Abstract
Summary
This manuscript describes the role of low vitamin D in bone metabolism of Klinefelter subjects. Low vitamin D is frequent in this condition and seems to be more important than testosterone in inducing low bone mineral density (BMD) and osteoporosis. Supplementation with vitamin D restores BMD after 2 years of treatment, whereas testosterone alone seems to be ineffective.
Introduction
Decreased bone mineral density (BMD) in Klinefelter syndrome (KS) is frequent, and it has been traditionally related to low testosterone (T) levels. However, low BMD can be observed also in patients with normal T levels and T replacement therapy does not necessarily increase bone mass in these patients. Nothing is known about vitamin D levels and supplementation in KS. In this study, we determine vitamin D status and bone mass in KS subjects and compare the efficacy of T therapy and vitamin D supplementation on BMD.
Methods
A total of 127 non-mosaic KS patients and 60 age-matched male controls were evaluated with reproductive hormones, 25-hydroxyvitamin D, PTH, and bone densitometry by dual-energy X-ray absorptiometry (DEXA). Patients with hypogonadism and/or 25-hydroxyvitamin D deficiency were treated with T-gel 2 % and/or calcifediol and re-evaluated after 24 months of treatment.
Results
25-hydroxyvitamin D levels were significantly lower in KS patients with respect to controls, and they had significantly lower lumbar and femoral BMD. The percentage of osteopenia/osteoporosis in subjects with 25-hydroxyvitamin D deficiency was higher with respect to subjects with normal 25-hydroxyvitamin D and was not related to the presence/absence of low T levels. Subjects treated with calcifediol or T + calcifediol had a significant increase in lumbar BMD after treatment. No difference was found in T-treated group.
Conclusions
These data highlight that low 25-hydroxyvitamin D levels seem to have a more critical role than low T levels in inducing low BMD in KS subjects. Furthermore, vitamin D supplementation seems to be more effective than T replacement therapy alone in increasing BMD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Klinefelter syndrome (KS) is the most common sex chromosomal disorder, with a prevalence of 1:600, and it is a frequent form of male hypogonadism and infertility [1]. The clinical features of KS commonly include hypergonadotropic hypogonadism, gynecomastia, small testes, and azoospermia [2], but KS may be associated also with an increased risk of systemic diseases including malignancies, venous thromboembolism [3], diabetes, cardiovascular diseases [4, 5], and osteoporosis [6].
In particular, reduced bone mass affects 25–48 % of cases and full-blown osteoporosis is present in 6–15 % of cases [7–9]. Alterations in KS bone mass have an early onset, with a decreased pubertal peak bone mineral density (BMD) and accelerated bone loss during adulthood [7], and are attributable to both reduced bone formation and higher bone resorption [10].
Reduced bone mass in KS has been usually ascribed to low testosterone (T) plasma level [7, 8], but its pathogenetic role is not well-established [11], and conflicting data are reported about this hypothesis [11–16]. In particular, the prevalence of low BMD is similar in KS men with low and normal T levels [9, 15] and even prolonged androgen replacement therapy may not reverse decreased bone mass in men with KS, low T levels and low bone mass [6, 8, 16].
Vitamin D levels might be another possible modulator of bone metabolism in KS. Vitamin D is a key regulatory factor of bone mineralization and calcium homeostasis in both men and women [17]. In order to be biologically active, vitamin D must be converted to its active form, 1,25-dihydroxyvitamin D3, by two sequential hydroxylation steps catalyzed by 25-hydroxylase and 1α-hydroxylase. In the presence of inadequate vitamin D status, calcium absorption is lower than optimal and there is a compensatory increase in PTH levels (secondary hyperparathyroidism), with a subsequent stimulation of bone reabsorption and accelerated bone loss. The high bone turnover associated with elevated PTH levels is characterized by a lower degree of mineralization [18].
