Abstract
Summary
Loop diuretic use has been shown to be associated with an increased fracture risk, but the findings have been inconsistent. The present meta-analysis suggests that loop diuretics show a significant positive association with the overall risk of total fractures and, specifically, hip fractures.
Introduction
Despite being widely used, there is limited, prospective randomized trial evidence regarding the skeletal effects of loop diuretics. Previous observational studies have reported conflicting findings regarding the association between loop diuretic use and the risk of fractures.
Methods
This meta-analysis of observational studies assessed the association between loop diuretic use and the risk of fractures. The PubMed, EMBASE, and OVID databases were searched for prospective cohort and case–control studies. Relative risks (RR) with 95 % confidence intervals (CI) were derived using random-effects models throughout the analysis.
Results
Thirteen studies (4 cohort studies and 9 case–control studies) were included, involving 842,644 participants and 108,247 fracture cases. Compared with non-users, people who had taken loop diuretics had an approximately 15 % higher risk of total fractures (95 % CI, 1.04–1.26; p < 0.01), with high heterogeneity between studies (I 2 = 80.5 %; p < 0.01). The RR was 1.14 (95 % CI, 1.08–1.19) for hip fractures and 0.99 (95 % CI, 0.93–1.05) for lower arm or wrist fractures. The RR was 1.05 (95 % CI, 1.00–1.11) in prospective cohort studies and 1.22 (95 % CI, 1.00–1.44) in case–control studies. There was no evidence of publication bias.
Conclusion
The results suggest that loop diuretics show a significant positive association with the overall risk of total fractures and hip fractures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fractures are major causes of morbidity, including pain and loss of function, and also cause considerable mortality. The medical costs associated with fractures also cause a tremendous financial burden on the family, and the overall society [1–3]. Therefore, identifying and confirming the risk factors for preventing fractures has significance for public health and clinical medicine [4–6]. Known factors associated with the incidence of fractures include physical activity [7], age [8], smoking [9], alcohol consumption [8], and body mass index (BMI) [10]. However, the relationship between drug use and the risk of fractures requires more attention [11].
Loop diuretics are typically prescribed to manage hypertension, especially when associated with renal insufficiency (glomerular filtration rate, <30 mL/min) or resistant hypertension [12, 13]. In the USA, loop diuretics are the third and sixth most commonly prescribed medications among community-dwelling men and women, ≥65-years-old, respectively; 12 % of men and 9 % of women in this demographic are estimated to use these medications [14]. Moreover, loop diuretic use is associated with significantly increased urinary calcium excretion [15, 16]. Previous studies have shown that loop diuretics affect bone turnover and increase the rate of bone loss and the risk of falls [12, 17, 18]. However, whether treatment with loop diuretics increases the fracture risk is controversial. Several epidemiological studies have reported that loop diuretics appear to increase the risk of fractures [19, 20], but the findings have been inconsistent [21, 22]. Therefore, the objectives of the present meta-analysis were to quantitatively assess the available observational studies that have examined the association between loop diuretics and fracture risks and to evaluate the association between their use and fracture subtypes.
Methods
We conducted this study according to the Meta-analysis Of Observational Studies in Epidemiology group guidelines and used the Preferred Reporting Items for Systematic reviews and Meta-Analyses statement to guide our methods [23–25].
Search strategy
We conducted a systematic search of the MEDLINE, EMBASE, and OVID databases, from their dates of inception to March 1, 2014, and identified all potentially relevant articles. Although the search was limited to humans, language restrictions were not employed. The searches were performed using either Medical Subject Headings or free-text words. We combined search terms for the outcome (fracture) and the influencing factor (loop diuretics, diuretics, furosemide, bumetanide, ethacrynic, torasemide, piretanide, azosemide, indacrinone, etozolin, ozolinone, cicletanine, tienilic, and tizolemide). We also searched the reference lists of the full-text papers and reviewed studies from all of the relevant publications to identify any omitted studies. Moreover, we searched the conference abstracts in the ISI Proceedings database, the American Society for Bone and Mineral Research, and the proceedings of the International Osteoporosis Foundation World Conference on Osteoporosis from 2000 to 2014. However, none of the meeting abstracts were included in this meta-analysis.
