Abstract
Summary
The aim of this systematic review and meta-analysis is to study the utility of the commonly used bone turnover markers in evaluating disease activity in patients with Paget’s disease of bone before and after treatment with bisphosphonates. We found good correlation between the bone turnover marker concentrations and disease activity assessed by bone scintigraphy.
Introduction
Paget’s disease of bone is a common skeletal disorder of the elderly. Bone turnover marker concentrations are used for diagnosis and follow-up. We aimed to compare the available bone turnover markers and determine their utility in assessing disease activity when compared to quantitative bone scintigraphy.
Methods
We conducted a systematic review and meta-analysis searching MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Scopus. We evaluated total alkaline phosphatase (total ALP), bone-specific alkaline phosphatase (bone ALP), procollagen type 1 amino-terminal propeptide (P1NP), serum, and urine C-terminal telopeptide (uCTx and sCTx, respectively), and urine N-terminal telopeptide (uNTx). The main outcome of interest was the correlation of disease activity with concentrations of bone turnover markers in Paget’s disease patients before and after treatment with bisphosphonates. Correlation coefficients were pooled across studies using the random effects model.
Results
We included 17 observational studies and one trial reporting on 953 patients. Prior to treatment, all studied bone turnover markers had moderate to strong correlation with scintigraphic indices (correlation coefficients ranging from 0.58 to 0.80) with no statistically significant difference between the bone turnover markers overall (p = 0.08). P1NP, uNTx, and bone ALP tend to have higher correlation with scintigraphy. After starting treatment with bisphosphonate, there was moderate to strong correlation with disease activity with all markers except bone ALP (correlation coefficients ranging from 0.43 to 0.70).
Conclusion
The findings of this meta-analysis suggest the Paget’s disease activity is best monitored by following P1NP levels. However, total ALP, bone ALP, and uNTx are good alternatives as markers of disease activity in untreated patients. Total ALP and uNTx can be useful in following patients with Paget’s disease after treatment if P1NP is not available. Clinicians, however, should take availability, cost, and the presence of liver disease into consideration when deciding which bone turnover marker is most appropriate when evaluating patients with Paget’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paget’s disease of bone is a common skeletal disorder of the elderly. This disorder could be isolated to one bone (monostotic) or affecting multiple bones (polyostotic). Paget’s disease is slightly more common in men compared to women and the prevalence is highest among the elderly [1]. The most frequently affected sites are the pelvis, vertebrae, and the femur [2]. The prevalence of this disease significantly differs by geographic region [1]. The highest rate has been reported in the UK (5.4 %) and the lowest in Japan [1]. Prevalence rate of pelvic Paget’s disease in the US among patients older than 65 years is 2.3 % based on a population-based study [3]. The fact that relatives of patients with Paget’s disease are at increased risk of experiencing the disease [4, 5] and the effect of geographic distribution suggest a genetic and environmental component to its pathophysiology. Studies have shown that a high proportion of patients with familial cases of Paget’s disease have mutations in the sequestosome 1 (SQSTM1) gene [6, 7].
Plain radiographs are the primary method to diagnose Paget’s disease. However, when these are equivocal, computed tomography can be used to evaluate the internal bone structure and confirm the diagnosis [8, 9]. Bone scintigraphy is more sensitive than plain radiographs in detecting active pagetic lesions [9–11].
Because Paget’s disease is characterized by a high rate of bone remodeling that results in abnormal bone formation [12], biochemical markers of bone turnover have widely been utilized as an objective tool to evaluate disease activity and monitor response to treatment. Currently, the most commonly used treatment for Paget’s disease is bisphosphonates. Treatment with a bisphosphonate often leads to normalization of bone turnover markers [13]. Due to its good sensitivity, low cost, and wide availability, total alkaline phosphatase (total ALP) has been the most frequently used marker to detect Paget’s disease activity and follow its progression. There are many other bone turnover markers that have been utilized to assess disease activity. Bone-specific alkaline phosphatase (bone ALP) and procollagen type 1 amino-terminal propeptide (P1NP) are markers of osteoblast activity [14–16]. On the other hand, markers of osteoclast activity include serum and urine C-terminal telopeptide (sCTx and uCTx, respectively) and urine N-terminal telopeptide (uNTx). In this systematic review and meta-analysis, we summarize the available evidence regarding the utility of markers of bone turnover in evaluating disease activity in patients with Paget’s disease prior to treatment and during the follow-up period after treatment.
