Abstract
Summary
High-frequency loading via whole body vibration promotes bone formation and increases bone strength. Whether this translates to positive titanium implant osseointegration in osteoporotic bone was explored in this animal study. An anabolic effect of not only bisphosphonate treatment but also high-frequency loading on implant osseointegration in osteoporotic bone was observed.
Introduction
The present study investigated the impact of high-frequency (HF) loading, applied via whole body vibration (WBV), on titanium implant osseointegration in healthy versus ovariectomy-induced compromised versus pharmacologically treated compromised bone.
Methods
A custom-made Ti implant was inserted into the metaphyseal tibia of 59 rats and left to heal for either 4 or 14 days. Rats were divided into six groups according to their hormonal and mechanical status. WBV, consisting of 10 consecutive frequency steps at an acceleration of 0.3g, was applied daily for either 4 or 14 days. Tissue samples were processed for quantitative histology at the tibial cortical and medullar level. Data were analyzed by three-way ANOVA and by post hoc pairwise comparisons.
Results
The bone healing response at the interface and surrounding titanium implants was negatively influenced by osteoporotic bone conditions, mainly at the trabecular bone level. Furthermore, the administration of bisphosphonates for preventing the ovariectomy-induced impaired peri-implant response was successful. Finally, the effect of HF WBV loading on the peri-implant bone healing was dependent on the bone condition and was anabolic solely in untreated osteoporotic trabecular bone when applied for an extended period of time.
Conclusions
The bone healing response to implant installation is compromised in osteoporotic bone conditions, in particular at the trabecular bone compartment. Meanwhile, not only pharmacological treatment but also mechanical loading via HF WBV can exert a positive effect on implant osseointegration in this specific bone micro-environment. The peri-implant cortical bone, however, seems to be less sensitive to HF WBV loading influences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Implant therapy is a well-established treatment for replacing missing teeth in the elderly population [1]. Treatment success versus failure factors include the biomechanical condition, the microbial environment, and the host bone quality and quantity [2]. The latter factor is closely associated with systemic diseases associated with ageing, such as osteoporosis and diabetes. Postmenopausal osteoporosis is a skeletal disease characterized by reduced bone mass and deterioration of the bone micro-architecture, mainly due to increased bone resorption elicited by estrogen deficiency [3]. This medical condition is an important risk factor for implant failure in orthopedic and oral surgery, both during the healing as well as during the implant functional status [4–6]. Anti-resorption drugs, such as bisphosphonates, selective estrogen receptor modulators, and hormone therapy have been used to treat osteoporosis for years. These agents suppress bone resorption and bone turnover, resulting in a relative increase of bone formation and bone mineral density and subsequently lowering fracture rates in hip and spine [7]. However, there seems to be limited information available with regard to the long-term usage of oral bisphosphonates. This may represent a challenge when investigating bone healing and regeneration, in particular during implant therapy. As bisphosphonates are not metabolized, their concentrations are maintained within the bone for longer periods of time [8]. Their ability to affect systemic bone remodeling raises questions on the drug’s impact on dental implant osseointegration. Therefore, implant therapy in patients diagnosed with osteoporosis may prove to be challenging when bisphosphonate therapy is coupled with the process of osseointegration [9].
In the field of orthopedic and dental implant surgery, it is known that bone adjusts itself by responding to mechanical loading through alteration of its mass and microstructure [10, 11]. In line with the latter concept, the effect of mechanical loading on bone regeneration and remodeling also pertains to the bone surrounding titanium implants [12, 13]. Previous in vivo studies have shown that well-controlled mechanical loading at low frequency can improve bone apposition onto the implant surface and bone formation and mineralization in the implant’s vicinity [12–15]. The importance of using high-frequency (HF) mechanical loading, i.e., at a frequency beyond the physiological frequency (approx ∼3 Hz for mastication), is increasing because of the evidenced positive effect of HF loading on bone formation and fracture healing [16–18]. Furthermore, based on the clinical outcome of exercise studies [10, 19, 20], the advantages of using HF mechanical loading are considered to be safe and efficient. HF mechanical loading improves the bone’s mechanical properties while being able to withstand the physiological demands [11]. This makes HF mechanical loading a powerful non-pharmacological intervention not only for the treatment or prevention of osteoporosis but also for fracture healing and beyond [1, 19, 20].
Numerous in vivo systems employing HF loading have been successfully used, such as whole body vibration (WBV) devices [16–18] and individual limb compressive loading setups [21, 22]. WBV experiments have evidenced the advantageous effects of HF loading on bone [16–18] and on titanium implant osseointegration [23]. Ogawa and co-workers recently reported, by means of rat animal experiments and quantitative histology, that HF loading via WBV can promote peri-implant bone healing and enhance implant osseointegration [23, 24]. However, the latter-referred experiments did not make use of an impaired bone model. Therefore, it is unclear whether HF loading could positively affect titanium implant osseointegration in compromised bone conditions, in particular in osteoporotic host bone.