It has been demonstrated that the male reproductive tract expresses most of the enzymes involved in vitamin D metabolism. In particular, the testis is featured by the highest expression of CYP2R1, a member of cytochrome P450 family [19], considered to be a key enzyme of vitamin D activation through its 25-hydroxylase activity [20].
The physiological importance of CYP2R1 expression in the testis has been highlighted in the past few years in studies that suggested a pathophysiological link between testicular damage, reduced levels of 25-hydroxyvitamin D, and reduced bone mass [21]. Although definitive data are not available, there is an increasing evidence suggesting that an impairment of testicular function leads to low levels of 25-hydroxyvitamin D and consequently to an increased risk of osteopenia and osteoporosis [21].
No studies evaluated the possible role of vitamin D levels and vitamin D supplementation in bone metabolism in KS. The aim of this study was to determine vitamin D status and bone mass in a cohort of KS subjects and to compare the efficacy of T therapy and vitamin D supplementation on BMD.
Materials and methods
Subjects
We retrospectively studied 127 non-mosaic KS patients (mean age 31.5 ± 8.5 years; range 20–53 years), diagnosed at the Centre for Human Reproduction Pathology at the University of Padova from January 2007 to November 2010 after referral for fertility problems or testicular hypotrophy. As controls, 60 age-matched healthy (good general condition) and fertile (who recently fathered) males (mean age 30.9 ± 8.4 years; range 19–45 years) were recruited through an andrological screening program. The study was approved by the Hospital Ethics Committee, and each participant gave his written informed consent. All subjects (patients and controls) underwent peripheral karyotype analysis, evaluating at least 50 peripheral blood lymphocyte metaphases, and had never received T or vitamin D substitution at the time of evaluation.
Patients with more than one supernumerary X chromosome, mosaicisms, or with any endocrine dysfunction different from hypogonadism, and subjects assuming any drug were excluded from the study.
Subjects’ evaluation included complete medical history (pubertal history, lifestyle, physical activity, smoking, alcohol misuse), physical examination (weight, height, body mass index (BMI), waist circumference, arm span), lipid and glucose metabolism (total cholesterol, HDL, triglycerides, fasting glucose), hemochrome, serum insulin, and PSA.
Serum levels of total T, estradiol, LH, FSH, PTH, and 25-hydroxyvitamin D were measured in all subjects, and 25-hydroxyvitamin D levels were evaluated in the same period of the year between November and February.
Homeostasis model assessment for insulin resistance (HOMA-IR) was calculated as fasting insulin (μIU ml)−1 × fasting glucose (mmol l)−1⁄22.5.
Hypogonadism was defined as total testosterone <10.4 nmol/l [22]. Vitamin D deficiency was defined as 25-hydroxyvitamin D levels <50 nmol/l [23].
Measurement of bone densitometry was done by dual-energy X-ray absorptiometry (DEXA) using a Hologic QDR 4500 C densitometer (Hologic, Waltham, MA, USA) in the femoral neck and lumbar spine (L1–L4), by the same technician, and a spine phantom was used before each exam. The mean BMD index (total BMD CV was 1 %) and the mean T-score were considered. T-score was calculated as the number of SD that the bone mineral density (BMD) was above or below the mean for young healthy adults of the same race and gender. Low bone mass was defined based on T-score (−2.5 SD < score <−1 SD for osteopenia; score ≤−2.5 SD for osteoporosis) [24].
