereas the present study is in agreement with the studies reporting an increase of Tb.Th [19, 21, 22]. Bone remodeling process associated with estrogen depletion in ovariectomized rats not only result in bone loss but also in the formation of new bones, as concluded by Waarsing et al. after 54 weeks of follow-up [22]. Thin trabeculae are more prone to performation by osteoclast activity and subsequent complete resorption than thicker ones [19, 22]. Furthermore, existing trabeculae merge together to form new and thicker trabecular structures as a result of mechanical adaptation [23]. Since the thicker trabeculae remain present, the average Tb.Th will increase.
At the cortical level, no apparent effect of OVX (10 weeks post-surgery) compared to that of shOVX was observed. It is hypothesized that periods of evaluation longer than those applied in this study are necessary to assess the cortical bone changes in response to ovariectomy since the cortical bone is more mineralized and presents a decreased surface ratio compared to the trabecular bone [24], resulting in a lower resorption rate.
The mechanical treatment with HF loading via WBV was not able to substantially oppose the established (10 weeks without treatment following the OVX surgery) negative effects of ovariectomy on the trabecular bone. The lack of a bone-inducing effect of HF treatment in trabecular bone was also demonstrated previously by Hatori et al. using a different loading protocol (same loading frequency applied for 2 weeks starting at 6 weeks post-ovariectomy) [10]. Thus, the trabecular bone response to loading seems to be more related to the role of estrogen receptors than to loading. At these regions, the α-form of estrogen receptor (ER-α) is involved in the mechanosensing pathway of bone cells and its expression in osteoblasts and osteocytes depends on the estrogen concentration [25–27]. Thus, a failure to maintain bone mass after estrogen withdrawal might be due to a reduction in the activity or number of ER-α in bone cells limiting their anabolic response to mechanical loading and allowing bone loss comparable to that associated with disuse [25]. While at the periosteal surface of cortical bone, the lack of inhibitory ER-β signaling might activate a compensatory mechanism promoting an osteogenic response [28, 29], represented in this study by a slightly but not statistically significant increase in Ps.Pm (OVX-shWBV versus OVX-WBV).
Besides mechanical treatment, pharmacological treatment with bisphosphonate alendronate is still the most widely used therapy for the treatment of postmenopausal osteoporosis. In the present study, the ALN treatment, regardless of the dosage used, was able to fully recover one specific trabecular bone microstructure: the trabecular thickness after ALN treatment reaching the same values as the ones presented by that of the shOVX group. Furthermore, compared to the OVX group, positive changes could be observed for Tb.Sp and SMI in ALN(1) group and for Tb.Pf in both ALN(1) and ALN(2) groups, indicating a partial reconstitution of the bone microstructure by bone apposition on the existing topology [18].
At the cortical bone level, differences between the shOVX and the ALN groups were not observed for none of the parameters. However, when comparing OVX with the ALN(1) group, a slightly but significant increase in cortical thickness was noticed as a possible anabolic response to the administration of alendronate. The same trend, i.e., an increase in Ct.Th, is also seen for the other ALN dosage. Future longitudinal studies are necessary to assess the role of alendronate dosage on the cortical bone. In general, the few changes in the cortical region can be explained by the cortical anatomy that limits the accessibility of the mineralized cortical bone matrix volume to being remodeled and may limit the accessibility of drugs. The opposite is found in the trabecular region, which has a high surface area/bone matrix volume configuration and provides a large area facilitating the initiation of remodeling or facilitating the accessibility of drugs [24].
As no significant difference was observed between the different alendronate dosages [ALN(2) or ALN(1)] for bone microarchitecture parameters evaluated, the selection of a reduced dosage can be considered in the treatment of osteoporosis. Furthermore, a reduced alendronate dosage [ALN(1)] showed additional effects on Tb.Sp (trabeculae closer to each other), SMI (change of rod-like shape to plate-like shape) and Ct.Th (cortical thickening). Using a lower dose seems to provide adequate but less complete suppression of bone turnover and is likely to provide an equal fracture and osteonecrosis risk reduction compared to a higher dose [30]. In addition, the combination of ALN administration with HF loading did not produce any additive effect on bone microstructural parameters, regardless of ALN dosage employed. It seems that the anabolic effect of HF loading was masked by the major influence of ALN, even when a moderate ALN dosage (3 mg/kg/week), twofold reduction standard ALN dosage (6 mg/kg/week) [10], was considered. Further studies using less elevated ALN dosages than those used in present study (e.g., tenfold reduction of standard ALN dosage of 6 mg/kg/week) should be performed to clarify what is the optimal effective dose of alendronate which, when combined with HF loading, is able to enhance bone microstructural parameters after ovariectomy with good treatment cost-effectiveness and safety. Besides microstructural analysis, an evaluation at cellular and molecular levels should be addressed in future studies. This could give a better insight of HF loading anabolic effects on bone, once structural bone changes in response to mechanical stimulation take more time to be detected at the microstructural level than those induced by alendronate administration considering its potent and immediate antiresorptive effect.