In an effort to further exploit the anabolic effect of HF loading on titanium implant osseointegration, we tested the hypothesis that HF loading has a beneficial effect on implant osseointegration independently of the micro-environmental (i.e., bone status) conditions. More specifically, the aim of the present study was to investigate the impact of HF loading, indirectly applied through WBV, on the peri-implant bone response for healthy versus ovariectomy-induced compromised versus pharmacologically treated compromised bone. It was anticipated that the application of HF loading in implant dentistry for the osteoporotic compromised bone situation is of significant clinical relevance and may lead to defining optimized clinical protocols for specific conditions and hence implant prognosis.
Materials and methods
Animals and experimental design
Fifty-nine female adult Wistar rats at 12 weeks of age were used in the present study. Thirty-nine rats underwent ovariectomy surgery (OVX), of which 20 rats were treated with the anti-resorptive bisphosphonate drug alendronate (OVX-ALN). The remaining 19 rats received sham-ovariectomy surgery (shOVX). (Sham)-ovariectomy surgery was performed by veterinary surgeons at Charles River Laboratories (Charles River, L’Arbresle, France). For the rats subjected to sham surgery, the bilateral ovaries were lifted up and returned to their original position, while for the ovariectomized rats, the ovaries were removed. Rats arrived 5 days post-(sh)OVX surgery ranging between 215 and 23,057g for shOVX rats and for OVX rats, respectively. Each group (shOVX versus OVX versus OVX-ALN) was further divided into groups relative to the loading condition (sham-WBV loaded versus WBV loaded) and to the duration of healing (4 versus 14 days) (Table 1). For the OVX-ALN group, alendronate sodium trihydrate (A4978-100MG, Sigma-Aldrich, Bornem, Belgium) was injected subcutaneously 3 days/week at a dose of 2 mg/kg body weight, starting 5 days post-OVX surgery. Saline administration (0.9 % NaCl) was performed according to the same time schedule for the rats of the groups OVX and shOVX. Injections were administered till the day of euthanasia. The animals were fed standard laboratory diet (or chow) containing 0.7 % phosphorus and 1 % calcium (SSNIFF, Soest, Germany) and allowed tap water. Pair-feeding regiment was initiated for OVX and OVX-ALN animals at the day of arrival. The average daily food consumption of the shOVX animals was determined, and the quantified amount was then provided to all animals in an attempt to control the weight gain for all groups throughout the study. Animals were weighted at the start and once a week during the study. Despite pair-feeding, the body weight of OVX and OVX-ALN rats increased, but independently from the mechanical condition. All experiments were conducted according to institutional guidelines for animal welfare; the experimental protocols were approved by the ethical committee of KU Leuven (P130/2010) and followed the ARRIVE guidelines.
Implants and surgical procedure
Implant surgery was performed 6 weeks post-(sh)OVX. A commercially pure titanium, cylindrical one-piece percutaneous implant (2 × 8 mm) (GC Corporation, Tokyo, Japan) (Fig. 1a) was used. Implants were installed unilaterally in the proximal metaphyseal region of the tibia (Fig. 1b). The contralateral tibia served as control for validation of the experimental osteoporosis animal model by μCT imaging [25]. A 5-mm incision was made to provide access to the medio-proximal side of the tibia. Consecutively, larger drills perforated both cortices of the tibia until a final diameter of 0.3 mm smaller than the implant’s diameter was reached. This discrepancy between implant and osteotomy dimensions provided the primary implant stability. The implants were inserted by means of a custom-fit wrench with manual torque, with the screw threads at both implant ends in contact with the medial and lateral cortical bone.
Loading protocols
HF mechanical loading was initiated the day of implant installation and was applied by means of WBV via a custom-made vibration device (in collaboration with the Department of Mechanical Engineering, Division of Biomechanics KU Leuven, Leuven, Belgium). As outlined above, the animals were randomly divided into two groups with different experimental periods. In one group (n = 30), the experiment lasted 4 days, while in the other group (n = 29) for 14 days. Each group was subdivided into two groups, a sham-WBV-loaded (shWBV) and a WBV-loaded group (Table 1). The WBV protocol consisted of ten consecutive frequency steps (130, 135, 140, 145, 150, 130, 135, 140, 145, and 150 Hz), each of these applied for 1 min at an acceleration of 0.3g. In this way, a WBV session lasted 10 min. This particular loading protocol has shown to enhance the peri-implant bone healing and osseointegration in non-compromised bone. Vibration loading started the day following surgery (“immediate implant loading” corresponding to the classification of the consensus report on clinical loading protocols) [26] and was applied daily for either 4 or 14 days. WBV was applied to the animals individually, taking into account the animal’s body weight. Intervals of 24 h between the loading sessions were respected.
