Abstract
Summary
A 12-month extension phase of DIRECT in Japanese subjects with osteoporosis showed that total 3 years of denosumab treatment in Japanese postmenopausal women and men with osteoporosis was associated with low fracture rates, persistent bone turnover marker (BTM) reductions, continuous bone mineral density (BMD) increases, and a favorable overall benefit/risk profile.
Introduction
The DIRECT trial demonstrated that 2 years of treatment with denosumab 60 mg subcutaneously every 6 months significantly reduced the incidence of vertebral fracture compared to placebo in Japanese postmenopausal women and men with osteoporosis. The purpose of this study is to evaluate the efficacy and safety of denosumab treatment for up to 3 years.
Methods
This study includes a 2-year randomized, double-blind, placebo-controlled phase and a 1-year open-label extension phase in which all subjects received denosumab. The data correspond to 3 years of denosumab treatment in subjects who received denosumab (long-term group) and 1 year of denosumab treatment in subjects who received placebo (cross-over group) in the double-blind phase.
Results
Eight hundred and ten subjects who completed the double-blind phase enrolled into the extension phase, and 775 subjects completed the study. All subjects received denosumab with daily supplements of calcium and vitamin D. The cumulative 36-month incidences of new or worsening vertebral fractures and new vertebral fractures were 3.8 and 2.5 %, respectively, in the long-term group. In this group, the BMD continued to increase, and the reduction in BTMs was maintained. In the cross-over group, comparable BMD increases and BTMs reductions to those of in their first year of the long-term group were confirmed. Adverse events did not show a notable increase with long-term denosumab administration. One event of osteonecrosis of the jaw occurred in the cross-over group.
Conclusions
Three-year denosumab treatment in Japanese subjects with osteoporosis showed a favorable benefit/risk profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a chronic disease characterized by compromised bone strength mainly due to decreased bone mass and microarchitectural deterioration of the skeleton, leading to an increased risk of fracture [1], requiring long-term treatment. Bone mineral density (BMD) and bone turnover markers (BTMs) are the main biomarkers associated with anti-fracture efficacy of antiresorptive drugs for the treatment of osteoporosis [2, 3].
Denosumab, a fully human monoclonal IgG2 antibody, binds RANK ligand with high affinity and specificity, resulting in the inhibition of osteoclast formation, function, and survival [4–7]. In the 3-year, placebo-controlled FREEDOM study [8], administration of 60-mg denosumab subcutaneously every 6 months to predominantly postmenopausal Caucasian women with osteoporosis significantly reduced the risk of new vertebral, hip, and nonvertebral fractures by 68, 40, and 20 %, respectively, associated with significant increase in BMD at all skeletal sites measured and BTM reduction compared with placebo. The ongoing 7-year FREEDOM extension study [9–11] has shown that denosumab treatment for up to 8 years (up to 5 years in the extension study) was associated with continued increases in BMD, persistent reduction of bone turnover, low fracture incidence, and no increase in adverse event incidence, thus, indicating that the benefit/risk profile for denosumab remains favorable with extended exposure to denosumab [9–11].
The anti-fracture efficacy of denosumab has also been demonstrated in Japanese subjects in DIRECT (Denosumab Fracture Intervention Randomized Placebo Controlled Trial) [12]. Denosumab 60 mg given as a subcutaneous injection every 6 months for 2 years significantly decreased the risk of new or worsening vertebral fractures by 65.7 % in Japanese subjects with osteoporosis, compared with placebo. Characterization of the long-term efficacy and safety of denosumab in Japanese patients with osteoporosis is essential for clinical practice because osteoporosis is a chronic disease requiring long-term treatment. For this reason, the DIRECT study also included a 1-year open-label extension to evaluate the efficacy and safety of up to 3 years of denosumab treatment.
