Abstract
Summary
Zolpidem is a representative of non-benzodiazepine hypnotics. Recent epidemiologic studies have reported increased fracture risk in patients taking zolpidem, but the results have been inconsistent. The present meta-analysis shows that the use of zolpidem is associated with an increased risk of fractures.
Purpose
Previous studies have reported inconsistent findings regarding the association between the use of zolpidem and the risk of fractures. We performed a systematic literature review and meta-analysis to assess the association.
Methods
We identified relevant studies by searching MEDLINE, EMBASE, Cochrane Library, and PsycINFO without language restrictions (until August 2014). Methodological quality was assessed based on the Newcastle-Ottawa Scale (NOS).
Results
A total of 1,092,925 participants (129,148 fracture cases) were included from 9 studies (4 cohort, 4 case-control, and 1 case-crossover study). Overall, the use of zolpidem was associated with an increased risk of fracture (relative risk [RR] 1.92, 95 % CI 1.65–2.24; I 2 = 50.9 %). High-quality subgroups (cohort studies, high NOS score, adjusted for any confounder, or adjusted for osteoporosis) had higher RRs than the corresponding low-quality subgroups (high quality, 1.94–2.76; low quality, 1.55–1.79). Of note, the risk for hip fracture was higher than that for fracture at any site (hip fracture, RR 2.80, 95 % CI 2.19–3.58; fracture at any site, RR 1.84, 95 % CI 1.67–2.03; P < 0.001).
Conclusions
The use of zolpidem may increase the risk of fractures. Clinicians should be cautious when prescribing zolpidem for patients at high risk of fracture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insomnia is a common condition in the adult population [1]. In the past, benzodiazepines were the major class of drugs prescribed for treating insomnia. However, the development of addiction with long-term use and accidents due to residual hypnotic effects led to serious social issues related to these drugs [2]. Thereafter, since 1990, the non-benzodiazepine hypnotics, known as Z-drugs (zolpidem, zaleplon, zopiclone, and eszopiclone), were developed and have dominated the market [3]. Zolpidem, a representative of these new hypnotics, is the most popular medicine to treat insomnia in the USA [4] and many Asian countries [5, 6]. It has potential advantages over benzodiazepines, including quick onset (within 15 min), short half-life (2–3 h), relatively preserved sleep architecture, and less tolerance [7].
However, by interacting with the γ-aminobutyric acid type A (GABAA) receptor in the thalamus, zolpidem exhibits common central nervous system (CNS) side effects such as dizziness, confusion, poor motor coordination, and postural imbalance [8]. In particular, residual, or hangover, effects may persist into the day following nighttime administration [7]. These characteristics can increase the risk of accidents, notably falls and subsequent fractures [9]. Since the first report on the risk of fracture associated with the use of zolpidem in 2001 [10], Bakken et al. [11] and many other investigators have studied this issue directly and indirectly [12–19]. However, the results have been inconsistent, with some studies finding that zolpidem use is associated with an increased risk of fractures [10, 12–17] and others finding no significant association [18, 19].
Thus, we aimed to investigate the association between zolpidem use and risk of fractures through a comprehensive literature review and meta-analysis.
Materials and methods
Search strategy and data sources
For this systematic review, we adhered to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [20, 21]. We searched MEDLINE (PubMed), EMBASE, Cochrane Library, and PsycINFO from their inception to July 20, 2015, without any language restrictions. We also searched bibliographies of relevant articles to identify additional studies. The following keywords or corresponding Medical Subject Heading (MeSH) terms were used: “zolpidem” or “non-benzodiazepine” or “hypnotics” as the exposure factors and “fracture” as the outcome factor.
Study selection
We included studies that published original data relevant to a possible association between the use of zolpidem or non-benzodiazepine and incident bone fractures. We included any study that met all of the following criteria: randomized controlled trial, cohort study, case-control study, or case-crossover study. We investigated the association between the use of zolpidem and the risk of fractures and quantified the outcome with odds ratios (ORs), relative risks (RRs), hazard ratios (HRs), and corresponding 95 % confidence intervals (CIs). If studies provided ORs/RRs/HRs related to, but not exclusive to, zolpidem (i.e., ORs/RRs/HRs for Z-hypnotics or non-benzodiazepine hypnotics), we contacted the authors for relevant information. Two investigators (SMP and JR) independently evaluated the eligibility of all the studies retrieved from the databases based on predetermined selection criteria. We resolved any disagreements either by mutual discussion or by consulting a third investigator.