Selection criteria
Two reviewers independently assessed the content of the studies to identify potentially eligible articles. Any discrepancies between the two reviewers regarding study inclusion and data interpretation were resolved by arbitration with a third reviewer; consensus was reached after discussion. Studies were eligible for inclusion in this meta-analysis if they (1) were observational studies (case–control or cohort), (2) involved an adult population, (3) investigated the association between loop diuretic use and the risk of fractures, and (4) provided risk estimates, such as relative risks (RRs), odds ratios, hazard ratios, or other measures that could be transformed into RRs, with 95 % confidence intervals (CIs). If different papers came from the same cohort, the paper with the most comprehensive design, based on a quality assessment, was included in the analysis.
Data extraction and quality assessment
Two reviewers independently extracted data for analysis using a standardized data collection form. Discrepancies were resolved by consensus, involving another two reviewers, after consulting the original article(s). The following data were collected from each study: the first author’s last name, publication year, country where the study was performed, study design, duration of follow-up, recruitment time, sample size, participant sex and age, methods of fracture determination, adjustment variables, types of fracture (e.g., hip, wrist, or all fractures), and the risk estimates with their corresponding CIs. The study quality was assessed by two reviewers, based on a previously published 10-point scale corresponding to the five methodological characteristics of either cohort or case–control studies [26]. A third reviewer was enlisted to resolve discrepancies regarding the abstracted data.
Data synthesis and statistical analysis
RRs were used as the common measure of association between the loop diuretic use and fracture risk [27]; odds ratios were transformed into RRs [27, 28]. We only extracted the RRs and 95 % CIs that reflected the greatest degree of control for potential confounders for loop diuretic use in our main analyses.
For the meta-analysis, we used a random-effects model to calculate pooled RRs and 95 % CIs, as this model best accounts for heterogeneity between studies [29]. Heterogeneity between studies was assessed using I 2 statistics; values of 25, 50, and 75 % were defined as low, moderate, and high, respectively [30]. We used subgroup analyses to identify associations between the risk of fractures and the study characteristics (design of study, sex, number of participants, and adjustment for other drugs, prior fracture, and falls) that may have served as possible sources of heterogeneity.
Publication bias was detected by inspecting funnel plot asymmetry, and the Egger’s and Begg’s regression tests were applied to measure funnel plot asymmetry [31, 32]. We also performed a “trim and fill” procedure to further assess the possible effect of publication bias on our meta-analysis [33]. This method considers that hypothetical, “missing” studies exist, imputes their RRs, and recalculates a pooled RR that incorporates the hypothetical missing studies as though they had actually been performed. All of the analyses were conducted using Stata 12 (StataCorp, College Station, TX, USA); p values <0.05 were considered to be statistically significant.
Results
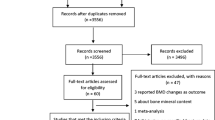
The strategy used to identify the relevant studies is presented in Fig. 1. There were 773 potentially relevant citations that were obtained from the database search. After an evaluation of the titles and abstracts, we excluded 735 citations that were duplicated or that included patients, interventions, or outcomes that did not satisfy the inclusion criteria. As a result, 38 articles were included in the detailed evaluation. Of these, 25 studies were excluded because of insufficient data or the absence of data on loop diuretics, as opposed to the use of other diuretics. After the evaluation, we included four cohort studies [34–37] and nine case–control studies [19–22, 38–42] in the meta-analysis. The observers demonstrated good agreement on the selection of studies appropriate for inclusion (Cohen’s unweighted κ = 0.92).
Included study characteristics
The included studies were published between 1986 and 2013 (Table 1), describing 842,644 participants from 13 studies, and involving 108,247 fractures. The studies were from different countries (five from the USA [35–38, 42], seven from Europe [19–21, 39–41], and one from Australia [22]). Nine studies recruited mixed-sex groups [19–21, 34, 37–39, 41, 42], and the other four recruited only women [34–36, 40]. The follow-up durations for the cohort studies ranged from 1 to 9.6 years. Fractures were ascertained using self-reporting, radiographic reports, medical records, questionnaires, or administrative data. The most frequent confounders, primarily age, sex, and body mass index, were adjusted in the studies.