Methods
Study design
This 3-year prospective multicenter intervention study, consisted of a 2-year randomized, double-blind, placebo-controlled phase with an open-label alendronate referential arm and a 1-year open-label extension phase. Generally, healthy Japanese subjects with osteoporosis including postmenopausal women and men were randomly assigned in a 2:2:1 ratio to receive one of the following three treatments for a period of 2 years: denosumab 60 mg given as a subcutaneous injection every 6 months, matching placebo, or open-label alendronate 35 mg taken orally every week. Randomization was stratified by gender. The eligibility criteria were age ≥50 years, 1–4 prevalent vertebral fractures, and low bone mineral density (BMD) (T-score < −1.7 at lumbar spines [L1–L4] or < −1.6 at total hip). All subjects in the denosumab and placebo groups who completed the 2-year double-blind phase were eligible to enter the 1-year extension phase. The study duration in the alendronate group was 2 years and was not included into the extension phase. During the open-label extension phase, all subjects received 60 mg denosumab subcutaneously every 6 months for 1 year with daily supplements containing at least 600 mg calcium and 400 IU vitamin D.
The institutional review boards at all study sites approved the protocol and consent process for this study, and all subjects provided written informed consent for the entire study duration before participation in the double-blind phase. This study was conducted in compliance with the ethical principles of the Declaration of Helsinki and in accordance with Good Clinical Practice.
Study procedures
In the 1-year open-label extension phase, all safety and efficacy assessments continued with the same methodology used during the double-blind phase [12].
Fracture assessment
X-ray images were taken to identify the vertebral fractures at the baseline, 6, 12, 18, 24, and 36 months or study withdrawal. To identify a morphometric vertebral fracture, the vertebral bodies of the lateral projection from Th4 to L4 were assessed using both the semi-quantitative (SQ) method and quantitative morphometry (QM) method by the experts of the central committee [13, 14]. For assessment of a nonvertebral fracture, investigators took radiographs during the study to identify the fracture whenever a subject reported clinical symptoms. Then, the committee reviewed the radiographs to identify the fracture.
BMD assessment
BMD measurement as evaluated by dual X-ray absorptiometry was conducted at the lumbar spine (L1–L4), total hip and femoral neck at the baseline, 3, 6, 12, 18, 24, and 36 months or study withdrawal. BMD at a distal 1/3 radius was also measured at the baseline, 6, 12, 18, 24, and 36 months or study withdrawal. The QDR scanner (Hologic, Bedford, MA, USA) was used in this study. Quality control and BMD scan analysis were performed centrally (Synarc, Portland, OR, USA).
BTM assessment
Serum and urinary samples were obtained under fasting conditions at the baseline, 1, 3, 6, 12, 24, 25, and 36 months or study withdrawal. All serum and urinary samples were taken before administration of the investigational product (IP) and were measured in a central laboratory. Serum C-telopeptide of type 1 collagen (CTX-1) was evaluated by enzyme-linked immunosorbent assay. Serum bone specific alkaline phosphatase (BSAP) was evaluated by chemiluminescent enzyme immunoassay. Serum intact PTH was evaluated by electro chemiluminescence immunoassay. All BTMs were assayed by Mitsubishi Chemical Medience Corporation, Tokyo, Japan.
Safety assessment
All subjects were questioned concerning AEs at each visit, and all AEs were assessed regardless of the determination of causality by the investigator. The Medical Dictionary for Regulatory Activities (MedDRA, Version 14.0) was used to categorize reported AEs. Laboratory tests including blood chemistry, hematology, and urinalysis were assessed at the baseline, 1, 6, 12, 18, 24, 25, 30, and 36 months and were measured in the central laboratory. Serum albumin-adjusted calcium (mg/dL) was calculated as follows: actual serum calcium (mg/dL) – 0.8 × [serum albumin [g/dL] -4 ] (only when albumin was <4.0 [g/dL]). Safety was assessed by recording all AEs, serious AEs, fatal AEs, AEs leading to study discontinuation, and AEs leading to discontinuation of IP. AEs of interests such as hypocalcemia, bacterial cellulitis, infection, eczema, events potentially related to hypersensitivity, cardiovascular disorder, malignant or unspecified tumors, fracture healing complication, atypical fracture of femur, and osteonecrosis of the jaw (ONJ) were specified in advance. A dental expert of this study reviewed each potential case of ONJ in a blinded manner. Study investigators clinically assessed the healing of nonvertebral fractures within 6 months after their occurrence. Denosumab-specific antibodies were assessed in those samples of subjects randomized in the denosumab or placebo group. The anti-denosumab antibodies were assessed at the baseline, 1, 6, 12, 18, 24, 25, 30, and 36 months. A validated electrochemiluminescent immunoassay (PPD Inc., VA, USA and Amgen Inc., CA, USA) was used to detect denosumab-binding antibodies; samples with binding antibodies were later screened for denosumab-neutralizing antibodies by a cell-based assay (Amgen Inc., CA, USA).