Specimen preparation and analyses
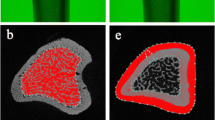
At euthanasia, the implants and surrounding bone tissues were isolated and immediately fixed in a CaCO3-buffered formalin solution, dehydrated in an ascending series of ethanol concentration, and embedded in methylmethacrylate resin. The tissue blocks containing the implants were cross-sectioned along the direction of the tibia and the implant’s axis by means of a diamond saw (Leica SP1600, Wetzer, Germany). Commercially pure (c.p.) titanium implants were used (cf supra) because of facilitated sawing and grinding procedures compared to Ti6Al4V alloy materials, After polishing to a final thickness of 20–30 μm (Exakt 400 CS, Exakt Technologies Inc., Germany), the sections were stained with a combination of Stevenel’s blue and Von Gieson’s picrofuchsin red, visualizing mineralized (red) and non-mineralized (blue) tissues. In total, 109 sections were obtained (Table 1). The histological analyses of these sections were performed using a light microscope (Leica Laborlux, Wetzlar, Germany) and a high sensitivity video camera (AxioCam MRc5, Zeiss, Gottingen, Germany). Histomorphometric analyses were carried out on the medial part of the implant (i.e., the implant part closest to its insertion port) (Fig. 2a), at both cortical and medullar level (Fig. 2b). The implant cortical region of interest was defined as the implant part extending from the first implant screw thread located in the medial outer cortex till the inner surface of the cortex (i.e., endosteum); the implant medullar region of interest was defined as the implant part starting from the endosteum till the last screw thread extension. The following parameters were measured by using image analyzing software (Axiovision 4.0, Zeiss, Göttingen, Germany):
-
Bone-to-implant contact (BIC, %)—summation of the lengths of bone tissue in direct contact with the implant/the considered implant length (i.e., from the 1st implant screw thread till endosteal level and from endosteal level till the last implant screw thread for BIC-cortical (BIC-C) and BIC-medullar (BIC-M), respectively).
-
Peri-implant bone area relative to tissue area (BA/TA, %)—the amount of bone in a specific reference area. Two different regions of interest (ROI) were defined: 0–100 μm (ROI1, hereafter named as “interfacial”) and 100–500 μm (ROI2; hereafter referred to as “remote”) wide zones extending from the implant surface. The area encompassed the peri-implant tissues of the screw-threaded medial part of the implant, thus including cortical (BA/TA-C) and medullar (BA/TA-M) tissue areas (Fig. 2c).
Illustration of the implant part (a) and of the cortical and medullar implant regions (b) considered for histomorphometrical analyses. c Illustration of the defined regions of interest (ROI) for histomorphometrical analysis of the bone area relative to the tissue area (BA/TA). The different ROIs defined according to their distance relative to the implant surface (ROI1, 0–100 μm and ROI2, 100–500 μm) are indicated. Scale bar 0.5 mm
Statistical analysis
Three-way ANOVA and post hoc pairwise comparison tests were performed to assess the effect of hormonal status, mechanical status, duration of healing (i.e., the three independent variables), and their interactions on the parameters BIC-C, BIC-M, BA/TA-C-ROI1, BA/TA-M-ROI1, BA/TA-C-ROI2, and BA/TA-M-ROI2 (i.e., the dependent variables). It consisted of seven significance tests: a test for each of the three main effects, a test for each of the three two-way interactions, and a test of the three-way interaction. A three-way interaction between the main effects (hormonal status, mechanical status, and duration of healing) was found, and subsequent statistical analyses made use of the full factorial model. Differences were considered significant at p < 0.05. Post hoc tests for pairwise comparisons were performed using the Tukey HSD test with Bonferroni corrections up to a significance level of p < 0.00417 for the hormonal status and p < 0.0083 for the mechanical status and for the duration of the period of healing. Data were represented as mean ± standard error of the mean (SEM). All the statistical analyses were performed using statistical software (SPSS ver. 13.0, Chicago, IL, USA).
Results
For the 59 rats participating in the study, all implants were clinically stable and healed uneventfully.