The limitations of the present study should not be denied. First, only evaluations at the bone microstructural level were included, as discussed above. In addition, the study period was limited to 17 weeks, including 10-week post-ovariectomy surgery prior to treatment and 6 weeks of treatment. Longer periods than 10-week post-ovariectomy surgery should be performed to test the efficacy of the treatment in a severe osteoporotic bone. Also, extended periods (>6 weeks) of treatment are advised to further the effects of HF loading and ALN administration on osteoporotic bone (especially at the cortical level), considering the different mechanisms of action of both treatments. To the author’s knowledge, the present report is the first evaluating the effect of specific ALN dosage associated or not with HF loading on bone microstructure. Therefore, a reduction twofold (50%) of the ALN dosage applied by the author’s group in previous studies was selected [7, 10]. Further studies using longer evaluation periods and lower ALN dosages combined with HF are necessary to elucidate the role of this combined treatment in osteoporotic conditions.
Conclusion
Considering the results and the limitations of the present study, the use of a reduced ALN dosage in the ovariectomy-induced osteoporotic rat model displayed the same efficacy as a standard ALN dosage on bone, with further positive effects on the trabecular bone microarchitecture. Additionally, the bone anabolic potency of HF loading was overruled by ALN dosages applied in the present study. Further reducing the ALN dosage combined with robust mechanical stimuli is worth considering in osteoporosis research and therapy.
References
Rachner T, Khosla S, Hofbauer L, Manuscript A (2011) New horizons in osteoporosis. Lancet 377:1276–1287
Hernlund E, Svedbom A, Ivergård M et al (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8:1–115
Manolagas SC (2000) Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 322:305–311
Iwamoto J, Sato Y, Takeda T, Matsumoto H (2012) Whole body vibration exercise improves body balance and walking velocity in postmenopausal osteoporotic women treated with alendronate: Galileo and Alendronate Intervention Trail (GAIT). J Musculoskelet Neuronal Interact 12:136–143
Roschger P, Misof B, Paschalis E, Fratzl P, Klaushofer K (2014) Changes in the degree of mineralization with osteoporosis and its treatment. Curr Osteoporos Rep 12:338–350
Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B (2009) American Association of Oral and Maxillofacial Surgeons Position Paper on bisphosphonate-related osteonecrosis of the jaw—2009 update. J Oral Maxillofac Surg 67:2–12
Im S, Lim SH, Lee JI et al (2010) Effective dosage and administration schedule of oral alendronate for non-nociceptive symptoms in rats with chronic constriction injury. J Korean Med Sci 25:938–944
Chang CH, Wang CZ, Chang JK, Hsu CY, Ho ML (2014) The susceptive alendronate-treatment timing and dosage for osteogenesis enhancement in human bone marrow-derived stem cells. PLoS One 9:1–9
Chatterjee M, Hatori K, Duyck J, Sasaki K, Naert I, Vandamme K (2014) High-frequency loading positively impacts titanium implant osseointegration in impaired bone. Osteoporos Internat 26:281–290
Hatori K, Camargos GV, Chatterjee M et al (2015) Single and combined effect of high-frequency loading and bisphosphonate treatment on the bone micro-architecture of ovariectomized rats. Osteoporos Int 26:303–313
Chen B, Li Y, Yang X, Xie D (2013) Comparable effects of alendronate and strontium ranelate on femur in ovariectomized rats. Calcif Tissue Internat 93:481–486
Judex S, Lei X, Han D, Rubin C (2007) Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech 40:1333–1339
Giro G, Gonçalves D, Sakakura CE, Pereira RMR, Marcantonio Júnior E, Orrico SRP (2008) Influence of estrogen deficiency and its treatment with alendronate and estrogen on bone density around osseointegrated implants: radiographic study in female rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 105:162–167