Methods
Reporting this systematic review is based on the preferred reporting items for systematic reviews and meta-analyses (PRISMA) [17]. This review was conducted based on a priori established protocol.
Inclusion and exclusion criteria
Eligible studies were any type of study that evaluated the utility of using bone turnover markers in patients diagnosed with Paget’s disease. In this review, we consider bone scintigraphy as the gold standard to determine disease activity. The bone turnover markers of interests are total ALP, bone ALP, P1NP, sCTx and uCTx (alpha or beta isoforms), and uNTx. Because we are also aiming to evaluate the usefulness of these markers in assessing disease activity after treatment with bisphosphonates, studies that evaluated the utility of bone turnover markers in patients undergoing bisphosphonate treatment are included. Studies that do not report the outcome of interest (correlation coefficient factor between the markers and bone scintigraphy or sensitivity of bone turnover markers to detect Paget’s disease) are excluded. We also excluded publications without original data (clinical reviews and editorials), as well as studies with no available full-text paper. No language or country restrictions are used.
Data sources and search strategy
A comprehensive search of several databases from each database’s earliest inception to October 2012, which was updated to include studies to December 2014, in any language, was conducted. The databases include Ovid MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus. The search strategy was designed and conducted by an experienced librarian with input from the study’s principle investigator. Controlled vocabulary supplemented with keywords was used to search for serum and urinary biological markers of Paget’s disease. The electronic search was supplemented with manual search and review of bibliographies of included studies. The actual strategy is detailed as Appendix 1.
Study selection and data extraction
Pairs of reviewers independently assessed each abstract for eligibility. Disagreement yielded an automatic inclusion in the upper level of screening. Included studies were retrieved as full text and they were screened in duplicate. Disagreement at this level was resolved by consensus. Information (baseline characteristics and results) were extracted by reviewers independently in duplicate. The PI resolved conflicts in the reviewers’ data by referring to the full-text article.
Reviewers independently extracted study details from the full-text articles using a predesigned online form. The following data were abstracted: study design, country, patient characteristics (number of patients in each arm, sex, and age), follow-up period, details about their Paget’s disease (treatment, disease progression, and/or response to treatment definition and markers used).
Assessment of study quality
The quality of included observational studies was assessed using the Newcastle–Ottawa scale [18] by determining outcome ascertainment, adjustment for confounders, and proportion of patients lost to follow-up as well as sample selection. Quality of the randomized controlled trials (RCT) was assessed using Cochrane’s Collaboration’s tool [19] by determining the randomization method, blinding, allocation concealment, lost to follow–up, and source of funding.
Statistical analysis
The main outcome of interest was the correlation between bone turnover marker levels and scintigraphic activity at baseline and after bisphosphonate treatment. The correlation coefficient value and the number of subjects included in the study analysis were extracted. The correlation coefficient values range from −1,+1. We considered the correlation to be weak if the correlation coefficient value is less than 0.3, moderate if the value is ranged between 0.3 and 0.7, and strong if the correlation coefficient value is greater than 0.7 [20].
Other outcomes of interest were the correlation between different bone turnover marker levels and sensitivity of bone turnover markers to detect disease activity. Correlation coefficients were pooled across studies using the random effects model. Statistical analysis was conducted using Comprehensive Meta-Analysis (CMA) software.
Results
Study selection
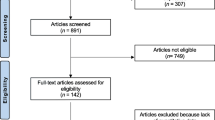
The search identified 637 abstracts of which 18 studies met all the inclusion criteria and are included in this systematic review (see Fig. 1). Seven of the included 18 studies assessed the utility of bone turnover markers in patients with Paget’s disease before treatment [8, 9, 21–25]. Three were cross-sectional, two cohort prospective, one cohort retrospective, and one case-control study. The characteristics of the studies are summarized in Table 1. Six other studies followed patients from the period prior to treatment throughout treatment [10, 11, 26–29]. One is a randomized controlled trial (RCT) [29], and the rest are cohort prospective studies. The total number of untreated patients with Paget’s disease in these 12 studies was 483.