Loop diuretic use and fracture risk
The multivariate-adjusted RRs for each study are presented in Fig. 2. Compared with participants who had not used loop diuretics, those who had taken loop diuretics had an approximately 15 % higher risk of total fractures (95 % CI, 1.04–1.26; p < 0.01), with high heterogeneity across studies (p < 0.01; I 2 = 80.5 %). The analysis of fracture subtypes showed an increased risk of hip fractures (RR, 1.14; 95 % CI, 1.08–1.19; p < 0.01). However, the association between loop diuretic use and the risk of lower arm or wrist fractures was not statistically significant (RR, 0.99; 95 % CI, 0.93–1.05; p < 0.01) (Table 2).
Subgroup and sensitivity analyses
In the subgroup analyses, we examined study design, participant numbers, participant sex, adjustments for other drugs, prior fracture, and falls as possible sources of heterogeneity (Table 2). The analyses indicated that the study design did not influence the associations between loop diuretic use and fracture risk. The RR was 1.05 (95 % CI, 1.00–1.11; p < 0.01) for prospective cohort studies and 1.22 (95 % CI, 1.00–1.44; p < 0.01) for case–control studies; no significant interactions were observed between subgroups (p = 0.57), and no heterogeneity was found (p = 0.49; I 2 = 0 %) for the analysis of case–control studies. With respect to sex, the RRs were 1.10 (95 % CI, 0.53–1.66; p < 0.01) for men, 1.09 (95 % CI, 1.01–1.16; p < 0.01) for women, and 1.07 (95 % CI, 1.05–1.09; p < 0.01) for mixed-sex studies. Significant interactions were not observed between subgroups (p = 0.89), and heterogeneity was not found for the men (p = 0.77, I 2 = 0 %) or women (p = 0.37, I 2 = 7.1 %), but was found for the mixed-sex studies (p < 0.01, I 2 = 89.4 %). We also examined the number of participants as a possible source of heterogeneity. The RRs were 0.99 (95 % CI, 0.69–0.1.29; p < 0.01) for studies involving fewer than 3000 participants and 1.17 (95 % CI, 1.05–1.29; p < 0.01) for those including ≥3000 participants; no significant interactions were observed between subgroups (p = 0.60).
To examine the effects of adjustments for potentially confounding factors, we considered four studies that had provided both unadjusted and multiple-adjusted coefficients. The unadjusted RR for the association between loop diuretic use and fracture risk was 1.37 (95 % CI, 1.21–1.53; p < 0.01) with high heterogeneity across studies (p < 0.01; I 2 = 91.4 %). There was no evidence of attenuation of unadjusted and multiple-adjusted coefficients of the RR for loop diuretic use associated with fractures. However, further subgroup analyses showed that adjustment for other drug use and prior fractures were possible sources of heterogeneity. A sensitivity analysis showed that the exclusion of any one study from the pooled analysis did not substantially vary the results (RRs ranged from a low of 1.22 [95 % CI, 1.09–1.35] to a high of 1.38 [95 % CI, 1.16–1.60]) (Fig. 3).
Publication bias
The funnel plot did not show asymmetry, suggesting the absence of a publication bias among the included studies. Egger’s test (p = 0.329) and Begg’s test (p = 0.154) further confirmed the absence of statistical evidence of publication bias, and the “trim and fill” method showed that there were no missing studies (Supplemental Fig. S1).
Discussion
Main findings
This meta-analysis included data from 13 observational studies, revealing that the use of loop diuretics was associated with an increased risk of fractures (approximately 15 %). An analysis, stratified by fracture subtype, suggested that the use of loop diuretics was associated with a 14 % greater risk of hip fracture. However, we did not observe an increased risk of lower arm or wrist fractures associated with the use of loop diuretics.
Implications
The present study highlights important aspects of the relationship between the use of loop diuretics and the risk of fractures, with several plausible mechanisms for this relationship possible. An obvious explanation for the increased fracture risk is the increased urinary loss of calcium. Persistent calcium loss, induced by loop diuretics, might result in higher rates of bone loss and increased bone porosity [43, 44]. A randomized-controlled trial that included 87 postmenopausal women revealed that 1-year treatment with a loop diuretic (bumetanide) decreased bone mineral density and increased bone turnover marker levels compared with placebo [17]. Recently, a cohort study involving 2980 older women showed that loop diuretic use was associated with a small, but significantly higher, rate of hip bone loss than was observed among non-users [36]; the findings were also replicated in older men [12]. Since substantial evidence indicates that loop diuretics are associated with an increased risk of bone loss, and lower bone mineral density is an important risk factor for fractures [9], loop diuretics may increase the risk of fractures by increasing the risk of bone loss. A recent study investigating the relationship between diuretic-induced hyponatremia and osteoporotic fractures indicated a clinical association between hyponatremia during loop diuretic use and an increased risk of osteoporosis-associated fractures. The authors suggested that loop diuretic therapy exerts negative long-term effects on calcium homeostasis and increases the risk of fall-related fractures [19].