Statistical analysis
The objective of the extension was to describe the efficacy including evaluation of the incidence of vertebral and nonvertebral fractures, and the changes in BMD and BTMs, and safety and tolerability of denosumab for 3-years.
Efficacy analyses were performed using the full analysis set (FAS) which includes all randomized subjects except for those who did not have osteoporosis at screening, did not receive the IP, or had no available efficacy data after the first dose of the IP. For post hoc analyses, a p value of less than 0.05 was considered as statistically significant.
The cumulative incidence rates for new or worsening vertebral fractures, new vertebral fractures, nonvertebral fractures, and major nonvertebral fractures, consisting of proximal humerus, forearm, ribs/clavicle, pelvis, hip, distal femur, and proximal tibia, were estimated by the Kaplan-Meier method. The annual crude subject incidence rates for new or worsening vertebral fracture and new vertebral fracture were also provided. As a post hoc analysis, the number of new vertebral fractures for each year (i.e., year 1, year 2, and year 3) was analyzed by the generalized estimating equation (GEE) approach using a Poisson regression model with treatment, year and treatment by year interaction. The results for comparisons between treatment groups at year 1 and year 2 and between years in each treatment group were presented as rate ratios, the corresponding 95 % confidence intervals (CIs), and p values based on empirical variance. The percent changes from the baseline in BMD were presented at the time points of interest. In post hoc analysis, comparisons of BMD between the baseline and each time point, and between 24 and 36 months were conducted by Student’s t test. The results were presented as means with 95 % CIs. The percent changes from the baseline in BTM at the time point of interest were presented as medians and interquartile ranges. In post hoc analysis, comparisons of BTM between the baseline and each time point, and between 24 months and 36 months were conducted by the Wilcoxon signed rank test.
Safety analyses included subjects who received at least 1 dose of IP; however, the summary of AEs in a 1-year open-label extension phase included subjects who received at least 1 dose of IP in this phase. AEs were summarized by subject incidence rates. The concentration of the serum albumin-adjusted calcium at the baseline and subsequent time points were presented as means and SDs.
Results
Disposition and characteristics
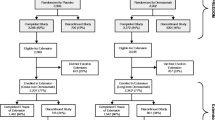
Of the 1262 subjects (500 in the denosumab group, 511 in the placebo group, and 251 in the alendronate group) enrolled in the double-blind phase for 2 years [12], 810 (404 in the denosumab group and 406 in the placebo group) were enrolled in the 1-year open-label extension phase. A total of 775 subjects (389 from the denosumab group [long-term group] and 386 from the placebo group [cross-over group]) completed the extension phase. Subjects in the alendronate group were not transferred to the 1-year open-label extension phase based on the protocol definition.
Subject characteristics at the baseline and month 24 are shown in Table 1. At the start of the 1-year open-label extension (i.e., month 24 of DIRECT), BMD T-scores were increased, and BTM values decreased, relative to the baseline (i.e., day 1 of DIRECT) in the long-term group, whereas they were comparable to the baseline (i.e., day 1 of DIRECT) in the cross-over group.
Fractures
The 2-year incidence of new vertebral fractures was 2.2 % in the denosumab group and 8.6 % in the placebo group, with the reduction in risk by 74.0 % (p < 0.0001) [12], and the rate ratios comparing the number of new vertebral fractures for the denosumab group in year 1 and 2 with those for the placebo group were 0.44 (p = 0.0937) and 0.14 (p = 0.0001), respectively (Table 2).
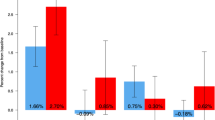
In the long-term group, the cumulative incidences of new or worsening vertebral and new vertebral fractures at 36 months were 3.8 % (95 % CIs, 2.4; 6.1) and 2.5 % (95 % CIs, 1.4; 4.5), respectively. The crude incidences (annual rate) of new or worsening vertebral and new vertebral fractures were 1.9 and 1.3 %, respectively, in the first year, 1.6 and 0.9 %, respectively, in the second year, and 0.3 and 0.3 %, respectively, in the third year (Fig. 1a, b). The rate ratios comparing the number of new vertebral fractures for year 2 and 3 with that for year 1 were 0.89 (p = 0.8327) and 0.19 (p = 0.1261), respectively (Table 2). The cumulative incidences of nonvertebral fracture and major nonvertebral fracture at 36 months were 5.1 % (95 % CIs, 3.4; 7.7) and 2.1 % (95 % CIs, 1.1; 4.0), respectively (Fig. 1c, d).