Treatment
The treatment protocol included T supplementation when T levels were <8 nmol/l or between 8 and 12 nmol/l associated with hypogonadal symptoms (n 39) and calcifediol supplementation when 25-hydroxyvitamin D levels were <50 nmol/l (n 21). Combined treatment was used for men with low T and 25-hydroxyvitamin D levels (n 39). Data presented here refer to 34 patients for whom complete data were obtained after 2 years of treatment, including 12 treated with T, eight treated with calcifediol, and 14 treated with both T and calcifediol. T treatment was performed with T-gel 2 % (Tostrex®, ProStrakan), dose-adapted to maintain plasma T levels between 12 and 26 nmol/l. Calcifediol treatment was performed with Didrogyl (Bruno Farmaceutici), from a starting dose of 4000 IU/week, adjusting the dosage to maintain 25-hydroxyvitamin D levels above 50 nmol/l, by determining the levels every 6 months. Total T, estradiol, LH, FSH, 25-hydroxyvitamin D, PTH, hemochrome, PSA, bone-specific alkaline phosphatase (BAP), and carboxyl-terminal telopeptide of collagen type I (ICTP), and DEXA were compared before and after treatment.
Hormone assays
Blood was collected in the fasting state between 0800 and 1000 hours. Serum FSH, LH, total T, and estradiol were evaluated by commercial electrochemiluminescence immunoassay methods (Elecsys 2010, Roche Diagnostics, Mannheim, Germany). For all parameters, the intraassay and interassay coefficients of variation were 8 and 10 %, respectively. PTH serum levels were determined with a direct, two-site, sandwich-type chemiluminescent immunoassay (LIAISON N-TACT PTH, DiaSorin Inc., Stillwater, MN, USA). 25-hydroxyvitamin D was determined with direct, competitive chemiluminescent immunoassay (LIAISON 25 OH Vitamin D TOTAL Assay, DiaSorin Inc.). Bone-specific alkaline phosphatase plasma levels were determined with OCTASE, a paramagnetic particle-linked chemiluminescent immunoassay (Access Immunoassay System Beckmann Coulter, Roma, Italy). ICTP were quantified by electrochemiluminescent immunoassay (CrossLaps/serum, Cobas, Roche diagnostics, Mannheim, Germany). All determinations were performed according to manufacturer’s instructions.
Statistical analysis
Data in the tables are reported as mean ± SD of the mean. Data in the figures are reported as median and interquartile range. Differences between KS patients and controls were evaluated by unpaired two-sided Student’s t test and Wilcoxon sum of ranks test, where appropriate. Relationships between continuous variables were assessed using linear regression model with partial correlation corrected for BMI, and Pearson correlation coefficients are reported in figures. Differences between subgroups of KS patients were analyzed by ANOVA using BMI as covariate. Differences between basal and post-therapy were evaluated by paired two-sided Student’s t test. Regression analysis for the relation between the change in 25-hydroxyvitamin D levels and the change in T levels vs the change in BMD after correction for BMD was performed to assess the difference in BMD increase after the different treatment. Comparisons between the percentages were performed with χ 2 test. The significance level was set to P < 0.05.
All tests were performed with SAS version 9.2 (SAS Institute, Cary, NC, USA) for Windows.
Results
Table 1 reports that KS patients had, as expected, significantly higher weight, waist circumference, BMI, gonadotropins, estradiol, and LH/T ratio and significantly reduced levels of total T and estradiol/T ratio with respect to controls.
Age, height, arm span, PSA, and metabolic parameters (total and HDL cholesterol, triglycerides, glycemia) did not show significant differences between patients and controls. Only insulin and HOMA-IR were significantly higher in KS patients with respect to controls.
25-hydroxyvitamin D plasma levels were significantly lower and PTH significantly higher in KS patients with respect to controls (P < 0.0001).
DEXA scans showed a significantly lower BMD at both lumbar and femoral sites in KS patients with respect to controls. T-score was also lower, both at lumbar and femoral site. Out of 127 KS patients, 10/127 (7.9 %) had osteoporosis, 50/127 (39.4 %) osteopenia, and 67/127 (52.8 %) normal BMD.