Five studies evaluated the role of bone turnover markers in managing patients with Paget’s disease only after treatment [30–34]. One of these five studies was a prospective clinical trial [30], whereas the rest were cohort prospective studies. In total, the 11 studies included 401 patients with Paget’s disease. The characteristics of the 11 studies are summarized in Table 2. The RCT compared treatment with ibandronate to placebo. Patients in other studies received several bisphosphonates including tiludronate, zoledronate, and pamidronate. Bone scintigraphic indices (visual or quantitative) were the methods used to assess Paget’s disease activity in all studies.
Of the studies that evaluated urine and serum CTx, five evaluated the beta isoform [8, 11, 21, 29, 35], one studied both alpha and beta isoforms [36], and one study did not report which isoform was evaluated [24].
Comparative analysis of bone turnover markers in untreated patients
We conducted a meta-analysis on studies that evaluated the correlation between disease activity assessed by quantitative scintigraphy and bone turnover marker concentrations (Fig. 2). All bone turnover markers had moderate to strong correlation with scintigraphic indices. There is no statistically significant difference between the markers overall (p = 0.08). P1NP, uNTx, and bone ALP tend to have higher correlation with scintigraphy at baseline.
Patients with polyostotic disease showed significantly higher bone turnover biochemical markers compared to patients with monostotic disease patients [9, 11, 21, 29].
The sensitivity of bone turnover markers for detecting Paget’s disease varied between the studies. Sensitivity of bone formation markers ranged between 77 and 100 % for P1NP, 69–100 % for total ALP and 82–100 % for bone ALP [9, 21, 22, 24, 27, 29, 37]. uNTx had the highest sensitivity of the bone resorption markers with sensitivity ranging from 94 to 100 % [22, 24, 30]. Of all the bone turnover markers, one study showed that uNTx had the greatest sensitivity for low levels of disease activity, since it was the only marker that discriminated fully from the reference range [30]. We were unable to calculate the sensitivity of simultaneously using more than one bone turnover marker, since such analysis requires individual patient data.
Correlation between bone turnover markers was studied at baseline (Fig. 3). Generally, there was a moderate to strong correlation between bone ALP, total ALP, P1NP, and uNTx. bone ALP had a strong correlation with total ALP and P1NP; but a moderate correlation with uNTx and a weak correlation with uCTx . P1NP had a strong correlation with bone ALP, uNTx, and total ALP. P1NP had a weak to moderate correlation with uCTx. Total ALP had a strong correlation with bone ALP and P1NP, but a moderate correlation with uNTx. Total ALP had a weak correlation with uCTx. uNTx had a strong correlation with P1NP, but a moderate correlation with total ALP, bone ALP, and uCTx. uCTx had weak correlation with bone ALP and total ALP, weak to moderate correlation with P1NP, and a moderate correlation with uNTx and sCTx [9, 29].
Comparative analysis of bone turnover markers in treated patients
All bone turnover marker concentrations decreased significantly after treatment with bisphosphonates. Among markers of bone formation, serum P1NP and bone ALP showed the most marked decrease after treatment. uNTx had the most marked decrease among markers of bone resorption [11, 29, 31].
We conducted a meta-analysis on studies that evaluated the correlation between disease activity after treatment assessed by quantitative scintigraphy and bone turnover marker concentrations (Fig. 4). There was moderate to strong correlation with disease activity with all markers except bone ALP. The meta-analysis detected a statistically significant difference between the bone turnover markers (p = 0.019) in patients with Paget’s disease after treatment. Due to the small number of studies, we are unable to assess the effect of time and magnitude of response.
Bone ALP
Serum concentrations of bone ALP decreased significantly from day 10 after treatment with zoledronic acid and continued to be low after 2 months [35]. It continued to decrease significantly after 3 months of treatment with tiludronate and with pamidronate [27, 30]. There was a significant reduction in the serum concentration of bone ALP after 5 months of treatment with neridronate [28]. At 6 months after treatment, Alvarez et al. [31] reported that 76 % of the patients had a normal bone ALP concentration. One study concluded that bone ALP was the most sensitive marker for Paget’s disease relapse after 24 months of discontinuing treatment (100 % of the patients with relapsed disease had an increase in their bone ALP). However, the meta-analysis that we conducted showed a weak correlation between bone ALP and scintigraphic indices after treatment (r = 0.24, p = 0.047).