Incidences of fractures during the overall treatment period. The crude incidences of new or worsening vertebral fractures (a) and new vertebral fractures (b). The cumulative incidence of nonvertebral fractures (c) and major nonvertebral fractures (d) For panels c and d, the percentages given were calculated using the Kaplan-Meier estimate over a 36-month treatment period
In the cross-over group, the cumulative incidences of new or worsening vertebral and new vertebral fractures at 36 months were 11.8 % (95 % CIs, 9.1; 15.2) and 10.3 % (95 % CIs, 7.8; 13.5), respectively. The crude incidences (annual rate) of new or worsening vertebral and new vertebral fractures in the first, second, and the third years were 2.7, 5.9, and 1.9 %, respectively (Fig. 1a, b). The rate ratio comparing the number of new vertebral fractures for year 2 with that for year 1 was 2.87 (p = 0.0027), and the rate ratio comparing the number of new vertebral fractures for year 3 with that for year 2 was 0.23 (p = 0.0003) (Table 2). The cumulative incidences of nonvertebral fractures and major nonvertebral fractures at 36 months were 6.6 % (95 % CIs, 4.6; 9.5) and 5.5 % (95 % CIs, 3.7; 8.1), respectively (Fig. 1c, d).
BMD
In the long-term group, increases from the baseline in BMD after 3 years of continued denosumab treatment were 11.0 % at the lumbar spine, 5.3 % at the total hip, 4.8 % at the femoral neck, and 0.9 % at the distal 1/3 radius (p < 0.001) (Fig. 2a–d). From 24 months to 36 months BMD increased by 1.8 % at the lumbar spine, 0.6 % at the total hip, 0.8 % at the femoral neck, and 0.4 % at the distal 1/3 radius (p < 0.001).
Mean BMD percentage changes from the baseline. Mean BMD percentage changes from the baseline over a 36-month treatment period at the lumbar spine (L1–L4) (a), total hip (b), femoral neck (c), and distal one third radius (d) are shown. a p < 0.001 based on the Student’s t test at each time point from the baseline. b p < 0.05 based on the Student’s t test at each time point from the baseline. c p < 0.001 based on the Student’s t test at 36-month vs 24-month. The bars show 95 % CIs of the mean values at each time point. A central vender (Synarc Inc.) performed all BMD analyses. Abnormal vertebrae, such as those with an abnormality, fracture, or artifact, were excluded from analyses
In the cross-over group, BMD changes at 36 months (1 year after the first dose of denosumab) were 5.4 % at the lumbar spine, 1.4 % at the total hip, 1.1 % at the femoral neck, and −1.3 % at the distal 1/3 radius from the baseline (p < 0.001) (Fig. 2a–d). The BMD increased from 24 to 36 months by 5.3 % at the lumbar spine, 2.6 % at the total hip, 2.3 % at the femoral neck, and 0.5 % at the distal 1/3 radius (p < 0.001).
BTMs
In the long-term group, serum CTX-1 was greatly decreased by 70.9 % at 1 month with relatively sustained reductions thereafter. Serum BSAP was reduced by 9.8 % at 1 month and 50.2 % at 3 months, and remained relatively stable thereafter (Fig. 3a, b).
Median BTM mean serum and calcium changes during the overall treatment period. Median BTM percentage changes from the baseline. Median BTM changes from the over a 36-month treatment period for serum CTX-1 (a), serum BSAP (b) and iPTH (d) are shown. a p < 0.01 and b p< 0.05 based on the Wilcoxon signed rank test for each time point from the baseline. c p < 0.01 based on the Wilcoxon signed rank test at 25- or 36- vs 24-month. The bars show the interquartile range of the percentage changes from the baseline at each time point. Mean changes from the baseline over a 36-month treatment period for albumin-corrected serum Ca (c) are shown. a p < 0.01 and b p < 0.05 based on paired t test for each time point from the baseline. c p < 0.01 and d p < 0.05 based on paired t test at 25-, 30 or 36- vs 24-month. The bars show SD of changes from the baseline at each time point
In the cross-over group, serum CTX-1 was greatly decreased by 77.9 % at 25 months (1 month after the first dose of denosumab) and remained relatively stable thereafter. Serum BSAP was also reduced by 19.6 % at 25 months and reached the same level as the long-term group at 36 months (Fig. 3a, b).