Histological observations
At 4 days post-implantation, bone healing was ongoing though without bone regeneration discernible at tissue level, for both shWBV and WBV conditions. For the 14-day experimental period, the histological images displayed bone neoformation, lamellar organization, and apposition onto the implant for both loading conditions. No obvious differences between sham-loaded and loaded implants could be noticed.
Histomorphometrical analysis
BIC was significantly influenced by the animal’s hormonal status as well as by the duration of the (sham)-loaded healing period, with a significant interaction between these two factors for BIC-M (ANOVA; p < 0.05) (Table 2). Post hoc tests investigating the differences between the hormonal conditions (shOVX versus OVX versus OVX-ALN) indicated that, in the medullar region, there were significantly higher BIC values for the shOVX and the OVX-ALN hormonal conditions compared to the OVX condition, in the 14-day sham-loaded (shWBV) experimental regime (p < 0.001 and p < 0.001, respectively). Furthermore, no effect of HF WBV loading on the bone formation and mineralization at the implant surface could be found. Finally, BIC significantly increased over time, both at cortical and medullar level for all three hormonal conditions and for the two loading regimes (Fig. 3a, d).
Histomorphometrical results of bone-to-implant contact (BIC) at cortical (a) and medullar (d) level and of the bone area relative to the tissue area (BA/TA) at cortical (b, c) and medullar (e, f) level, in the 0–100 μm “interfacial” zone (b–e) and in the 100–500 μm “remote” zone (c–f) surrounding the implant for the two loading conditions and the two experimental terms. Means and standard deviations are shown. An asterisk denotes a statistically significant difference between the hormonal conditions. A double asterisk denotes a statistically significant difference relative to the corresponding 4-days group. A triple asterisk indicates a statistically significant difference between the shWBV and the WBV-loaded conditions
BA/TA was significantly influenced by the animal’s hormonal status and by the duration of the (sham)-loaded healing period, with a significant interaction not only between these but also with the other independent variable, i.e., the mechanical status. These ANOVA results were found for all BA/TA results except for BA/TA-C-ROI2 (Table 2). The full factorial ANOVA analysis was applied, followed by post hoc tests, with the application of stringent Bonferroni correction factors, resulting in the setting of the significance level at p < 0.0083 for the variables mechanical status and duration and p < 0.0042 for the variable hormonal status. Differences between conditions through pairwise comparisons were investigated. At the cortical interfacial bone (C-ROI1—Fig. 3b), no differences in the amount of bone could be observed between shOVX versus OVX versus OVX-ALN animals. Furthermore, an overall increase of BA/TA-C-ROI1 over time (4 versus 14 days) was seen (p < 0.001, p < 0.005, and p < 0.004 for the sham-loaded shOVX, OVX, and OVX-ALN group, respectively; p < 0.005, p < 0.001, and p < 0.001 for the loaded shOVX, OVX, and OVX-ALN group, respectively). Finally, WBV loading for 14 days resulted in a significantly inferior amount of bone near the implant surface for the shOVX group compared to the corresponding sham-loaded group (p < 0.005). The latter result was not found for the OVX nor for the OVX-ALN group. Concerning the amount of cortical bone remote from the implant (C-ROI2; Fig. 3c), significantly higher BA/TA values for the shOVX and the OVX-ALN hormonal conditions compared to the OVX condition in the 14-day shWBV loading regime were observed (p < 0.003 and p < 0.001, respectively). Furthermore, an increase in the bone volume over time was only observed for the shWBV shOVX and shWBV OVX-ALN groups (p < 0.005 and p < 0.001, respectively). Finally, mechanical loading did not affect the BA/TA-C-ROI2 outcomes.
In the medullar implant interfacial region (M-ROI1; Fig. 3e), significantly lower BA/TA values were recorded for the OVX group compared to the shOVX group in two out of four conditions (shWBV—14 days and WBV—4 days; p < 0.001 and p < 0.004, respectively). Furthermore, an increase of BA/TA-M-ROI1 over time (4 versus 14 days) was observed (p < 0.001 and p < 0.003 for the sham-loaded shOVX and OVX-ALN group, respectively; p < 0.001 for the loaded shOVX, OVX, and OVX-ALN group). Finally, WBV loading for 14 days resulted in an increase of the amount of implant interfacial bone for the OVX group (p < 0.003 compared to no loading) (Fig. 3e). For the amount of medullar bone remote from the implant (ROI2 zone; Fig. 3f), the results of the effect of the hormonal condition on BA/TA-M-ROI2 were analogous as those found for ROI1. Furthermore, selected increases in BA/TA over time were recorded (p < 0.001 for the sham-loaded shOVX group; p < 0.001 and p < 0.002 for the loaded OVX and OVX-ALN group, respectively). Finally, WBV loading did not result in distinct BA/TA-M-ROI2 outcomes relative to the hormonal condition and to the duration of (sham)-loaded healing.