Data extraction and quality assessment
We extracted data from the selected articles on the following items: name of first author, publication year, country where the study was conducted, study design, study population and baseline characteristics, type of outcome (fracture of the hip or any site), ORs/RRs/HRs with CIs, and adjusted covariates. When a study presented estimates of fracture risk according to the defined daily dose (DDD) of zolpidem (that is, the dose of the drug that a person uses on average in 1 day), we recalculated the RR corresponding to 1 DDD by estimating the dose-response slope [22, 23]. We assessed the methodological quality based on the Newcastle-Ottawa Scale (NOS), which ranges from 0 to 9, with high quality defined as a NOS score ≥7 [24].
Main and subgroup analysis
We investigated the association between the use of zolpidem and the risk of fracture using adjusted data, if available. When the RRs for overall and individual anatomical sites of fractures were presented, the overall data were used in the main analysis. We performed subgroup analysis by type of study design (cohort, case-control, or case-crossover study), sex (male or female), location (Europe/USA or Asia), latitude (25–34° N, 35–44° N, or 45–60° N), methodological quality (high or low), adjustment for any confounder (crude or adjusted), adjustment for osteoporosis (unadjusted vs. adjusted), and anatomical fracture site (hip vs. any). We also assessed the pooled results by applying different exclusion criteria (elderly group ≥65 years old and community-based population).
Sensitivity analysis
Robustness of the pooled results was examined by subtracting the results of each published study in turn. In addition, the influence of unpublished data received by author contact was tested by adding it to the pooled estimates.
Statistical analysis
We computed the pooled RR and 95 % CI from adjusted ORs/RRs and 95 % CIs reported in the studies. We assumed that the ORs approximated RRs because the incidence of the outcomes of interest was sufficiently rare (<5 % per year in Asia and Latin America) [25]. For studies that reported outcomes stratified by subgroups only, the overall effect size across the subgroups was estimated by the inverse-variance weighted estimation method [26]. To quantify the heterogeneity across studies (overall and within-group heterogeneity), we computed the Higgins I 2 value, which measures the percentage of total variance in the summary estimate due to between-study heterogeneity [27]. Based on the Higgins I 2 value, heterogeneity was divided into two categories (low, <50; high, ≥50). Differences between subgroups (between-subgroup heterogeneity) were assessed using Q statistics; a P value of <0.10 was considered statistically significant. Given the heterogeneity of the study and population characteristics, we calculated the summary effect using the DerSimonian-Laird method for random-effects models [28]. We examined the publication bias by visual inspection of the asymmetry of a funnel plot, in which log RRs were plotted against their standard errors. The publication bias was also assessed statistically by Begg’s test [29] and Egger’s test [30]. All analyses were performed using Stata software version 13 (StataCorp LP, College Station, TX, USA).
Results
Literature search
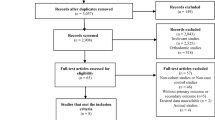
Figure 1 shows the strategy used to identify the relevant studies for meta-analysis. After applying the search strategy, 661 potentially relevant citations were identified in our initial literature search. This was reduced to 601 articles after excluding duplication. After screening the titles and abstracts, 551 studies were excluded, mainly because they were not relevant to our analysis. After reviewing the full texts, nine studies met the inclusion criteria [10, 12–19]. A flow chart showing the study selection is presented in Fig. 1. No additional studies were identified through our manual search of references from published studies. We contacted the authors of seven articles to ask for data exclusive to zolpidem use [6, 11, 31–35]; the author of one article provided recalculated results with limited information, which we included only in the sensitivity analyses [11]. Table 1 summarizes the main characteristics of the nine published studies that were included in the analysis.