Albumin-adjusted serum calcium and intact PTH
In the long-term group, mean albumin-adjusted serum calcium was 9.09 and 9.14 mg/dL at month 1 and month 25, and median intact PTH was 54 pg/ml at 1 month and 44 pg/ml at month 25 (Fig. 3c, d).
In the cross-over group, mean albumin-adjusted serum calcium was 9.09 mg/dL at 25 months, and median intact PTH was 56 pg/ml at 25 months. These changes in albumin-adjusted serum calcium and intact PTH had tendency to return to the baseline level during follow-up period.
Safety
The incidences of all AEs, serious AEs, fatal AEs, AEs leading to study discontinuation, AEs leading to discontinuation of IP, AEs of interest, and serious AEs of interest did not show a notable increase with longer denosumab treatment from 2 to 3 years, irrespective of the different duration of the observation periods. During the additional 1-year extension phase, the incidences of all AEs, serious AEs, fatal AEs, AEs leading to study discontinuation, and AEs leading to discontinuation of IP in the long-term group were similar to those in the cross-over group. The incidences of AEs of interest and serious AEs of interest including hypocalcemia, bacterial cellulitis, infection, eczema, hypersensitivity, cardiovascular disorder, or malignant or unspecified tumors, were similar between the long-term and cross-over groups (Table 3). One oral AE (osteomyelitis) in the cross-over group was adjudicated by a medical expert as consistent with ONJ based on the definition of the protocol. This case occurred at the site of tooth extraction (tooth extraction was conducted during the open-label phase) after receiving 2 doses of denosumab. The subject reported the symptoms such as pus discharge, pain and swelling, and observations such as sequestrum. The subject was treated with antibiotics, intraoral irrigation, and curettement, and the event was confirmed resolved within the follow-up period. No cases of delayed fracture healing or atypical fracture of the femur were reported in either treatment groups. No subjects developed neutralizing antibodies to denosumab in the long-term or cross-over group.
Discussion
The extension phase of DIRECT involved Japanese subjects with osteoporosis who had received denosumab or placebo in the double-blind phase for 24 months, providing an opportunity to evaluate the long-term efficacy and safety of continued denosumab administration up to 36 months. In this extension phase, continuous denosumab treatment maintained a low rate of vertebral fractures and BTMs reduction, increased BMD at all sites, and was well tolerated.
The incidences of the new or worsening and new vertebral fractures at 36 months in the long-term group were low and showed no difference between the 2nd and 3rd year of treatment. A post hoc analysis, conducted to evaluate the prolonged treatment effect of denosumab on new vertebral fracture risk reduction, showed that the rate of new vertebral fractures for year 2 was comparable with that for year 1, and that for year 3 trended to be lower than that for year 1. Therefore, the effect of denosumab treatment on vertebral fracture risk persists through 3 years of treatment. In the FREEDOM study, the crude incidences (annual rate) of new vertebral fractures in the first, second, and third years were 0.9, 0.7, and 1.1 %, respectively, in the denosumab group [8]. Thus, the results of the extension phase of DIRECT, conducted in a Japanese population, are consistent with those from FREEDOM extension, conducted in a predominantly Caucasian population. Additionally, denosumab shows low incidence of new vertebral fractures until 8 years [9–11]. These data suggest consistent long-term anti-fracture efficacy of denosumab, independent of race/ethnicity.