The main findings of the histomorphometrical results relative to the hormonal and to the mechanical condition, for the 14-day experimental period, are schematically displayed in Fig. 4.
A schematic representation of the significant bone changes according to the hormonal and the mechanical status for the 14-day groups. Hatched areas represent negative changes (bone loss); plain gray-colored area represents positive changes (bone gain). The red-colored line indicates the negative changes in bone-to-implant contact occurring at the implant surface
Discussion
In order to explore the effect of osteoporotic impaired bone on titanium implant osseointegration, more in particular the impact of mechanical and/or pharmacological interventions herein, the present animal study was performed where titanium implants were installed in the tibial bone. It was hypothesized that HF loading exerted via WBV has a positive effect on implant osseointegration, irrespective of the micro-environment (i.e., the bone condition, compromised (OVX and OVX-ALN) or not (shOVX)). To test this hypothesis, the effect of HF loading and of its duration on the peri-implant bone response was assessed in healthy versus impaired bone. The effect of the animal’s hormonal health status in se on implant osseointegration was also considered in order to provide data on whether osteoporotic bone presents a challenge for implant therapy. The main findings of the study are as follows: (i) the bone healing response at the interface and surrounding titanium implants is negatively influenced by osteoporotic bone conditions, mainly at the trabecular bone level; (ii) the administration of bisphosphonates for preventing the OVX-induced impaired peri-implant response is successful; and (iii) the effect of HF WBV loading on the peri-implant bone healing is dependent on the bone condition and was shown to be anabolic solely in untreated osteoporotic trabecular bone when applied for an extended period of time. Hence, the study hypotheses could only be partially confirmed.
Literature of clinical studies investigating the increased risk for implant failure in osteoporotic patients reveals contradictory outcomes [27–29]. The study of Friberg et al. [30] reported an overall 97 % success rate of implants installed in maxilla and mandible of osteoporotic patients after a 3-year follow-up period, thereby suggesting that osteoporosis is not affecting implant success. Likewise, Minsk and Polson [31] evaluated 450 maxillary and mandibular implants placed in 116 postmenopausal women aged >50 years and reported an overall success of 92 %. It was observed that all failures occurred early, i.e., at the time of abutment connection. The study by August et al. [32], assessing implant failure rates in pre- and postmenopausal women, did not report differences in implant success rates for the mandibular implants, but revealed significantly more maxillary implant failures in the postmenopausal subjects compared to their premenopausal counterparts. Even though the latter study did not mention the patient’s osteoporosis status, it highlights the fact that postmenopausal patients may have a higher risk for implant failure, notably during the implant healing stage.
For the present animal study, the rat tibia was chosen as experimental template for studying the peri-implant bone healing response, owing to its similar bone remodeling rate with humans [33], the accessibility for implant surgery, and for mechanical loading. Clinical and histological observations revealed an uneventful implant osseointegration for both loaded and sham-loaded implants, with good implant stability. However, quantitative assessment revealed a negative impact of the osteoporotic bone state, primarily at the trabecular (medullar) bone level at the implant surface and in its surroundings for the advanced healing state. After 4 days of healing, peri-implant bone differences between the hormonal conditions could not be captured at cortical level. Although evidence has been provided that bone resorption takes place concomitant with bone neoformation in the early osseointegration healing process [34], this dual-event early tissue healing cascade could not be distinguished at tissue level in cortical bone. In contrast, at the medullar interfacial and remote region, areas initially devoid of bone tissue, an impaired peri-implant bone response for OVX implants compared to shOVX could be quantified for the 4-day healing setup. Differently, after 14 days of implant healing, the newly formed bone was mineralized and structurally rearranged, and more and more pronounced differences between the hormonal conditions could now be observed at tissue level. It was observed that the estrogen deficiency-induced bone loss occurring in the OVX state is accompanied by deficient bone modeling, i.e., the bone regeneration in the initial bone-free medullar region. Likewise, the OVX status altered the bone remodeling, resulting in a decreased amount of cortical bone. However, at the implant surface itself and in the implant interfacial region, the negative effect of OVX on the cortical shell could no longer be distinguished and may have been masked by the important periosteal reaction related to implant surgery [35]. This finding for the titanium implant setting corresponds with available data on the effect of OVX status on bone regeneration and on the thinning of the cortical bone shelf [36, 37].