An additional explanation for the relationship between loop diuretic use and the increased risk of fractures is an increased incidence of falls. Loop diuretics may potentially cause orthostatic hypotension [45], which might be positively associated with an increased risk of falls and, subsequently, an increased risk of fractures [37]. However, a large meta-analysis failed to confirm an independent relationship between orthostatic hypotension and falls [46]. Additionally, urinary urgency and frequency is commonly associated with the initiation of loop diuretics, and could result in an increased number of falls when patients are hurrying to the toilet [20]. Berry et al. demonstrated that following a new prescription or increased dose of a loop diuretic drug, patients in nursing home residents had an increased risk of day time falls [47]. However, a prospective cohort study, including 6244 participants, failed to show an association between loop diuretic use among older women and a greater risk of falls [36]. Furthermore, a meta-analysis showed there was no relationship between loop diuretic use and the risk of falls (odds ratio, 0.90; 95 % CI, 0.73–1.12) [18]. Thus, further investigations are needed to better understand whether loop diuretics increase the risk of falls.
The analysis of fracture subtypes showed an increased risk of hip fractures, but not of lower arm or wrist fractures. A possible explanation for the lack of such an association in this study is the limited amount of relevant data pertaining to lower arm or wrist fractures; the absence of an association might be ascribed to chance effects or it may result from systematic errors (e.g., residual confounding or selection bias). Therefore, the results should be interpreted with caution, and further well-designed and stratified cohort studies should be conducted to examine the association between fractures and loop diuretic use.
Strengths and limitations
There are several strengths associated with the present study. First, we conducted the most comprehensive literature search, to date, using the MEDLINE, EMBASE, OVID databases, as well as related conference abstracts and reference lists describing the effect of loop diuretics on the risk of fractures. The size of the study and the absence of a language restriction also minimized the possibility of selection bias. Second, we included a substantial number of participants (842,644) and cases involving fractures (108,247). Compared with separate case–control or cohort studies, our analysis significantly enhanced the study’s statistical power. Third, literature retrieval, data extraction and analysis, and methodological quality assessments were conducted by two independent investigators, and an experienced arbitrator verified the consistency of these two sets of reports, ensuring the accuracy of the data used in our meta-analysis.
Despite these strengths, several limitations must be considered. First, the quality of individual studies varied; several included studies had limited adjustment for potential statistical confounding, including three studies without clear adjustment [21, 39, 42]. The present study was also subject to confounding factors within the selected studies, which is an innate limitation of all observational studies and meta-analyses. Although most of the included studies were adjusted for age and sex, confounding by other risk factors remains a potential explanation for the observed findings. We examined the effect of adjustment in studies that provided unadjusted coefficients, but there was no evidence of attenuation of the RR of fractures associated with loop diuretics use after adjustment for multiple factors. Second, not all articles involved prospective cohort studies, which reduced the reliability of the conclusions to a certain extent. However, our sensitivity analyses and subgroup analyses show that different types of study designs were not the main sources of heterogeneity. Third, there was a high degree of heterogeneity among the included studies, which might have reduced the strength of our conclusions. We should interpret the results discreetly. The Egger’s and Begg’s tests confirmed the absence of statistical evidence of a publication bias, and the “trim and fill” method showed that there were no missing studies.
Suggestions for future studies
Based on our meta-analysis, several key points should be considered in future studies. First, because our study did not perform a dose–response analysis, the existence of a dose–response relationship between loop diuretics and fracture risk remains unknown. Second, we could not stratify the population based on current or past use of these drugs, nor on the duration of use. Therefore, several well-designed and stratified cohort studies are needed to clarify the relationship between dose and fracture risk, duration of treatment and fracture risk, and the use of loop diuretics and the fracture site (hip, pelvis, humerus, vertebrae, and other sites). Meanwhile, future studies should also adjust for other factors that may potentially increase the risk of fracture risk, including patient age, bone mineral density, body mass index, diabetes, cardiovascular disease, supplementary vitamins, alcohol consumption, and amount of exercise. Notwithstanding, this study found a small, statistically significant increase in the risk of hip fracture among users of loop diuretics. However, the results should be interpreted discreetly, and evaluated with respect to the cost-effectiveness of loop diuretics and their clinical significance.