Total ALP
Serum concentrations of total ALP decreased significantly after the first and second month of treatment, then remained stable for another month in patients treated with tiludronate [27]. There was a significant reduction noticed 5 months after treatment with noridronate [28]. Strong correlation was found between bone ALP and total ALP after treatment [28]. Pooling the results of the studies that reported correlation between total ALP and bone scintigraphy showed moderate correlation between total ALP and scintigraphic indices after treatment (r = 0.427, p = 0.000).
P1NP
Along with bone ALP, P1NP showed the most marked decrease after treatment. P1NP showed a strong correlation with scintigraphic activity after treatment (r = 0.704, p = 0.000) [11, 31].
uNTx
Among markers of bone resorption, uNTx showed the highest response to treatment [11, 30, 31]. uNTx concentrations decreased significantly from day 10 after treatment with one injection of zoledronic acid and continued to be reduced 2 months after treatment [35] and 6 months after treatment [11, 31]. uNTx has moderate correlation with scintigraphic activity in Paget’s disease patients after treatment (r = 0.674, p = 0.000).
uCTx
Concentrations of uCTx decreased by 40 % within 5 days, then increased between days 10 and 60, and returned to baseline values by 2 months after one zoledronic acid infusion [35]. One study evaluated the correlation between uCTx and bone scintigraphic activity in patients with Paget’s disease after treatment and showed a moderate correlation between them (r = 0.563, p = 0.000) [11].
sCTx
sCTx showed a significant decrease in monostotic patients only at 1 month after treatment, but continued to decrease in the first 6 months after treatment in patients with polyostotic disease, as reported by Alvarez et al. [11]. Alvarez et al. [11] reported moderate correlation between sCTx and bone scintigraphic indices in Paget’s disease patients after treatment (r = 0.639. p = 0.000).
One study assessed the bone turnover marker values after treatment in patients with monostotic vs. polyostotic disease. In this study, the bone turnover markers decreased significantly in both groups. However, all bone turnover marker values continued to be higher than control in patients with polyostotic disease after 6 months of treatment, whereas total ALP was the only marker that was different from the control group in patients with monostotic disease [11].
Quality assessment
One RCT was included in our systematic review [29] and had an unclear method of randomization and allocation concealment. The authors did not report whether the patients or the investigators were blinded from the interventions or outcomes (Table 3). The observational studies had overall fair quality (Table 4).
Discussion
The diagnosis of Paget’s disease is often incidental during the evaluation of another health condition. The diagnosis is confirmed radiographically [38]. The best available method to evaluate disease activity is bone scintigraphy [9–11]. Measuring bone turnover markers, specifically total and bone-specific alkaline phosphatase, has been recommended and widely used by clinicians to assist in diagnosing and following patients with Paget’s disease. However, these recommendations are mainly based on expert opinion or clinical experience [39]. The excessive bone remodeling that occurs in patients with Paget’s disease results in nonlamellar bone formation and disorganized collagen maturation and deposition [40]. So breakdown products of type 1 collagen, such as CTx and NTx [41], as well as markers of collagen formation that result from conversion of type 1 procollagen to collagen, such as P1NP, have been widely used as markers of disease activity [42]. In this systematic review and meta-analysis, we summarized the current available evidence in regard to the usefulness of bone turnover markers in the management of Paget’s disease of the bones.
Main findings and clinical implications
We found that all the bone turnover markers we evaluated have high sensitivity to pagetic bone changes considering bone scintigraphy as a gold standard to evaluate disease activity. However, normal concentrations do not completely rule out Paget’s disease.
All bone turnover markers showed moderate to strong correlation with scintigraphic indices prior to bisphosphonate treatment. This suggests that bone turnover markers are good surrogate for the degree of disease activity in the untreated state.
P1NP , bone ALP, total ALP, and uNTx have good sensitivity for detecting Paget’s disease. We also demonstrated that these bone turnover markers have moderate to strong correlation between each other at baseline. These facts make any of these markers a reasonable option for assessing disease activity in patients with Paget’s disease at baseline. However, although the available evidence does not demonstrate clear superiority of a particular marker, the highest correlation with bone scintigraphy was found for P1NP for bone formation and uNTx for bone resorption. After treatment, the P1NP concentration demonstrated the highest correlation with disease activity. Although this makes P1NP an attractive option for monitoring response to treatment, it is not as widely available and is more expensive to perform compared to total ALP. Due to its availability and moderate correlation with bone scintigraphy, total ALP is a useful marker to assess disease activity after treatment. Total ALP remains as one of the least expensive and most available markers with the limitation of decreased accuracy in patients with liver disease.