Study characteristics
The selected studies were published between 2001 and 2014. Of these, four were retrospective cohort studies [12, 14, 16, 17], four were case-control studies [10, 15, 18, 19], and one was a case-crossover study [13]. All nine studies reported sex-mixed data, and three studies provided data separately for men and women [12–14]. The cohort studies achieved relatively high scores on the quality assessment (6–8 in total); conversely, the case-control studies achieved relatively low scores (4–7 in total) (Table 2). Geographically, the studies were distributed relatively evenly across western and eastern countries: three from the USA [10, 12, 16], two from Europe [15, 19], and four from Asia [13, 14, 17, 18]. The potential confounding effect of osteoporosis was adjusted in two studies [12, 14].
Main analysis
Figure 2 shows the pooled estimate of the nine included studies assessing the risk of fracture associated with the use of zolpidem. The use of zolpidem was strongly correlated with the risk of fracture with borderline high heterogeneity (RR 1.92, 95 % CI 1.65–2.24; I 2 = 50.9 %).
Subgroup analysis
We performed subgroup analysis by type of study design, methodological quality, zolpidem medication, ethnicity, latitude, fracture site, age, drug interval from the index date, medication duration, and osteoporosis. Overall, an increase in fracture risk associated with zolpidem use was observed in all subgroups. The heterogeneity of subgroups was low (I 2 < 50 %) when studies were divided by sex and with adjustment for a diagnosis of osteoporosis, and heterogeneity decreased greatly (I 2 ≤ 7 %) when the subgroup analysis was carried out with adjustment for a diagnosis of osteoporosis. In contrast, subgroup analysis by other variables did not change the heterogeneity substantially.
Based on between-group heterogeneity, no significant difference was found between the RRs for the subgroups except in the diagnosis of osteoporosis. Analyses focused on the anatomical fracture site also did not reveal a substantial difference in the pooled RRs between studies examining hip fracture and those examining fracture at any site (hip fracture, RR 2.06, 95 % CI 1.40–3.04; fracture at any site, RR 1.84, 95 % CI 1.66–2.03; P = 0.30); however, when two studies with unadjusted results were excluded, the difference was statistically significant (hip fracture, RR 2.80, 95 % CI 2.19–3.58; fracture at any site, RR 1.84, 95 % CI 1.67–2.03; P < 0.001).
Of note, we found that the subgroups with high methodological quality, such as cohort studies, NOS >7, adjustment for any confounder, and adjustment for diagnosis of osteoporosis, had higher RRs than the corresponding low-quality subgroups (high quality, 1.94–2.76; low quality, 1.55–1.79).
Restricting the analysis to the selected groups of elderly (>65 years old) or community-based populations did not produce a substantial change in the summary RR (1.73–1.91) (Table 3).
Sensitivity analysis
By excluding each study in turn, the pooled RRs held their significance without substantial change (range 1.80–1.95) (detailed data not shown). When including unpublished data from the study by Bakken et al. [11], the pooled RR remained similar and significant (RR 1.78, 95 % CI 1.36–2.34).
Publication bias
Figure 3 presents the funnel plot of log RR versus variance of log RR for all the studies included in the meta-analysis. Publication bias was not suspected from visualization of the funnel plot, Begg’s test (P = 0.75), or Egger’s test (P = 0.85).
Discussion
Our results suggest that the zolpidem use is associated with a 92 % increased risk of fracture. This increased risk was consistent in various subgroup and sensitivity analyses. If we assume that 139 cases of fracture occur for every 100,000 person-years not receiving zolpidem [14], and if we assume a 1.92-fold increased risk of fracture due to zolpidem, as determined in this study, an additional 127 cases of fracture can be expected for every 100,000 recipients of these drugs annually (the 1-year number needed to harm = 747).
In subgroup and sensitivity analyses, as expected, high heterogeneity was observed. This is plausible because these individual studies were conducted among populations with differences in factors such as dose and duration of zolpidem use, medical conditions, and bone health, which were not adjustable by current information. However, our result was robust in various circumstances. Especially in the osteoporosis-focused subgroup analysis, heterogeneity was very low (Table 2). This is very likely because a large body of evidence has shown that osteoporosis is one of the most influencing factors for fractures. Interestingly, as indicated in the results, the subgroups with high methodological quality revealed higher RRs than did low-quality subgroups. This observation suggests that zolpidem use may elevate the risk of fractures much more than expected in the overall analysis.