In the cross-over group, the incidences of new or worsening vertebral fracture and new vertebral fracture in the third year were lower, as expected, than those in the first and second years (when subjects received placebo) and were comparable to those observed in the first year of the long-term group. The post hoc analysis revealed that the rate of new vertebral fractures for year 3 treatment was statistically lower than that for year 2. The rate ratio of that for year 2 compared to that for year 1 was significantly higher, and this result indicates that vertebral fracture risk would increase in a time-dependent manner. Together, these results indicated that the early onset of the effect on the vertebral fracture risk reduction would be expected by denosumab treatment. The rate ratio comparing the number of new vertebral fracture for the denosumab group with that for the placebo group showed a tendency for lower rates in year 1 and a significantly lower rate in year 2. These results suggest that the magnitude of effect on fracture risk reduction by denosumab depends on treatment duration.
In this study, continuous BMD increases and BTM reductions were observed for 36 months in the long-term group. These results are consistent with those from the FREEDOM study [8]. There are no significant differences in the percent changes from the baseline to 36 months in the lumbar spine and total hip BMD with denosumab between the DIRECT and FREEDOM study (11.0 vs ca. 9 % at the lumbar spine, 5.3 % vs ca. 6 % at the total hip) [8]. Similarly, the CTX reduction was maintained through 36 months in both studies. Continuous BMD increase and BTM reductions in the FREEDOM extension study [9–11], the comparability of the effect on BMD and BTMs, and no marked differences in the pharmacokinetic profile between Japanese and non-Japanese subjects [15] were reported. Thus, long-term efficacy on Japanese patients with osteoporosis could also be expected after 36 months from the results of denosumab treatment. In the cross-over group, rapid BMD increases and BTM reductions were observed, which were consistent with the results in the cross-over group from FREEDOM extension previously reported [9, 10].
Mild decreases in albumin-adjusted serum calcium levels and increases in intact PTH concentrations in the long-term group were observed at 1 and 25 months (i.e., 1 month after the previous denosumab dose). In the cross-over group, similar changes in albumin-adjusted serum calcium and intact PTH were also observed at 25 months (i.e., 1 month after the first denosumab dose). These increases in serum intact PTH occur concurrently with decreases in the albumin-adjusted serum calcium level and, therefore, they most likely represent a compensatory response to reductions in serum calcium with denosumab treatment.
The subject incidences of all AEs, serious AEs, fatal AEs, AEs leading to study discontinuation, and AEs leading to discontinuation of IP for 36 months treatment with denosumab were not notably increased compared to those for 24 months irrespective of the longer duration of the observation period. One case was adjudicated consistent with ONJ in the cross-over group of the extension phase. In the extension phase for 12 months, no apparent difference in AEs of interest between the long-term and cross-over group was observed. The incidences of AEs of interest showed no trend toward increases throughout the study period. All confirmed hypocalcemia were mild in severity and did not lead to discontinuation of the IP or study withdrawal. There was no delayed fracture healing or atypical femoral fractures in this study. Treatment with denosumab for 36 months in Japanese subjects with osteoporosis was well tolerated based on the findings of the extension phase in all, as was also demonstrated with up to 8 years of exposure in the FREEDOM study (3 years) and the FREEDOM extension study (5 years) [9–11].
This extension phase in DIRECT has several limitations. The number of patients was relatively small and the extension did not have a placebo arm. Due to the small number of subjects, this study is considered to have inadequate power to detect adverse events with very low incidence such as atypical femoral fractures. Additionally, since all subjects received denosumab in the 12-month extension phase, it is difficult to rigorously investigate long-term vertebral fracture risk reduction. This study was not designed to observe the significant difference in non-vertebral and hip fractures and could not provide the direct effects on these fractures in Japanese patients but the positive relationship between total hip BMD increase and nonvertebral fracture risk reduction has been demonstrated in the FREEDOM study [16] and continuous BMD increase at the total hip was observed in DIRECT. Therefore, nonvertebral fracture risk reduction in Japanese patients with osteoporosis would be also expected.
In conclusion, a 12-month extension phase of DIRECT in Japanese subjects with osteoporosis showed that 3 years of denosumab treatment in Japanese postmenopausal women and men with osteoporosis was associated with low fracture rates, persistent BTM reductions, continuous BMD increases, and a favorable overall benefit/risk profile.