Study limitations
There are multiple limitations to this review. The methods used to calculate bone scintigraphic index differed between the studies. For example, among the studies that evaluated the correlation between bone turnover markers and scintigraphic activity, Pons et al. [22], Alexandersen et al. [10], and Alvarez et al. [11] followed the same protocol in calculating the scintigraphic indices. This protocol is described in detail in the study of Pons et al. [22]. Whereas, Alvarez et al. [21], Griffith et al. [33], and Meunier et al. [23] followed different protocols. All these methods are at risk of subjectivity as well. Bonnin et al. [9] did not describe the method used to calculate bone scintigraphic index. One other limitation to our systematic review and meta-analysis is that most of the available studies in the literature are observational studies with different follow-up periods.
An important limitation to this review is that we were not able to analyze the correlation between disease activity based on the gold standard and the bone turnover marker concentrations in patients with monostotic disease vs. patients with polyostotic disease. That is because in most of the studies, even when the authors reported higher bone turnover marker concentrations in patients with polyostotic disease, they did not separately report the correlation with bone scintigraphy in patients with monostotic vs. polyostotic disease. A similar problem was recognized when we attempted to calculate the sensitivity of bone formation markers together
Conclusion
The current available evidence suggests good correlation between bone turnover marker concentrations and disease activity as assessed by bone scintigraphy in patients with Paget’s disease before treatment and during follow-up of patients after bisphosphonate therapy.
Based on the findings of our meta-analysis, disease activity is best assessed by following P1NP concentrations both initially and after therapy with bisphosphonates. However, because this marker is not universally available, and due to its strong correlation with total ALP, bone ALP, and uNTx in untreated patients, these markers can be used as markers for disease activity in patients with Paget’s disease before treatment.
Because of their moderate to strong correlation with disease activity after treatment, total ALP and uNTx can be useful in following patients with Paget’s disease after treatment if P1NP is not available. Clinicians, however, should take availability, cost, and the presence of liver disease in consideration when deciding the bone turnover markers when following patients with Paget’s disease.
References
Corral-Gudino L, Borao-Cengotita-Bengoa M, Del Pino-Montes J, Ralston S (2013) Epidemiology of Paget’s disease of bone: a systematic review and meta-analysis of secular changes. Bone Meta-Anal Rev 55(2):347–352
Wermers RA, Tiegs RD, Atkinson EJ, Achenbach SJ, Melton LJ 3rd (2008) Morbidity and mortality associated with Paget’s disease of bone: a population-based study. J Bone Miner Res. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. 23(6):819–25
Altman RD, Bloch DA, Hochberg MC, Murphy WA (2000) Prevalence of pelvic Paget’s disease of bone in the United States. J Bone Miner Res. [Research Support, Non-U.S. Gov’t]. 15(3):461–5
Siris ES, Ottman R, Flaster E, Kelsey JL (1991) Familial aggregation of Paget’s disease of bone. J Bone Miner Res. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. 6(5):495–500
Merlotti D, Gennari L, Galli B, Martini G, Calabro A, De Paola V et al (2005) Characteristics and familial aggregation of Paget’s disease of bone in Italy. J Bone Miner Res 20(8):1356–1364
Laurin N, Brown JP, Morissette J, Raymond V (2002) Recurrent mutation of the gene encoding sequestosome 1 (SQSTM1/p62) in Paget disease of bone. Am J Hum Genet. [Research Support, Non-U.S. Gov’t]. 70(6):1582–8