Regarding the association between zolpidem use and fracture risk, most researchers have proposed CNS side effects by zolpidem as the main mechanism [10, 12–14, 17]. Zolpidem can cause drowsiness, dizziness, poor posture control, and lack of coordination by modulating the GABAA receptor [8]. These common zolpidem-related CNS side effects may contribute to falling and subsequent fracture, especially in the hip area [10, 12, 14]. In our subgroup analysis by anatomical site, hip fracture tended to occur more commonly than fracture at any site. Given that the term “fracture at any” also includes hip fractures, a more differentiated analysis (hip vs. non-hip) may potentiate the anatomical difference of fracture risk, which also supports our hypothesis. Moreover, a recent meta-analysis revealed that benzodiazepine, another class of GABAA receptor modulator, was associated with excess fracture risk [36], which is in line with our finding. On the other hand, this association may be confounded by underlying insomnia. Insomnia can impair daytime function and psychological status and, therefore, is likely to increase the risk of accidental falls and fractures [37, 38].
However, there are plausible explanations that support the causal association between zolpidem use and fracture risk. First, there is a dose-dependent relationship between them. Based on a study by Vestergaard et al. [15], increasing the dose of zolpidem from <0.1 to >0.25 DDD was associated with a 13 % increase in fracture risk. Second, a significant decline in the risk of fracture was observed with time after initial prescription of zolpidem [12, 14]. Other data showed that the fracture risk is greatest in the first few weeks following prescription of zolpidem and declines thereafter. These phenomena may be explained by the initial expression and subsequent downregulation of the zolpidem receptor, which also supports our findings. Third, some classes of medications, which are not prescribed as hypnotics (i.e., not related to insomnia), increase the risk of fractures owing to CNS-related side effects. For example, antimuscarinic agents, usually prescribed for those with overactive bladder, have drowsiness as a common side effect and increase the risk of fractures [39]. This is also in accordance with our results and supports an independent biologic mechanism linking zolpidem use and fracture risk, excluding the confounding effect of insomnia.
Considering all these findings together, the use of zolpidem most likely increases the risk for falls and fractures. Taking into account the high prevalence of insomnia (particularly in the elderly) and the huge social costs of fractures, the risk of fractures in zolpidem users may be an important public health issue [1, 4, 40]. Clinicians should, therefore, carefully prescribe zolpidem, especially in patients at high risk of fracture. In addition, individuals taking zolpidem should receive relevant education to prevent fall-related fractures. Regular bone health monitoring should be considered for chronic zolpidem users. Patients prescribed other Z-hypnotics should also be evaluated for fall-related injuries such as head trauma and fractures.
Our study has some limitations. First, observational studies have the potential for bias owing to uncontrolled confounders. To compensate for this limitation, we conducted subgroup analyses according to various components. Second, many other possible confounders (e.g., bone mineral density, medications, alcohol consumption, and comorbidities) were not fully accounted for in several studies. Third, the exposure to zolpidem was not accurate in some studies that assessed the exposure only using medical records, which may impair the precision of our results. Fourth, because of limited data, the interactions between zolpidem use and other factors (e.g., age category or the use of other psychotropics) were not examined.
Despite these limitations, our study has several strengths. First, to the best of our knowledge, the present study is the first systematic review and meta-analysis to investigate the correlation between the use of zolpidem and the risk of fractures. Second, we specifically analyzed the association between zolpidem and fracture by stratification according to the type of study design, sex, geographic location, latitude, methodological quality, adjustment for any confounder, adjustment for osteoporosis, and anatomical fracture site. Third, there was little evidence of publication bias by visual inspection and statistical analyses. Along with these strengths, the overall estimates were consistent and significant in all subgroup and sensitivity analyses, and the association was stronger in the analyses confined to studies with high methodological quality, all of which make our study more noteworthy.