In the clinics, the dosage of ALN and its frequency of administration for treating osteoporosis have undergone important changes in recent years, targeting respectively optimal efficiency to prevent postmenopausal-induced bone deterioration and increased patient adherence. Based on previous literature reports using ALN administration in OVX rats [38–40], it was anticipated for the present study that administration of ALN three times per week at a concentration of 2 mg/kg body weight/dose to the rats would be sufficient to prevent the OVX-related bone changes to occur. Indeed, no differences were observed in none of the quantified parameters between shOVX and OVX-ALN groups. We therefore anticipate that ALN, via its evidenced role in inhibiting osteoclast-mediated bone resorption [41], also acted on bone healing related to titanium osseointegration. However, a significant positive effect of ALN on the interfacial and remote bone, compared to the OVX status, was only found at the cortical peri-implant level, where mainly bone remodeling is occurring. Furthermore, ALN treatment was not able to attain the same level of bone regeneration in the interfacial and remote peri-implant medullar region as the shOVX groups, thereby not significantly differing from the OVX results. This is in accordance with literature findings on the protective effect of ALN not only in implant osseointegration, but also in bone regeneration settings such as fracture healing [40].
The anabolic effects of HF loading on bone and bone healing have been reported in several animal [16–18, 42] and clinical trials [19, 20]. It is well established that bone has the potential of sensing and responding to very small mechanical signals when applied at HF [43]. Since the process of osseointegration of oral implants involves the bone healing process, it was assumed that HF loading might positively affect peri-implant bone healing and osseointegration. Ogawa and co-workers have confirmed a bone anabolic response of HF loading, even when applied indirectly, on the peri-implant tissue mineralization in non-compromised rat bone [24]. This was discernible during the implant osseointegration cascade at tissue level already after 7 days of healing. Other research groups reported an osteogenic effect of HF WBV loading on the peri-implant response in an ovariectomized animal model [23, 44]. In the present study, the anabolic effect of HF loading was only seen in the impaired bone micro-environment (OVX) and solely in the bone marrow region, a region where bone neoformation occurred. Significantly more newly formed bone was observed in the OVX implant interfacial region subjected to HF loading compared to the sham-loaded implant. Although not significantly different, the same trend was found for the two other medullar sub-regions (at the implant surface and at the implant’s remote region). It could be speculated that HF stimuli differentially affect the bone cell activities during healing relative to the estrogen environment. Estrogens are known to regulate the rate and extent of bone remodeling, but the bone homeostasis balance is probably modulated by the prevailing mechanical environment [45]. Also, this phenomenon was observed only in the region where bone formation (modeling) takes place and not at the cortical, remodeling region [43]. The differential effect of HF WBV on the OVX cortical versus trabecular bone could be explained by the presence of decreased cortical bone mass and the correlation of the tissue response to HF loading to the bone mass, as observed by Judex et al. [46]. Likewise, because shOVX have higher bone mass, they may require a different vibration stimulus to trigger a greater osteogenic healing response. A recent computational study by Coughlin et al. reported that the mechanism of the osteogenic impact of WBV on the trabecular bone is based on the stimulation of the bone cells by the fluid shear stress of the bone marrow on the trabeculae surface generated by HF loading [47]. They demonstrated that lower trabecular bone volume fraction resulted in higher stresses on the trabecular surface and therefore in increased stimulation of the bone cells. This is in accordance with our results as OVX healing trabecular bone displayed a greater response to HF WBV stimulation. The study of Rubinacci et al. also suggested that the difference in response of the trabecular versus cortical bone compartments to loading may be sex hormone dependent and inhibited by estrogen in the cortical compartment. Such observations support the view that vibration loses its osteogenic potential when the estrogen axis is intact which is in agreement with the fact that estrogen acts as negative modulator of the mechanotransduction process [48].
In contrast to the anabolic effect of HF WBV on impaired OVX bone, a catabolic effect of HF on shOVX healing bone was observed, again solely statistically significant in the interfacial region but supported with a same trend for the other two regions. A possible explanation for this observation could be the increased stress-shielding occurring at the implant-tissue interface in response to WBV. These stress concentrations may have evoked increased bone resorption at the implant neck [49]. Noteworthy mentioning and in agreement with the findings of a μCT imaging study assessing the bone micro-architecture in se relative to the hormonal and mechanical status [25], the association of pharmacological (ALN) with mechanical treatment (HF WBV) did not lead to a synergistic reaction influencing the bone healing response at any level (cortical/medullar) or region (implant surface, implant interfacial, and remote zone).