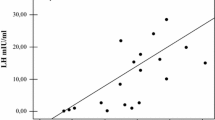
Since T levels correlated negatively with BMI (r = −0.53, P < 0.0001), the relationship between 25-hydroxyvitamin D levels, T, and BMD was corrected for BMI. This analysis showed that in KS, 25-hydroxyvitamin D was positively related to lumbar and femoral BMD and T (Fig. 1). On the contrary, no relation was found between lumbar and femoral BMD with T or LH/T ratio (data not shown). Also in control group, 25-hydroxyvitamin D was positively related to lumbar and femoral BMD (r = 0.32, P < 0.001 and r = 0.35, P < 0.001, respectively, data not shown).
We subgrouped KS patients on the basis of 25-hydroxyvitamin D plasma levels (<50 nmol/l or ≥50 nmol/l) and T levels (<10.4 nmol/l or ≥10.4 nmol/l) (Fig. 2). With respect to KS group with vitamin D ≥50 nmol/l (N = 67/127; 52.8 %), patients with 25-hydroxyvitamin D deficiency (N = 60/127; 47.2 %) had lumbar and femoral BMD (0.96 ± 0.15 vs 1.03 ± 0.14 g/cm2 and 0.96 ± 0.13 vs. 1.04 ± 0.13 g/cm2, respectively; P < 0.05) significantly reduced (Fig. 2). The percentage of patients with osteopenia or osteoporosis was higher in 25-hydroxyvitamin D deficient group (40/60; 66.7 %) with respect to the group of KS patients with vitamin D ≥50 nmol/l (20/67; 29.8 %; P < 0.0001), independently of T levels (30.8 % in men with low T, 28.6 % in men with normal T) (Table 2).
Lumbar and femoral BMD in KS with hypogonadism (T < 10.4 nmol/l) and eugonadism (T ≥ 10.4 nmol/l) and in KS with low (<50 nmol/l) and normal (≥50 nmol/l) 25-hydroxyvitamin D levels. The boxes represent the first and third quartiles; whiskers extend from minimum to maximum, and the square in the boxes represents the median
On the contrary, analysis of subgroups of KS patients with reduced T plasma levels (78/127; 61.4 %) with respect to the subgroup with normal T plasma levels (49/127; 38.6 %) showed no significant difference in lumbar and femoral BMD (0.99 ± 0.15 vs 1.00 ± 0.15 g/cm2 and 0.99 ± 0.13 vs 1.01 ± 0.13 g/cm2, respectively) (Fig. 2). Also, the percentage of patients with osteopenia or osteoporosis was similar between the two considered subgroups: 38/78 in hypogonadal group (48.7 %) vs 22/49 in eugonadal group (44.9 %). After adjusting for BMI, among patients with normal T, those with low vitamin D had significantly reduced lumbar and femoral BMD with respect to those with vitamin D ≥50 nmol/l (Table 2). Among patients with low T levels, those with low vitamin D had significantly reduced femoral BMD with respect to those with vitamin D ≥50 nmol/l (Table 2).
Table 3 reports the data of KS patients treated with T and/or calcifediol. In calcifediol or T + calcifediol groups, we found a significant increase in lumbar BMD and lumbar T- and Z-score after treatment (P < 0.05). On the contrary, in T-treated group, we did not find any significant difference in BMD before and after therapy. Figure 3 shows that the change in lumbar and femoral BMD after treatment with calcifediol was 12.5 and 3.8 % respectively, whereas no change in BMD was observed after treatment with T.
Discussion
This study showed for the first time that in KS subjects, low 25-hydroxyvitamin D levels have a more critical role than low T levels in inducing low BMD in KS subjects. Furthermore, vitamin D supplementation seems to be more effective than T replacement therapy alone in increasing BMD. We found that 25-hydroxyvitamin D levels are significantly lower in KS patients with respect to controls, and they had significantly lower lumbar and femoral BMD. The percentage of osteopenia/osteoporosis in subjects with 25-hydroxyvitamin D deficiency is higher with respect to subjects with normal 25-hydroxyvitamin D and is not related to the presence/absence of low T levels. Subjects treated with calcifediol or T + calcifediol significantly increased lumbar BMD after treatment. On the contrary, no difference was found in T-treated group.