Liu XL, Li CL, Lu WW, Cai WX, Zheng LW (2015) Skeletal site-specific response to ovariectomy in a rat model: change in bone density and microarchitecture. Clin Oral Implants Res 26:392–398
Khosla S, Oursler MJ, Monroe DG (2012) Estrogen and the skeleton. Trends Endocrinol Metab 23:576–581
Washimi Y, Ito M, Morishima Y et al (2007) Effect of combined humanPTH(1-34) and calcitonin treatment in ovariectomized rats. Bone 41:786–793
Yao W, Cheng Z, Koester KJ et al (2007) The degree of bone mineralization is maintained with single intravenous bisphosphonates in aged estrogen-deficient rats and is a strong predictor of bone strength. Bone 41:804–812
Campbell GM, Bernhardt R, Scharnweber D, Boyd SK (2011) The bone architecture is enhanced with combined PTH and alendronate treatment compared to monotherapy while maintaining the state of surface mineralization in the OVX rat. Bone 49:225–232
Brouwers JEM, Lambers FM, Gasser JA, van Rietbergen B, Huiskes R (2008) Bone degeneration and recovery after early and late bisphosphonate treatment of ovariectomized wistar rats assessed by in vivo micro-computed tomography. Calcif Tissue Int 82:202–211
Boyd SK, Davison P, Muller R, Gasser JA (2006) Monitoring individual morphological changes over time in ovariectomized rats by in vivo micro-computed tomography. Bone 39:854–862
Yang J, Pham SM, Crabbe DL (2003) Effects of oestrogen deficiency on rat mandibular and tibial microarchitecture. Dentomaxillofac Radiol 32:247–251
Waarsing JH, Day JS, Verhaar JAN, Ederveen AGH, Weinans H (2006) Bone loss dynamics result in trabecular alignment in aging and ovariectomized rats. J Orthop Res 24:926–935
Frost HM (2003). Bone’s mechanostat: a 2003 update. Anat Rec A Discov Mol.Cell Evol Biol 275:1081–1101
Seeman E (2013) Age- and menopause-related bone loss compromise cortical and trabecular microstructure. J Gerontol A Biol Sci Med Sci 68:1218–1225
Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L (2003) Endocrinology: bone adaptation requires oestrogen receptor-α. Nature 424:389–389
Lee KCL, Lanyon LE (2004) Mechanical loading influences bone mass through estrogen receptor alpha. Exerc Sport Sci Rev 32:64–68
Lim SK, Won YJ, Lee HC, Huh KB, Park YS (1999) A PCR analysis of ERalpha and ERbeta mRNA abundance in rats and the effect of ovariectomy. J Bone Min Res 14:1189–1196
Saxon LK, Turner CH (2005) Estrogen receptor beta: the antimechanostat? Bone 36:185–192
Rubinacci A, Marenzana M, Cavani F et al (2008) Ovariectomy sensitizes rat cortical bone to whole-body vibration. Calcif Tissue Int 82:316–326
You L, Sheng Z, Chen J (2011) The safety and efficacy of early-stage bi-weekly alendronate to improve bone mineral density and bone turnover in Chinese post-menopausal women at risk of osteoporosis. J Int Med Res 39:302–310
Acknowledgments
The authors would like to acknowledge Dr. A. Ivanova for the help with the statistical analysis. This work was supported by the Fund for Scientific Research Flanders (FWO-Vlaanderen) by Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP (2014/08912-1 for Postdoctoral researcher Correa CB) and the Brazilian Science Without Borders Program (246131/2012-8 for PhD student Camargos GV).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The protocol of the animal experiment was approved by the ethical committee of KU Leuven (P050/2011), complied with ARRIVE guidelines for preclinical studies and was performed according to the Belgian animal welfare regulations and guidelines.
Conflicts of interest
The authors Cassia Bellotto Correa, Germana De Villa Camargos, Marissa Chatterjee, Marcelo Ferraz Mesquita, Altair Antoninha Del Bel Cury, Ignace Naert, Joke Duyck, and Katleen Vandamme declare that there are no conflicts of interest related to the manuscript.
Additional information
Correa CB and Camargos GV shared first authorship.
Rights and permissions
About this article
Cite this article
Correa, C., Camargos, G., Chatterjee, M. et al. Can the alendronate dosage be altered when combined with high-frequency loading in osteoporosis treatment?. Osteoporos Int 28, 1287–1293 (2017). https://doi.org/10.1007/s00198-016-3859-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3859-1