Conclusion
In conclusion, loop diuretics show a significant positive association with the overall risk of total fractures and hip fractures. Additional well-designed and stratified cohort studies, with broad coverage of confounding factors, are needed to facilitate a more comprehensive understanding of the underlying biology of the association between loop diuretic use and the risk of fractures.
Abbreviations
- RR:
-
Relative risk
- CI:
-
Confidence interval
- HF:
-
Heart failure
References
Michaelsson K, Nordstrom P, Nordstrom A, Garmo H, Byberg L, Pedersen NL, Melhus H (2014) Impact of hip fracture on mortality: a cohort study in hip fracture discordant identical twins. J Bone Miner Res 29:424–431
Koh GC, Tai BC, Ang LW, Heng D, Yuan JM, Koh WP (2013) All-cause and cause-specific mortality after hip fracture among Chinese women and men: the Singapore Chinese Health Study. Osteoporos Int 24:1981–1989
Gronskag AB, Romundstad P, Forsmo S, Langhammer A, Schei B (2012) Excess mortality after hip fracture among elderly women in Norway. The HUNT study. Osteoporos Int 23:1807–1811
Sheng J, Qu X, Zhang X et al (2014) Coffee, tea, and the risk of hip fracture: a meta-analysis. Osteoporos Int 25:141–150
Qu X, Huang X, Jin F, Wang H, Hao Y, Tang T, Dai K (2013) Bone mineral density and all-cause, cardiovascular and stroke mortality: a meta-analysis of prospective cohort studies. Int J Cardiol 166:385–393
Qu X, Zhang X, Qin A, Liu G, Zhai Z, Hao Y, Li H, Zhu Z, Dai K (2013) Bone mineral density and risk of breast cancer in postmenopausal women. Breast Cancer Res Treat 138:261–271
Qu X, Zhang X, Zhai Z, Li H, Liu X, Li H, Liu G, Zhu Z, Hao Y, Dai K (2014) Association between physical activity and risk of fracture. J Bone Miner Res 29:202–211
Drake MT, Murad MH, Mauck KF et al (2012) Clinical review. Risk factors for low bone mass-related fractures in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 97:1861–1870
Kanis JA, Johnell O, Oden A et al (2005) Smoking and fracture risk: a meta-analysis. Osteoporos Int 16:155–162
De Laet C, Kanis JA, Oden A et al (2005) Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int 16:1330–1338
Shen C, Chen F, Zhang Y, Guo Y, Ding M (2014) Association between use of antiepileptic drugs and fracture risk: a systematic review and meta-analysis. Bone 64:246–253
Lim LS, Fink HA, Kuskowski MA, Taylor BC, Schousboe JT, Ensrud KE (2008) Loop diuretic use and increased rates of hip bone loss in older men: the Osteoporotic Fractures in Men Study. Arch Intern Med 168:735–740
Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D (2008) Resistant hypertension: diagnosis, evaluation, and treatment a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 51:1403–1419
Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA (2002) Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA 287:337–344
Rejnmark L, Vestergaard P, Heickendorff L, Andreasen F, Mosekilde L (2005) Effects of long-term treatment with loop diuretics on bone mineral density, calcitropic hormones and bone turnover. J Intern Med 257:176–184
Rose BD (1991) Diuretics. Kidney Int 39:336–352
Rejnmark L, Vestergaard P, Heickendorff L, Andreasen F, Mosekilde L (2006) Loop diuretics increase bone turnover and decrease BMD in osteopenic postmenopausal women: results from a randomized controlled study with bumetanide. J Bone Miner Res 21(1):163–170
Leipzig RM, Cumming RG, Tinetti ME (1999) Drugs and falls in older people: a systematic review and meta-analysis: II. Cardiac and analgesic drugs. J Am Geriatr Soc 47:40–50
Arampatzis S, Gaetcke LM, Funk GC, Schwarz C, Mohaupt M, Zimmermann H, Exadaktylos AK, Lindner G (2013) Diuretic-induced hyponatremia and osteoporotic fractures in patients admitted to the emergency department. Maturitas 75:81–86
Berry SD, Zhu Y, Choi H, Kiel DP, Zhang Y (2013) Diuretic initiation and the acute risk of hip fracture. Osteoporos Int 24:689–695
Rashiq S, Logan RF (1986) Role of drugs in fractures of the femoral neck. BMJ 292:861–863