References
NIH (2001) Consensus conference (2001) osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–795
Hochberg MC, Greenspan S, Wasnich RD, Miller P, Thompson DE, Ross PD (2002) Changes in bone density and turnover explain the reductions in incidence of nonvertebral fractures that occur during treatment with antiresorptive agents. J Clin Endocrinol Metab 87(4):1586–1592
Ivaska KK, Gerdhem P, Väänänen HK, Akesson K, Obrant KJ (2010) Bone turnover markers and prediction of fracture: a prospective follow-up study of 1040 elderly women for a mean of 9 years. J Bone Miner Res 25(2):393–403
Fuller K, Wong B, Fox S, Choi Y, Chambers TJ (1998) TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med 188(5):997–1001
Lacey DL, Tan HL, Lu J, Kaufman S, Van G, Qiu W, Rattan A, Scully S, Fletcher F, Juan T, Kelley M, Burgess TL, Boyle WJ, Polverino AJ (2000) Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am J Pathol 157(2):435–448
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93(2):165–176
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A 95(7):3597–3602
Cummings SR, Martin JS, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Christiansen C (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
Papapoulos S, Chapurlat R, Libanati C, Brandi ML, Brown JP, Czerwinski E, Krieg M-A, Man Z, Mellstrom D, Radominski SC, Reginster J-Y, Resch H, Ivorra JAR, Roux C, Vittinghoff E, Austin M, Daizadeh N, Bradley MN, Grauer A, Cummings SR, Bone HG (2012) Five years of denosumab exposure in women with postmenopausal osteoporosis: results from the first two years of the FREEDOM extension. J Bone Miner Res 27(3):694–701
Bone HG, Chapurlat R, Brandi ML, Brown JP, Czerwinski E, Krieg MA, Mellström D, Radominski SC, Reginster JY, Resch H, Ivorra JA, Roux C, Vittinghoff E, Daizadeh NS, Wang A, Bradley MN, Franchimont N, Geller ML, Wagman RB, Cummings SR, Papapoulos S (2013) The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J Clin Endocrinol Metab 98(11):4483–4492
Papapoulos S, Lippuner K, Roux C, Hall J, Kendler D, Lewiecki EM, Brandi ML, Czerwiński E, Franek E, Lakatos P, Mautalen C, Minisola S, Reginster JY, Jensen S, Daizadeh N, Wang A, Geller M, Wagman RB, Bone HG (2013) Eight Years of Denosumab Treatment in Postmenopausal Women With Osteoporosis: Results From the First Five Years of the FREEDOM Extension [Abstract LB-MO26] American Society for Bone and Mineral Research 2013 Annual Meeting
Nakamura T, Matsumoto T, Sugimoto T, Hosoi T, Miki T, Gorai I, Yoshikawa H, Tanaka Y, Tanaka S, Sone T, Nakano T, Ito M, Matsui S, Yoneda T, Takami H, Watanabe K, Osakabe T, Shiraki M, Fukunaga M (2014) Fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis: denosumab fracture intervention randomized placebo controlled trial (DIRECT). J Clin Endocrinol Metab 99(7):2599–2607
Genant HK, Jergas M, Palermo L, Nevitt M, Valentin RS, Black D, Cummings SR (1996) Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis. J Bone Miner Res 11(7):984–996
Orimo H, Sugioka Y, Fukunaga M, Muto Y, Hotokebuchi T, Gorai I, Nakamura T, Kushida K, Ikai H, Tanaka T, Oh-hashi Y (1998) Diagnostic criteria of primary osteoporosis. J Bone Miner Metab 16:139–150
Kumagai Y, Hasunuma T, Padhi D (2011) A randomized, double-blind, placebo-controlled, single-dose study to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of denosumab administered subcutaneously to postmenopausal Japanese women. Bone 49(5):1101–1107
Austin M, Yang YC, Vittinghoff E, Adami S, Boonen S, Bauer DC, Bianchi G, Bolognese MA, Christiansen C, Eastell R, Grauer A, Hawkins F, Kendler DL, Oliveri B, McClung MR, Reid IR, Siris ES, Zanchetta J, Zerbini CA, Libanati C, Cummings SR (2012) FREEDOM Trial. Relationship between bone mineral density changes with denosumab treatment and risk reduction for vertebral and nonvertebral fractures. J Bone Miner Res 27(3):687–693
Acknowledgments
The study was sponsored by Daiichi-Sankyo., Tokyo, Japan. The authors acknowledge Amgen for editorial assistance on the manuscript.