Some study limitations need to be highlighted. Firstly, biomechanical assessment of the implant osseointegration for the different conditions was not tested. These are important in evaluating the properties (e.g., strength) of the tissue surrounding the implant. Secondly, the provided data of the present study (as well as of recent studies from other groups) offer descriptive findings, whereas further exploration of the mechanistic links between HF loading and implant osseointegration is still lacking [50]. Furthermore, given the facts that no power analysis was performed and that the use of the three-way interaction model implicated the assessment of statistical differences in several subgroups, the results of the non-significant outcomes should be interpreted cautiously. Finally, it should be pointed out that the present study aimed to evoke an anabolic effect of HF loading on the implant osseointegration response independently of the bone condition. However, the applied loading regime failed to elicit an anabolic response to HF WBV in the cases of healthy and ALN-treated osteoporotic bone condition.
In conclusion, the bone healing response to implant installation is compromised in osteoporotic bone conditions, in particular at the trabecular bone compartment. Meanwhile, not only pharmacological treatment by means of bisphosphonate drug but also HF loading via WBV can exert a positive effect on implant osseointegration in this specific bone micro-environment. The healing cortical peri-implant bone, however, seems to be less sensitive to HF WBV loading influences. The challenges posed by compromised bone conditions (e.g., smoking, osteoporosis, diabetes, irradiated bone) in implant dentistry need to be taken into account when considering earlier function of the implant (immediate implant loading). Acceleration of the osseointegration process by means of a bioengineered implant surface is a most welcomed alternative strategy.
References
Feine JS et al (2002) The McGill consensus statement on overdentures. Mandibular two-implant overdentures as first choice standard of care for edentulous patients. Gerodontology 19(1):3–4
Alsaadi G et al (2008) Impact of local and systemic factors on the incidence of late oral implant loss. Clin Oral Implants Res 19(7):670–676
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377(9773):1276–1287
Ozawa S et al (2002) Ovariectomy hinders the early stage of bone-implant integration: histomorphometric, biomechanical, and molecular analyses. Bone 30(1):137–143
Mellado-Valero A et al (2010) Implant treatment in patients with osteoporosis. Med Oral Patol Oral Cir Bucal 15(1):e52–e57
Alghamdi HS, Jansen JA (2013) Bone regeneration associated with nontherapeutic and therapeutic surface coatings for dental implants in osteoporosis. Tissue Eng B Rev 19(3):233–253
Nijenhuis T et al (2008) Bone resorption inhibitor alendronate normalizes the reduced bone thickness of TRPV5(−/−) mice. J Bone Miner Res 23(11):1815–1824
Ruggiero SL et al (2004) Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 62(5):527–534
Wang HL, Weber D, McCauley LK (2007) Effect of long-term oral bisphosphonates on implant wound healing: literature review and a case report. J Periodontol 78(3):584–594
Judex S, Gupta S, Rubin C (2009) Regulation of mechanical signals in bone. Orthod Craniofacial Res 12(2):94–104
Ozcivici E et al (2010) Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol 6(1):50–59
Duyck J et al (2007) Effect of intermittent loading and surface roughness on peri-implant bone formation in a bone chamber model. J Clin Periodontol 34(11):998–1006
Duyck J et al (2006) The influence of micro-motion on the tissue differentiation around immediately loaded cylindrical turned titanium implants. Arch Oral Biol 51(1):1–9
Vandamme K et al (2007) The effect of micro-motion on the tissue response around immediately loaded roughened titanium implants in the rabbit. Eur J Oral Sci 115(1):21–29
Vandamme K et al (2008) Effect of implant surface roughness and loading on peri-implant bone formation. J Periodontol 79(1):150–157
Goodship AE, Lawes TJ, Rubin CT (2009) Low-magnitude high-frequency mechanical signals accelerate and augment endochondral bone repair: preliminary evidence of efficacy. J Orthop Res 27(7):922–930
Hwang SJ et al (2009) Extremely small-magnitude accelerations enhance bone regeneration: a preliminary study. Clin Orthop Relat Res 467(4):1083–1091
Judex S et al (2007) Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech 40(6):1333–1339
Gilsanz V et al (2006) Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res 21(9):1464–1474
Rubin C et al (2004) Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res 19(3):343–351
Christiansen BA, Kotiya AA, Silva MJ (2009) Constrained tibial vibration does not produce an anabolic bone response in adult mice. Bone 45(4):750–759
De Souza RL et al (2005) Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone 37(6):810–818