Hocking LJ, Lucas GJ, Daroszewska A, Mangion J, Olavesen M, Cundy T (2002) et al. Domain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget’s disease. Hum Mol Genet. [Research Support, Non-U.S. Gov’t]. 11(22):2735–9
Alvarez L, RicOs C, Peris P, GuaNabens N, Monegal A, Pons F, et al (2000) Components of biological variation of biochemical markers of bone turnover in Paget’s bone disease. Bone. [Comparative Study]. 26(6):571–6
Bonnin MR, Moragues C, Nolla JM, Liron FJ, Roig-Escofet D, Navarro MA (1998) Evaluation of circulating type I procollagen propeptides in patients with Paget’s disease of bone. Clin Chem Lab Med. [Comparative Study]. 36(1):53–5
Alexandersen P, Peris P, Guanabens N, Byrjalsen I, Alvarez L, Solberg H, et al (2005) Non-isomerized C-telopeptide fragments are highly sensitive markers for monitoring disease activity and treatment efficacy in Paget’s disease of bone. J Bone Miner Res. [Evaluation Studies]. 20(4):588–95
Alvarez L, Guanabens N, Peris P, Vidal S, Ros I, Monegal A, et al (2001) Usefulness of biochemical markers of bone turnover in assessing response to the treatment of Paget’s disease. Bone. [Clinical Trial]. 29(5):447–52
Roodman GD, Windle JJ (2005) Paget disease of bone. J Clin Invest. [Research Support, U.S. Gov’t, P.H.S. Review]. 115(2):200–8
Reid IR, Miller P, Lyles K, Fraser W, Brown JP, Saidi Y et al (2005) Comparison of a single infusion of zoledronic acid with risedronate for Paget’s disease. N Engl J Med 353(9):898–908
Reid IR, Miller P, Lyles K, Fraser W, Brown JP, Saidi Y et al (2005) Comparison of a single infusion of zoledronic acid with risedronate for Paget’s disease. New Engl J Med 353(9):898–908
Leung KS, Fung KP, Sher AH, Li CK, Lee KM (1993) Plasma bone-specific alkaline phosphatase as an indicator of osteoblastic activity. J Bone Joint Surg Br. [Comparative Study]. 75(2):288–92
Shankar S, Hosking DJ (2006) Biochemical assessment of Paget’s disease of bone. J Bone Miner Res 21:P22–P27
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. [Guideline Research Support, Non-U.S. Gov’t]. 151(4):264–9, W64
Wells G SB, O’connell D, Peterson J, Welch V, Losos M, Tugwell P (2014) The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed June, 2014 [cited 2014 May, 2014]
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al (2011) The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. [Research Support, Non-U.S. Gov’t]. 343:d5928
David Machine MJC, Stephen J (2007) Walters medical statistics — a text book for the health sciences, 4th edn
Alvarez L, Peris P, Pons F, Guanabens N, Herranz R, Monegal A et al (1997) Relationship between biochemical markers of bone turnover and bone scintigraphic indices in assessment of Paget’s disease activity. Arthritis Rheum 40(3):461–468
Pons F, Alvarez L, Peris P, Guanabens N, Vidal-Sicart S, Monegal A et al (1999) Quantitative evaluation of bone scintigraphy in the assessment of Paget’s disease activity. Nucl Med Commun 20(6):525–528
Meunier PJ, Salson C, Mathieu L, Chapuy MC, Delmas P, Alexandre C, et al (1987) Skeletal distribution and biochemical parameters of Paget’s disease. Clin Orthop Relat Res. [Comparative Study]. (217):37–44
Woitge HW, Pecherstorfer M, Li Y, Keck AV, Horn E, Ziegler R, et al (1999) Novel serum markers of bone resorption: clinical assessment and comparison with established urinary indices. J Bone Miner Res. [Comparative Study]. 14(5):792–801
Bachiller-Corral J, Diaz-Miguel C, Morales-Piga A (2013) Monostotic Paget’s disease of the femur: a diagnostic challenge and an overlooked risk. Bone 57(2):517–521
Woitge HW, Oberwittler H, Heichel S, Grauer A, Ziegler R, Seibel MJ (2000) Short- and long-term effects of ibandronate treatment on bone turnover in Paget disease of bone. Clin Chem 46(5):684–690