Cumming RG, Klineberg RJ (1993) Psychotropics, thiazide diuretics and hip fractures in the elderly. Med J Aust 158:414–417
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151:W65–W94
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med 3:e123–e130
Wells G, Shea B, O’connell D, Peterson J, Welch V, Losos M, Tugwell P (2011) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
McNutt LA, Wu C, Xue X, Hafner JP (2003) Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol 157:940–943
Zhang J, Yu KF (1998) What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 280:1690–1691
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L (2007) Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med 26:4544–4562
Tromp A, Ooms M, Popp-Snijders C, Roos J, Lips P (2000) Predictors of fractures in elderly women. Osteoporos Int 11:134–140
Carbone LD, Johnson KC, Bush AJ, Robbins J, Larson JC, Thomas A, LaCroix AZ (2009) Loop diuretic use and fracture in postmenopausal women: findings from the Women’s Health Initiative. Arch Intern Med 169:132–140
Lim LS, Fink HA, Blackwell T, Taylor BC, Ensrud KE (2009) Loop diuretic use and rates of hip bone loss and risk of falls and fractures in older women. J Am Geriatr Soc 57:855–862
Solomon DH, Mogun H, Garneau K, Fischer MA (2011) Risk of fractures in older adults using antihypertensive medications. J Bone Miner Res 26:1561–1567
Heidrich FE, Stergachis A, Gross KM (1991) Diuretic drug use and the risk for hip fracture. Ann Intern Med 115:1–6
Jensen J, Nielsen LH, Lyhne N, Hallas J, Brosen K, Gram LF (1991) Drugs and femoral neck fracture: a case–control study. J Intern Med 229:29–33
Partanen J, Heikkinen J, Jamsa T, Jalovaara P (2002) Characteristics of lifetime factors, bone metabolism, and bone mineral density in patients with hip fracture. J Bone Miner Metab 20:367–375
Rejnmark L, Vestergaard P, Mosekilde L (2006) Fracture risk in patients treated with loop diuretics. J Intern Med 259:117–124
Bilik D, McEwen LN, Brown MB et al (2010) Thiazolidinediones and fractures: evidence from translating research into action for diabetes. J Clin Endocrinol Metab 95:4560–4565
Rejnmark L, Vestergaard P, Pedersen AR, Heickendorff L, Andreasen F, Mosekilde L (2003) Dose-effect relations of loop- and thiazide-diuretics on calcium homeostasis: a randomized, double-blinded Latin-square multiple cross-over study in postmenopausal osteopenic women. Eur J Clin Invest 33:41–50
Power ML, Heaney RP, Kalkwarf HJ, Pitkin RM, Repke JT, Tsang RC, Schulkin J (1999) The role of calcium in health and disease. Am J Obstet Gynecol 181:1560–1569
Poon IO, Braun U (2005) High prevalence of orthostatic hypotension and its correlation with potentially causative medications among elderly veterans. J Clin Pharm Ther 30:173–178
Ganz DA, Bao Y, Shekelle PG, Rubenstein LZ (2007) Will my patient fall? JAMA 297:77–86
Berry SD, Mittleman MA, Zhang Y, Solomon DH, Lipsitz LA, Mostofsky E, Goldense D, Kiel DP (2012) New loop diuretic prescriptions may be an acute risk factor for falls in the nursing home. Pharmacoepidemiol Drug Saf 21:560–563
Acknowledgments
This study was supported by the National Nature Science Foundation of China (Grant No. 31170901), the Fund for Key National Basic Research Program of China (Grant No. 2012CB619101), and the Major Basic Research of Science and Technology Commission of Shanghai Municipality (Grant No. 11DJ1400303). The funding sources had no role in the study design, collection, analysis, or interpretation of the data, or in the writing of the report.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. S1
Funnel plot of relative risk vs standard error of the log of relative risk (GIF 339 kb)
Rights and permissions
About this article
Cite this article
Xiao, F., Qu, X., Zhai, Z. et al. Association between loop diuretic use and fracture risk. Osteoporos Int 26, 775–784 (2015). https://doi.org/10.1007/s00198-014-2979-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-014-2979-8