Reduced bone mass in KS has been usually ascribed to low T plasma levels [7, 8]. Hypogonadism, in fact, represents one of the most important causes of male osteoporosis, highlighting the important role of androgens in bone metabolism [25]. T promotes periosteal bone formation during puberty [26] and reduces bone reabsorption during adult life [27]; thus, early onset of T deficiency, as observed in KS, is considered an important risk factor for osteoporosis. However, the pathogenetic role of low T in osteoporosis of KS is not supported by robust data and additional mechanisms might be involved [11–16].
We found no relation between lumbar and femoral BMD with T or LH/T ratio, and lumbar and femoral BMD, T-score at lumbar and femoral sites in KS patients with and without hypogonadism, were similar. Furthermore, the percentage of patients with osteopenia or osteoporosis was similar between the two groups. These results confirm that T is not the key regulator of BMD in KS and are in accordance with previous data [9, 13, 15]. In fact, although a positive correlation between BMD and T concentrations has been demonstrated in normal men and KS subjects with osteoporosis, a considerable variation in the bone phenotype of men with KS is present [13–15]. More specifically, the findings of the present study confirm previous data showing that the prevalence of low BMD is similar in KS men with low and normal T levels [9, 15]. Altogether, these data suggest that bone loss in KS might be, at least in part, independent from the presence of hypogonadism and might be related to other hypothetical genetic (related to the chromosomal abnormality) and hormonal (estradiol, vitamin D) factors.
The function and sensitivity of the androgen receptor (AR) have been also postulated to modulate the effects of T on the bone in KS [25]. The AR gene is located on the X chromosome (and, therefore, it is present in double copy in 47, XXY KS), and a nonrandom X inactivation in men with more than one X chromosome has been reported [28]. Furthermore, the AR gene contains the highly polymorphic CAG repeat, the length of which is inversely correlated with sensitivity to androgens [29]. Although one study found a negative correlation between BMD evaluated by phalangeal ultrasound and the X-weighted biallelic mean of CAG repeats [30], we previously demonstrated that the CAG repeat length is not different between KS patients with normal and low bone mass [9].
Vitamin D is another obvious possible modulator of bone metabolism in KS, but previous studies never considered this parameter. Apart from our previous study [9], only one recent report determined 25-hydroxyvitamin D levels in KS [31]. Together with findings in the present study, these data agree that KS subjects have 25-hydroxyvitamin D levels lower than healthy controls, with mean levels of 50–55 nmol/l.
It has been demonstrated that the male reproductive tract expresses most of the enzymes involved in vitamin D metabolism. In particular, the testis is featured by the highest expression of CYP2R1, a member of cytochrome P450 family [19], considered to be a key enzyme of vitamin D activation from its precursor cholecalciferol through its 25-hydroxylase activity [20]. Patients with hypogonadism and testiculopathy have frequently low levels of 25-hydroxyvitamin D due to a lower expression of this enzyme [21, 32–34]. We also recently showed that the administration of the 25-hydroxylated form of vitamin D (calcifediol), and not the administration of the precursor cholecalciferol, restores 25-hydroxyvitamin D levels in subjects with Leydig cell dysfunction [35]. Furthermore, it reduces PTH levels, the best marker in assessing the effectiveness of supplementation. Therefore, calcifediol supplementation overcomes the decline in testis function and represents the ideal treatment in these cases, likewise in cases with a decline in hepatic function.
On the basis of these data, low 25-hydroxyvitamin D levels in KS subjects could well be interpreted as a consequence to the severe testicular hypotrophy and Leydig cell impairment, which are characteristic signs of these subjects. However, we cannot exclude that low vitamin D status in KS might be simply the results of different lifestyle and/or yet unexplored genetic factors related to the chromosomal abnormality.