de la Piedra C, Rapado A, Diaz Diego EM, Diaz Martin MA, Aguirre C, Lopez Gavilanes E, et al (1996) Variable efficacy of bone remodeling biochemical markers in the management of patients with Paget’s disease of bone treated with tiludronate. Calcif Tissue Int. [Comparative Study Research Support, Non-U.S. Gov’t]. 59(2):95–9
Ulivieri FM, Piodi LP, Marotta G, Marchelli D, Corradini C, Parravicini L et al (2006) Usefulness of osteoprotegerin in assessing responses to neridronate treatment in Paget’s disease of bone. J Orthop Traumatol 7(4):192–194
Reid IR, Davidson JS, Wattie D, Wu F, Lucas J, Gamble GD et al (2004) Comparative responses of bone turnover markers to bisphosphonate therapy in Paget’s disease of bone. Bone 35(1):224–230
Randall AG, Kent GN, Garcia-Webb P, Bhagat CI, Pearce DJ, Gutteridge DH et al (1996) Comparison of biochemical markers of bone turnover in Paget disease treated with pamidronate and a proposed model for the relationships between measurements of the different forms of pyridinoline cross-links. J Bone Miner Res 11(8):1176–1184
Alvarez L, Peris P, Guanabens N, Vidal S, Quinto L, Monegal A et al (2004) Long-term biochemical response after bisphosphonate therapy in Paget’s disease of bone. Proposed intervals for monitoring treatment. Rheumatology (Oxford) 43(7):869–874
Garnero P, Gineyts E, Schaffer AV, Seaman J, Delmas PD (1998) Measurement of urinary excretion of nonisomerized and beta-isomerized forms of type I collagen breakdown products to monitor the effects of the bisphosphonate zoledronate in Paget’s disease. Arthritis Rheum. [Clinical Trial Randomized Controlled Trial]. 41(2):354–60
Griffith K, Pearson D, Parker C, Thorpe S, Vincent RM, Hosking DJ (2001) The use of a whole body index with bone scintigraphy to monitor the response to therapy in Paget’s disease. Nucl Med Commun 22(10):1069–1075
Zati A, Colori BC, Bonfiglioli Stagni S, Mignani A (2011) Pain in Paget’s disease: a retrospective study of treatment efficacy. Neuro Endocrinol Lett 32(2):127–132
Garnero P, Gineyts E, Schaffer AV, Seaman J, Delmas PD (1998) Measurement of urinary excretion of nonisomerized and beta-isomerized forms of type I collagen breakdown products to monitor the effects of the bisphosphonate zoledronate in Paget’s disease. Arthritis Rheum 41(2):354–360
Alexandersen P, Peris P, Guanabens N, Byrjalsen I, Alvarez L, Solberg H et al (2005) Non-isomerized C-telopeptide fragments are highly sensitive markers for monitoring disease activity and treatment efficacy in Paget’s disease of bone. J Bone Miner Res 20(4):588–595
Ulivieri FM, Marchelli D, Como G, Valente G, Messa P, Raimondi AR, et al (2006) Increased osteoprotegerin in Italian haemodialysis patients. Osteoporos Int. [Comment Letter]. 17(12):1822–3; author reply 4
Ralston SH (2013) Clinical practice. Paget’s disease of bone. N Engl J Med. [Review]. 368(7):644–50
Selby PL, Davie MW, Ralston SH, Stone MD (2002) Guidelines on the management of Paget’s disease of bone. Bone. [Guideline Research Support, Non-U.S. Gov’t]. 31(3):366–73
Ingram RT, Collazo-Clavell M, Tiegs R, Fitzpatrick LA (1996) Paget’s disease is associated with changes in the immunohistochemical distribution of noncollagenous matrix proteins in bone. J Clin Endocrinol Metab 81(5):1810–1820
Shankar S, Hosking DJ (2006) Biochemical assessment of Paget’s disease of bone. J Bone Mineral Res: Of J Am Soc Bone Mineral Res 21(Suppl 2):P22–P27
Delmas PD (1999) Biochemical markers of bone turnover in Paget’s disease of bone. J Bone Miner Res Off J Am Soc Bone Miner Res 14(Suppl 2):66–69
Acknowledgments
This study was supported by a contract from the Endocrine Society.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Appendix 1
Appendix 1
Search strategy
Ovid
Database(s)
EMBASE 1988 to 2012 Week 42, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) 1946 to Present, EBM Reviews - Cochrane Central Register of Controlled Trials October 2012, EBM Reviews - Cochrane Database of Systematic Reviews 2005 to September 2012