Akca K et al (2007) Micro-morphologic changes around biophysically-stimulated titanium implants in ovariectomized rats. Head Face Med 3:28
Ogawa T et al (2011) Influence of whole-body vibration time on peri-implant bone healing: a histomorphometrical animal study. J Clin Periodontol 38(2):180–185
Hatori K, Camargos GV, Chatterjee M, Faot F, Sasaki K, Duyck J, Vandamme K (2014) Single and combined effect of high-frequency loading and bisphosphonate treatment on the bone micro-architecture of ovariectomized rats. Osteoporosis Int. In press
Esposito M et al (2009) Interventions for replacing missing teeth: horizontal and vertical bone augmentation techniques for dental implant treatment. Cochrane Database Syst Rev 4:CD003607
Mori H et al (1997) Osseointegration of dental implants in rabbit bone with low mineral density. J Oral Maxillofac Surg 55(4):351–361, discussion 362
Dao TT, Anderson JD, Zarb GA (1993) Is osteoporosis a risk factor for osseointegration of dental implants? Int J Oral Maxillofac Implants 8(2):137–144
Steiger P et al (1992) Age-related decrements in bone mineral density in women over 65. J Bone Miner Res 7(6):625–632
Friberg B et al (2001) Branemark implants and osteoporosis: a clinical exploratory study. Clin Implant Dent Relat Res 3(1):50–56
Minsk L, Polson AM (1998) Dental implant outcomes in postmenopausal women undergoing hormone replacement. Compend Contin Educ Dent 19(9):859–862, 864; quiz 866
August M et al (2001) Influence of estrogen status on endosseous implant osseointegration. J Oral Maxillofac Surg 59(11):1285–1289, discussion 1290–1
Baron R, Tross R, Vignery A (1984) Evidence of sequential remodeling in rat trabecular bone: morphology, dynamic histomorphometry, and changes during skeletal maturation. Anat Rec 208(1):137–145
Botticelli D et al (2005) Bone regeneration at implants with turned or rough surfaces in self-contained defects. An experimental study in the dog. J Clin Periodontol 32(5):448–455
Pazzaglia UE (1996) Periosteal and endosteal reaction to reaming and nailing: the possible role of revascularization on the endosteal anchorage of cementless stems. Biomaterials 17(10):1009–1014
Szulc P et al (2006) Bone fragility: failure of periosteal apposition to compensate for increased endocortical resorption in postmenopausal women. J Bone Miner Res 21(12):1856–1863
Seeman E (2003) Periosteal bone formation—a neglected determinant of bone strength. N Engl J Med 349(4):320–323
Viera-Negron YE et al (2008) Effect of ovariectomy and alendronate on implant osseointegration in rat maxillary bone. J Oral Implantol 34(2):76–82
Chen BL et al (2011) Comparison of the effects of alendronate sodium and calcitonin on bone-prosthesis osseointegration in osteoporotic rats. Osteoporos Int 22(1):265–270
Duarte PM et al (2005) Alendronate therapy may be effective in the prevention of bone loss around titanium implants inserted in estrogen-deficient rats. J Periodontol 76(1):107–114
Hughes DE et al (1995) Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res 10(10):1478–1487
Shi HF et al (2010) Low-magnitude high-frequency vibration treatment augments fracture healing in ovariectomy-induced osteoporotic bone. Bone 46(5):1299–1305
Rubin C et al (2002) Mechanical strain, induced noninvasively in the high-frequency domain, is anabolic to cancellous bone, but not cortical bone. Bone 30(3):445–452
Chen B et al (2012) Low-magnitude high-frequency loading via whole body vibration enhances bone-implant osseointegration in ovariectomized rats. J Orthop Res 30(5):733–739
Westerlind KC et al (1997) Estrogen regulates the rate of bone turnover but bone balance in ovariectomized rats is modulated by prevailing mechanical strain. Proc Natl Acad Sci U S A 94(8):4199–4204
Judex S, Donahue LR, Rubin C (2002) Genetic predisposition to low bone mass is paralleled by an enhanced sensitivity to signals anabolic to the skeleton. FASEB J 16(10):1280–1282
Coughlin TR, Niebur GL (2012) Fluid shear stress in trabecular bone marrow due to low-magnitude high-frequency vibration. J Biomech 45(13):2222–2229
Rubinacci A et al (2008) Ovariectomy sensitizes rat cortical bone to whole-body vibration. Calcif Tissue Int 82(4):316–326
Pilliar RM et al (1979) Bone ingrowth and stress shielding with a porous surface coated fracture fixation plate. J Biomed Mater Res 13(5):799–810
Chang PC, Lang NP, Giannobile WV (2010) Evaluation of functional dynamics during osseointegration and regeneration associated with oral implants. Clin Oral Implants Res 21(1):1–12
Acknowledgments
This study was funded by the Fund for Scientific Research—Flanders (FWO) (G.0726.09).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chatterjee, M., Hatori, K., Duyck, J. et al. High-frequency loading positively impacts titanium implant osseointegration in impaired bone. Osteoporos Int 26, 281–290 (2015). https://doi.org/10.1007/s00198-014-2824-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-014-2824-0