We found that lumbar and femoral BMD in KS were positively associated with 25-hydroxyvitamin D, and patients with 25-hydroxyvitamin D deficiency have lumbar and femoral BMD significantly reduced with respect to KS subject with 25-hydroxy vitamin D ≥50 nmol/l. These findings are supported by the significantly higher percentage of patients with osteopenia or osteoporosis among men with 25-hydroxyvitamin D deficiency with respect to KS patients with vitamin D ≥50 nmol/l. These data confirm previous studies in non-KS subjects in which 25-hydroxyvitamin D levels have been found related to BMD [36] and to cortical bone mineral density, total bone mineral content, cortical thickness, and the polar and axial strength strain index at the distal radius and tibia [37].
To better support our data, we also evaluated the efficacy of therapy with T, calcifediol, or combined treatment (T + calcifediol) on BMD in KS. In the T-treated group, we did not find any significant difference in lumbar and femoral BMD, in lumbar and femoral T-score before and after therapy. These data agree with most of the studies that demonstrated that T treatment does not reverse decreased bone mass in these patients [8, 19, 20]. On the contrary, we observed that only calcifediol or T + calcifediol replacement treatments increased BMD after 2 years of supplementation. Although limitations exist in our study (low number of treated subjects, retrospective data, 2 years of follow up), these findings, other than supporting a minor role of low T levels in determining low BMD in KS, further support the key role of vitamin D in bone metabolism. In fact, several trials demonstrated that vitamin D supplementation improves bone density and reduces fractures and falls, above all when baseline levels of 25-hydroxyvitamin D are in the 25 to 40 nmol/l range and furthermore has beneficial impacts on cortical thickness [38–42].
Indeed, the increase in BMD (12.5 % at lumbar spine and 3.8 % at femoral neck) after 2 years of vitamin D supplementation is remarkable. Apart from the very low number of treated cases that does not allow generalizing the results, comparison with other reports is not immediate because most of the data on vitamin D supplementation and BMD are obtained with cholecalciferol and not with calcifediol. Nevertheless, these men represent a special group of patients, represented by young KS with very low vitamin D levels, and we cannot exclude that they might have defects in mineralization (osteomalacia) due to vitamin D deficiency.
In conclusion, although the limitations listed above do not allow having definitive evidence and our findings should be confirmed prospectively in larger groups of KS (and possibly older KS subjects), we suggest that 25-hydroxyvitamin D has a determinant role in bone metabolism of these patients and that adequate supplementation should be considered to prevent bone loss or improve low BMD.
References
Lanfranco F, Kamischke A, Zitzmann M, Nieschlag E (2004) Klinefelter’s syndrome. Lancet 364:273–283
Klinefelter HF, Reifenstein EC, Albright F (1942) Syndrome characterized by gynecomastia, aspermatogenesis without a-Leydigism, and increased excretion of follicle-stimulating hormone. J Clin Endocrinol Metab 2:615–627
Campbell WA, Price WH (1981) Venous thromboembolic disease in Klinefelter’s syndrome. Clin Genet 19:275–80
Swerdlow AJ, Higgin CD, Schomaker MJ, Wright AF, Jacobs PA (2005) Mortality in patients with Klinefelter’s syndrome in Britain: a cohort study. J Clin Endocrinol Metab 90:6516–6522
Bojesen A, Gravholt CH (2011) Morbidity and mortality in Klinefelter syndrome (47XXY). Acta Paediatr 100:807–13