Scopus
-
1
TITLE-ABS-KEY(“Osteitis Deformans” or (paget W/4 bone) or (pagets W/4 bone) or “ostitis deformans”)
-
2
TITLE-ABS-KEY(“biological marker*” or biomarker* or “serum marker*” or “laboratory marker*” or “immunologicmarker*” or (surrogate W/1 endpoint*) or (surrogate W/1 “end point*”) or “biologic marker*” or “immune marker*” or “clinical marker*” or “biochemical marker*” or “biological indicator*” or bioindicator* or “Alkaline Phosphatase”or “alcalic phosphatase” or “alkali phosphatase” or “alkalic phosphatase” or “alkaline monophosphoesterase” or “alkaline phosphohydrolase” or “alkaline phosphomonoesterase” or “alkalinic phosphatase” or “basic phosphatase” or “orthophosphoric monoester phosphohydrolase” or procollagen or “collagen precursor” or “proto-collagen” or protocollagen or precollagen or “collagen type 1” or “collagen 1” or “collagen i” or “type 1 collagen” or vitrogen or “type I collagen” or P1NP or P1NP or telopeptide or creatinine or creatinin or kreatinine or methylglycocyamimine or “1 methylglycocyamidine” or “1 methylhydantoin 1 imide” or “2 imino 1 methyl 4imidazolinone”)
-
3
TITLE-ABS-KEY(serum or blood or urine or urinary)
-
4
TITLE-ABS-KEY( (evidence W/1 based) OR (meta W/1 analys*) OR (systematic* W/2 review*) OR guideline OR (control* W/2 stud*) OR (control* W/2 trial*) OR (randomized W/2 stud*) OR (randomized W/2 trial*) or random* or “latin square” or crossover or “cross-over” or placebo* or (doubl* N5 blind*) or (doubl* N5 mask*) or (singl* N5 blind*) or (singl* N5 mask*) or (tripl* N5 blind*) or (tripl* N5 mask*) or (trebl* N5 blind*) or (trebl* N5 mask*) or “comparative study” OR “comparative survey” OR “comparative analysis” OR “cohort study” OR “cohort survey” OR “cohort analysis” OR “longitudinal study” OR “longitudinal survey” OR “longitudinal analysis” OR “retrospective study” OR “retrospective survey” or “retrospective analysis” OR “prospective study” OR “prospective survey” OR “prospective analysis” OR “population study” OR “population survey” OR “population analysis” OR “concurrent study” OR “concurrent survey” OR “concurrent analysis” or “incidence study” OR “incidence survey” OR “incidence analysis” OR “follow-up study” OR “follow-up survey” OR “follow-up analysis” or “observational study” OR “observational survey” OR “observational analysis” OR “case study” OR “case series” OR “clinical series” OR “case studies” or “clinical study” OR “clinical trial” or “evaluation study” OR “evaluation survey” OR “evaluation analysis” or “twin study” OR “twin survey” OR “twin analysis” or “validation study” OR “validation survey” OR “validation analysis” or “experimental study” OR “experimental analysis” or “field study” OR “field survey” OR “field analysis” or “in vivo study” OR “in vivo analysis” or “panel study” OR “panel survey” OR “panel analysis” or “pilot study” OR “pilot survey” OR “pilot analysis” or “prevention study” OR “prevention survey” OR “prevention analysis” or “replication study” OR “replication analysis” or “theoretical study” OR “theoretical analysis” or “trend study” OR “trend survey” OR “trend analysis” or “multivariate analysis”)
-
5
1 and 2 and 3 and 4
-
6
PMID (0*) OR PMID (1*) OR PMID (2*) OR PMID (3*) OR PMID (4*) OR PMID (5*) OR PMID (6*) OR PMID (7*) OR PMID (8*) OR PMID (9*)
-
7
5 and not 6
-
8
DOCTYPE(le) OR DOCTYPE(ed) OR DOCTYPE(bk) OR DOCTYPE(er) OR DOCTYPE(no) OR DOCTYPE(sh)
-
9
7 and not 8
Rights and permissions
About this article
Cite this article
Al Nofal, A.A., Altayar, O., BenKhadra, K. et al. Bone turnover markers in Paget’s disease of the bone: A Systematic review and meta-analysis. Osteoporos Int 26, 1875–1891 (2015). https://doi.org/10.1007/s00198-015-3095-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3095-0