Our results show that the use of zolpidem may increase the risk of fractures. Clinicians should carefully prescribe zolpidem and consider bone health monitoring in those patients vulnerable to falls and fractures. Additional large-scale prospective studies are warranted to confirm our findings and to evaluate the interactions between zolpidem use and fracture-related factors. Patients prescribed other Z-hypnotics should be investigated for the risk of fractures.
References
Pandey S, Phillips BA (2015) Why is the prevalence of insomnia skyrocketing? And what can be done about it? Sleep Med 16:555–556
Gustavsen I, Brarnness JG, Skurtveit S, Engeland A, Neutel I, Morland J (2008) Road traffic accident risk related to prescriptions of the hypnotics zopiclone, zolpidem, flunitrazepam and nitrazepam. Sleep Med 9:818–822
Huedo-Medina TB, Kirsch I, Middlemass J, Klonizakis M, Siriwardena AN (2012) Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ 345, e8343
Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A (2007) Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res 22:465–475
Hsiao F-Y, Hsieh P-H, Gau C-S (2013) Ten-year trend in prescriptions of z-hypnotics among the elderly: A nationwide, cross-sectional study in Taiwan. J Clin Gerontol Geriatr 4:37–41
Tamiya H, Yasunaga H, Matusi H, Fushimi K, Ogawa S, Akishita M (2015) Hypnotics and the occurrence of bone fractures in hospitalized dementia patients: a matched case-control study using a national inpatient database. PLoS One 10, e0129366
Gunja N (2013) In the Zzz zone: the effects of Z-drugs on human performance and driving. J Med Toxicol 9:163–171
Greenblatt DJ, Roth T (2012) Zolpidem for insomnia. Expert Opin Pharmacother 13:879–893
Vermeeren A (2004) Residual effects of hypnotics: epidemiology and clinical implications. CNS Drugs 18:297–328
Wang PS, Bohn RL, Glynn RJ, Mogun H, Avorn J (2001) Zolpidem use and hip fractures in older people. J Am Geriatr Soc 49:1685–1690
Bakken MS, Engeland A, Engesaeter LB, Ranhoff AH, Hunskaar S, Ruths S (2014) Risk of hip fracture among older people using anxiolytic and hypnotic drugs: a nationwide prospective cohort study. Eur J Clin Pharmacol 70:873–880
Finkle WD, Der JS, Greenland S, Adams JL, Ridgeway G, Blaschke T, Wang Z, Dell RM, Vanriper KB (2011) Risk of fractures requiring hospitalization after an initial prescription for zolpidem, alprazolam, lorazepam, or diazepam in older adults. J Am Geriatr Soc 59:1883–1890
Kang DY, Park S, Rhee CW, Kim YJ, Choi NK, Lee J, Park BJ (2012) Zolpidem use and risk of fracture in elderly insomnia patients. J Prev Med Publ Health 45:219–226
Lin FY, Chen PC, Liao CH, Hsieh YW, Sung FC (2014) Retrospective population cohort study on hip fracture risk associated with zolpidem medication. Sleep 37:673–679
Vestergaard P, Rejnmark L, Mosekilde L (2008) Anxiolytics and sedatives and risk of fractures: effects of half-life. Calcif Tissue Int 82:34–43
Winkelmayer WC, Mehta J, Wang PS (2007) Benzodiazepine use and mortality of incident dialysis patients in the United States. Kidney Int 72:1388–1393
Chung SD, Lin CC, Wang LH, Lin HC, Kang JH (2013) Zolpidem use and the risk of injury: a population-based follow-up study. PLoS One 8, e67459
Chang CM, Wu ECH, Chang IS, Lin KM (2008) Benzodiazepine and risk of hip fractures in older people: a nested case-control study in Taiwan. Am J Geriatr Psychiatry 16:686–692
Pierfitte C, Macouillard G, Thicoipe M et al (2001) Benzodiazepines and hip fractures in elderly people: case-control study. BMJ 322:704–708
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62:1006–1012
Tang N, Wu Y, Ma J, Wang B, Yu R (2010) Coffee consumption and risk of lung cancer: a meta-analysis. Lung Cancer 67:17–22
Wu Q, Bencaz AF, Hentz JG, Crowell MD (2012) Selective serotonin reuptake inhibitor treatment and risk of fractures: a meta-analysis of cohort and case-control studies. Osteoporos Int 23:365–375