Kübler A, Schulz G, Cordes U, Beyer J, Krause U (1992) The influence of testosterone substitution on bone mineral density in patients with Klinefelter’s syndrome. Exp Clin Endocrinol 100:129–132
Breuil V, Euller-Ziegler L (2001) Gonadal dysgenesis and bone metabolism. Joint Bone Spine 68:26–33
van den Bergh JP, Hermus AR, Spruyt AI, Sweep CG, Corstens FH, Smals AG (2001) Bone mineral density and quantitative ultrasound parameters in patients with Klinefelter’s syndrome after long-term testosterone substitution. Osteoporos Int 12:55–62
Ferlin A, Schipilliti M, Vinanzi C, Garolla A, Di Mambro A, Selice R, Lenzi A, Foresta C (2011) Bone mass in subjects with Klinefelter syndrome: role of testosterone levels and androgen receptor gene CAG polymorphism. J Clin Endocrinol Metab 96(4):739–45
Luisetto G, Mastrogiacomo I, Bonanni G, Pozzan G, Botteon S, Tizian L, Galuppo P (1995) Bone mass and mineral metabolism in Klinefelter’s syndrome. Osteoporos Int 5:455–461
Bojesen A, Birkebæk N, Kristensen K, Heickendorff L, Mosekilde L, Christiansen JS, Gravholt CH (2011) Bone mineral density in Klinefelter syndrome is reduced and primarily determined by muscle strength and resorptive markers, but not directly by testosterone. Osteoporos Int 22:1441–50
Jo DG, Lee HS, Joo YM, Seo JT (2013) Effect of testosterone replacement therapy on bone mineral density in patients with Klinefelter syndrome. Yonsei Med J 54(6):1331–5
Foresta C, Ruzza G, Mioni R, Meneghello A, Baccichetti C (1983) Testosterone and bone loss in Klinefelter syndrome. Horm Metab Res 15:56–57
Horowitz M, Wishart JM, O’Loughlin PD, Morris HA, Need AG, Nordin BE (1992) Osteoporosis and Klinefelter’s syndrome. Clin Endocrinol (Oxf) 36:113–118
Seo JT, Lee JS, OhTH JKJ (2007) The clinical significance of bone mineral density and testosterone levels in Korean men with non mosaic Klinefelter’s syndrome. BJU Int 99:141–146
Wong FH, Pun KK, Wang C (1993) Loss of bone mass in patients with Klinefelter’s syndrome despite sufficient testosterone replacement. Osteoporos Int 3:3–7
Battault S, Whiting SJ, Peltier SL, Sadrin S, Gerber G, Maixent JM (2013) Vitamin D metabolism, functions and needs: from science to health claims. Eur J Nutr 52:429–441
Lips P (2001) Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 22:477–501
Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB (2005) Expression patterns of mouse and human CYP orthologs (families 1–4) during development and in different adult tissues. Arch Biochem Biophys 436:50–61
Blomberg Jensen M, Nielsen JE, Jørgensen A, Rajpert-De Meyts E, Kristensen DM, Jørgensen N, Skakkebaek NE, Juul A, Leffers H (2010) Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum Reprod 25:1303–11
Ferlin A, Selice R, Carraro U, Foresta C (2013) Testicular function and bone metabolism—beyond testosterone. Nat Rev Endocrinol 9:548–54
Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM (2006) Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 91:1995–2010
Grant WB, Holick MF (2005) Benefits and requirements of vitamin D for optimal health: a review. Altern Med Rev 10:94–111
Leib ES, Lewiecki EM, Binkley N, Hamdy RC, International Society for Clinical Densitometry (2004) Official positions of the international society for clinical densitometry. J Clin Densitom 7:1–6
Ferlin A, Schipilliti M, Di Mambro A, Vinanzi C, Foresta C (2010) Osteoporosis in Klinefelter’s syndrome. Mol Hum Reprod 16:402–410
Romeo JH, Ybarra J (2007) Hypogonadal hypogonadism and osteoporosis in men. Nurs Clin North Am 42:87–99, vii-viii
Dupree K, Dobs A (2004) Osteopenia and male hypogonadism. Rev Urol 6(Suppl 6):S30–S34
Iitsuka Y, Bock A, Nguyen DD, Samango-Sprouse CA, Simpson JL, Bischoff FZ (2001) Evidence of skewed X-chromosome inactivation in 47,XXY and 48,XXYY Klinefelter patients. Am J Med Genet 98:25–31