Conflicts of interest
T. Sugimoto has received consulting fees from Asahi-Kasei Pharma, and Daiichi-Sankyo. He has received research grants from Eli Lilly Japan, Eisai, MSD, Taisho Toyama Pharmaceutical, Chugai Pharmaceutical, Daiichi-Sankyo, and Pfizer. T. Matsumoto has received consulting fees from Daiichi-Sankyo, Ono Pharmaceutical., Asahi-Kasei Pharma., Chugai Pharmaceutical, Astellas Pharma, and Teijin Pharma. T. Hosoi, T. Miki, and I. Gorai have received consulting fees from Daiichi-Sankyo. H. Yoshikawa has received consulting fees from Ono Pharmaceutical, Asahi-Kasei Pharma, and Eisai. He has also received speaker’s bureau from Asahi-Kasei Pharma, Ono Pharmaceutical, Astellas Pharma, and Eli Lilly Japan. Y. Tanaka has received consulting fees from Mitsubishi-Tanabe Pharma, Otsuka Pharma, Pfizer, Eli Lilly Japan, Astellas Pharma, Janssen Pharma, Astra-Zeneca, Mitsubishi-Tanabe Pharma, Abbott Japan, MSD, Daiichi-Sankyo, GlaxoSmithKline, Actelion Pharma Japan, Chugai Pharmaceutical, Eisai, and Santen Pharma. He has also received research grants from Eisai, Pfizer, Janssen Pharma, Astellas Pharma, Bristol-Myers Squibb, Abbott Japan, and Chugai Pharmaceutical. S. Tanaka has received speaker's bureau from Eli Lilly Japan, Janssen Pharmaceutical, Eisai, Santen Pharmaceutical, Teijin Pharma, Nippon Zoki, Johnson & Johnson, Chugai Pharmaceutical, Daiichi-Sankyo, Ono Pharmaceutical, GSK, Pfizer, Mitsubishi Tanabe Pharma, Hisamitsu Pharmaceutical, Asahi Kasei Pharma, Taisho Toyama Pharmaceutical, Astellas Pharma, and Otsuka Pharmaceutical. He has also received consulting fees from Eisai, Astellas Pharma, Bristol-Myers Squibb, Pfizer, Takeda Pharmaceutical, Asashi Kasei Pharma, Ono Pharmaceutical, Dainippon Sumitomo Pharma and Kaken Pharmaceutical, and research grants from Sanofi Aventis, Daiichi-Sankyo, and MSD. T. Nakano has received consulting fees from Asahi-Kasei Pharma, Teijin Pharma, Daiichi-Sankyo, and Chugai Pharmaceutical. M. Ito has received consulting fees from Asahi-Kasei Pharma, Daiichi-Sankyo, and Chugai Pharmaceutical. M. Fukunaga has received consulting fees from Asahi-Kasei Pharma and Astellas Pharma. T. Sone has received research grants from Daiichi-Sankyo Astellas Pharma, Takeda Pharmaceutical, Taisho Toyama Pharmaceutical, and Pfizer and speaker’s bureau from Daiichi-Sankyo, Chugai Pharmaceutical, MSD, Taisho Toyama Pharmaceutical, Pfizer, and Eli Lilly Japan. T. Yoneda has received consulting fees from Daiichi-Sankyo. S. Matsui has received consulting fees from Chugai Pharmaceutical, Daiichi-Sankyo, Eisai, and Takada Pharmaceutical. H. Takami, K Watanabe, T. Osakabe, and N. Okubo are Daiichi-Sankyo employees and own Daiichi-Sankyo stock. M Shiraki has received consulting fees from Daiichi-Sankyo, Chugai Pharmaceutical, Teijin Pharma, Asahi-Kasei Pharma and MSD. T. Nakamura has received consulting fees from Teijin Pharma, Daiichi-Sankyo, Chugai Pharmaceutical, Asahi-Kasei Pharma, and Amgen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sugimoto, T., Matsumoto, T., Hosoi, T. et al. Three-year denosumab treatment in postmenopausal Japanese women and men with osteoporosis: results from a 1-year open-label extension of the Denosumab Fracture Intervention Randomized Placebo Controlled Trial (DIRECT). Osteoporos Int 26, 765–774 (2015). https://doi.org/10.1007/s00198-014-2964-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-014-2964-2