Wells GA, Shea BO, Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm Accessed 19 Sep 2015
Cooper C, Campion G, Melton LJ 3rd (1992) Hip fractures in the elderly: a world-wide projection. Osteoporos Int 2:285–289
Walter SD, Cook RJ (1991) A comparison of several point estimators of the odds ratio in a single 2 x 2 contingency table. Biometrics 47:795–811
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Berry SD, Lee Y, Cai S, Dore DD (2013) Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents. JAMA Intern Med 173:754–761
Cappelle S, Ramon I, Dekelver C, Paesmans M, Moreau M, Bergmann P, Karmali R, Peretz A, Rozenberg S, Body JJ (2013) Distribution of clinical risk factors for fracture in a brussels cohort: an interim-analysis of the frisbee study. Osteoporos Int 24:S176
Chung CH (2014) The insomnia treatment, drug usage and their side effects in Taiwan: analysis of 2009-2011 Nationwide Health Insurance Database. Pharmacoepidemiol Drug Saf 23:S88–89
Thorell K, Ranstad K, Midlov P, Borgquist L, Halling A (2014) Is use of fall risk-increasing drugs in an elderly population associated with an increased risk of hip fracture, after adjustment for multimorbidity level: a cohort study. BMC Geriatr 14:131
Vestergaard P, Prieto-Alhambra D, Javaid MK, Cooper C (2013) Fractures in users of antidepressants and anxiolytics and sedatives: effects of age and dose. Osteoporos Int 24:671–680
Xing D, Ma XL, Ma JX, Wang J, Yang Y, Chen Y (2014) Association between use of benzodiazepines and risk of fractures: a meta-analysis. Osteoporos Int 25:105–120
Koski K, Luukinen H, Laippala P, Kivela SL (1998) Risk factors for major injurious falls among the home-dwelling elderly by functional abilities. A prospective population-based study. Gerontology 44:232–238
Avidan AY, Fries BE, James ML, Szafara KL, Wright GT, Chervin RD (2005) Insomnia and hypnotic use, recorded in the minimum data set, as predictors of falls and hip fractures in Michigan nursing homes. J Am Geriatr Soc 53:955–962
Moga DC, Carnahan RM, Lund BC, Pendergast JF, Wallace RB, Torner JC, Li Y, Chrischilles EA (2013) Risks and benefits of bladder antimuscarinics among elderly residents of Veterans Affairs Community Living Centers. J Am Med Dir Assoc 14:749–760
Cho YW, Shin WC, Yun CH, Hong SB, Kim J, Earley CJ (2009) Epidemiology of insomnia in Korean adults: prevalence and associated factors. J Clin Neurol 5:20–23
Acknowledgments
This work was supported by Wonkwang University in 2015.
Author contributions
SM Park and J Ryu were responsible for the initial plan, study design, data collection, data extraction, data interpretation, manuscript drafting, and conducting the study. D Shin was responsible for analyzing the data. J Lee was responsible for data extraction and critical revision of the manuscript. JM Yoon was responsible for critical revision of the manuscript for important intellectual content. DR Lee was responsible for the initial plan, study design, data interpretation, manuscript drafting, supervision, and critical revision of the manuscript for important intellectual content. The content of this manuscript is solely the responsibility of the authors. DR Lee is the guarantor for this manuscript and has full responsibility for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Sang Min Park, Jihye Ryu, Dong Ryul Lee, Doosup Shin, Jae Moon Yun, and Jungun Lee declare that they have no conflicts of interest.
Additional information
Sang Min Park and Jihye Ryu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Park, S.M., Ryu, J., Lee, D.R. et al. Zolpidem use and risk of fractures: a systematic review and meta-analysis. Osteoporos Int 27, 2935–2944 (2016). https://doi.org/10.1007/s00198-016-3605-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3605-8