Chamberlain NL, Driver ED, Miesfeld RL (1994) The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res 22:3181–3186
Zitzmann M, Depenbusch M, Gromoll J, Nieschlag E (2004) X-chromosome inactivation patterns and androgen receptor functionality influence phenotype and social characteristics as well as pharmacogenetics of testosterone therapy in Klinefelter patients. J Clin Endocrinol Metab 89:6208–6217
Overvad S, Bay K, Bojesen A, Gravholt CH (2014) Low INSL3 in Klinefelter syndrome is related to osteocalcin, testosterone treatment and body composition, as well as measures of the hypothalamic-pituitary-gonadal axis. Andrology 2:421–7
Foresta C, Strapazzon G, De Toni L, Perilli L, Di Mambro A, Muciaccia B, Sartori L, Selice R (2011) Bone mineral density and testicular failure: evidence for a role of vitamin D 25-hydroxylase in human testis. J Clin Endocrinol Metab 96:646–52
Foresta C, Selice R, Di Mambro A, Strapazzon G (2010) Testiculopathy and vitamin D insufficiency. Lancet 376:1301
Foresta C, Selice R, De Toni L, Di Mambro A, Carraro U, Plebani M, Garolla A (2013) Altered bone status in unilateral testicular cancer survivors: role of CYP2R1 and its luteinizing hormone-dependency. J Endocrinol Invest 36:379–84
Foresta C, Calogero AE, Lombardo F, Lenzi A, Ferlin A (2014) Late-onset hypogonadism—beyond testosterone. Asian J Androl. doi:10.4103/1008-682X.135985
Hannan MT, Litman HJ, Araujo AB, McLennan CE, McLean RR, McKinlay JB, Chen TC, Holick MFS (2008) Serum 25-hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J Clin Endocrinol Metab 93:40–6
Barbour KE, Zmuda JM, Horwitz MJ, Strotmeyer ES, Boudreau R, Evans RW, Ensrud KE, Gordon CL, Petit MA, Patrick AL, Cauley JA (2011) Osteoporotic fractures in men (MrOS) research group. The association of serum 25-hydroxyvitamin D with indicators of bone quality in en of Caucasian and African ancestry. Osteoporos Int 22:2475–85
Reid IR, Bolland MJ (2014) Skeletal and nonskeletal effects of vitamin D: is vitamin D a tonic for bone and other tissues? Osteoporos Int. May 21. [Epub ahead of print]
Cranney A, Horsley T, O’Donnell S, Weiler H, Puil L, Ooi D, Atkinson S, Ward L, Moher D, Hanley D, Fang M, Yazdi F, Garritty C, Sampson M, Barrowman N, Tsertsvadze A, Mamaladze V (2007) Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep) 158:1–235
Jones G, Hogan DB, Yendt E, Hanley DA (1996) Prevention and management of osteoporosis: consensus statements from the Scientific Advisory Board of the osteoporosis Society of Canada. 8. Vitamin D metabolites and analogs in the treatment of osteoporosis. CMAJ 155:955–61
Papadimitropoulos E, Wells G, Shea B, Gillespie W, Weaver B, Zytaruk N, Cranney A, Adachi J, Tugwell P, Josse R, Greenwood C, Guyatt G, Osteoporosis Methodology Group and The Osteoporosis Research Advisory Group (2002) Meta-analyses of therapies for postmenopausal osteoporosis. VIII: Meta-analysis of the efficacy of vitamin D treatment in preventing osteoporosis in postmenopausal women. Endocr Rev 23:560–9
Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B (2005) Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 293:2257–64
Grant
This study is supported by the Italian Ministry of University and Research (grant no. 2009AMPA9C_001).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Ferlin and R. Selice contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ferlin, A., Selice, R., Di Mambro, A. et al. Role of vitamin D levels and vitamin D supplementation on bone mineral density in Klinefelter syndrome. Osteoporos Int 26, 2193–2202 (2015). https://doi.org/10.1007/s00198-015-3136-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3136-8