Abstract
Summary
In patients with systemic lupus erythematosus (SLE), low bone mineral density (BMD) is associated with increased age, prolonged disease, low body mass index (BMI), and overlap with rheumatoid arthritis (RA). Elevated fibroblast growth factor (FGF)-23 in cyclosporine A (CsA) users with SLE are associated with decreased active vitamin D and osteocalcin.
Introduction
The objective of this study was to investigate the steroid and CsA effect on bone metabolism and serum FGF-23 in SLE patients.
Methods
Seventy-two SLE patients and 10 age- and sex-matched healthy individuals underwent blood tests for bone metabolic biomarkers and FGF-23, and lumbar spine dual-energy X-ray absorptiometry for BMD.
Results
Comparisons between patients and controls were made in premenopausal women/men younger than 50 years and postmenopausal women/men older than 50 years separately. SLE patients had more frequent low Z-score (≤ − 2.0, 8.5 vs. 0 %), osteopenia (−2.5 < T-score < −1.0, 52 vs. 50 %), and osteoporosis (T-score ≤ −2.5, 12 vs. 0 %), than the healthy age-compatible counterparts. BMD was significantly lower in patients with advanced age, longer disease duration, lower BMI, and overlap with RA (all p < 0.05 by multiple linear regression analyses). Serum FGF-23 was significantly higher and 1,25-dihydroxyvitamin D (1,25(OH)2D3) lower in SLE patients treated with glucocorticoid and CsA than in those not taking both of them (p = 0.027 and 0.002, respectively). The cumulative dose of glucocorticoid was inversely correlated with serum intact parathyroid hormone (r = −0.299, p = 0.011), C-terminal telopeptide of type I collagen (r = −0.581, p < 0.001), and osteocalcin (r = −0.648, p < 0.001). FGF-23 and the cumulative dose of CsA were positively correlated (r = 0.38, p = 0.001) and both were negatively correlated with 1,25(OH)2D3 (r = −0.266, p = 0.016 and r = −0.55, p < 0.001) and osteocalcin (r = −0.234, p = 0.034 and r = −0.274, p = 0.02).
Conclusion
SLE patients treated with glucocorticoid and CsA exhibited markedly decreased bone turnover. Those taking CsA had higher serum FGF-23 associated with suppression of 1,25(OH)2D3 and bone formation. Such high-risk patients necessitate regular screening of osteoporosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucocorticoids (GC) and cyclosporine A (CsA) are widely used to control autoimmunity in patients with systemic lupus erythematosus (SLE). They are responsible for drug-induced osteoporosis by markedly increasing bone resorption and impairing osteoblast maturation as well as mineralization [1–3]. Bone mineral density (BMD) rapidly declines within the first 3 months of GC use, nadirs at 6 months, and then goes through a gradual but steady course of loss [2]. Long-term or high-dose GC treatment is a definite risk factor for GC-induced osteoporosis and fractures [4]. A recent cohort study revealed a significantly higher chance of osteoporotic fractures in SLE patients, especially when they take GC longer than 6 months [5].
Fibroblast growth factor-23 (FGF-23), derived from osteocytes and osteoblasts, has been found to facilitate urine phosphate excretion by inhibition of sodium/phosphate exchangers in renal epithelial cells [6, 7]. FGF-23 exhibits potential roles in bone metabolism. It can decrease the level of active vitamin D (1,25-dihydroxyvitamin D3, 1,25(OH)2D3) and increase its inactive metabolites, 24,25(OH)2D3, by inhibiting 1α-hydroxylase and enhancing 24-hydroxylase, respectively [8]. In addition, FGF-23 has been proven to inhibit the production of parathyroid hormone (PTH) in vitro [9]. Recent studies have shown the relationship between FGF-23 and osteoporosis. In women with postmenopausal osteoporosis, the serum level of FGF-23 is significantly higher than in normal controls [10]. Elevated FGF-23 is associated with increased overall osteoporotic fracture risk in elderly men [11]. The relationships among GC treatment, BMD, PTH, and vitamin D level in SLE patients have been elucidated in recent years [12–15]. However, the effects of GC and CsA treatment on bone metabolic profiles, especially the FGF-23 level, and their relationships to BMD in SLE patients have not been investigated, and the role of FGF-23 and other markers of bone metabolism in drug-induced osteoporosis are less described.
The aim of this study was to compare the markers of bone and mineral metabolism, including FGF-23, PTH, 1,25(OH)2D3, and BMD in SLE patients and healthy controls, focusing especially on the effect of GC and CsA treatments on bone metabolism in lupus patients.
Patients and methods
Study populations
The study was approved by the Institutional Review Board of Taipei Veterans General Hospital (VGHIRB No. 2011-07-007IC). Consecutive SLE patients from outpatient clinics of the rheumatology department in the hospital were screened. Patients fulfilling all of the following were recruited: (1) the classification criteria of SLE [16, 17], (2) adult men or women above 20 years of age, and (3) taking either GC or CsA longer than 3 months. Ten age- and gender-matched healthy controls were enrolled from among the hospital’s staffs.
The exclusion criteria included patients who (1) smoked or drank alcohol above three glasses (about 42 g of alcohol) per day; (2) were pregnant or lactating; (3) had taken thyroxine, aromatase inhibitors (such as anatrozole, letrozole), drug inhibiting gonadal functions (such as leuprolide, medroxyprogesterone acetate), thiazolidinediones, selective serotonin reuptake inhibitors, anticonvulsants, heparin, or warfarin within the past 1 year; or (4) had associated end-stage renal disease, malignant tumor, cirrhosis, hyperthyroidism, hyperparathyroidism, Cushing’s syndrome, type 1 diabetes, anorexia nervosa, Turner syndrome, or osteogenesis imperfecta. The same exclusion criteria were applied to the control group.
Clinical profiles, bone status, and sample collections
Basic demographic data including age, sex, medication history, prior history of nontraumatic fracture, smoking status, body mass index (BMI), SLE disease activity index (SLEDAI [18]), and cumulative dose of GC and/or CsA per kilogram of body weight before enrollment were collected from medical records and questionnaires. BMI was calculated as body weight (kg) divided by the square of height (m). BMD of the lumbar spine (g/cm2) was evaluated using dual-energy X-ray absorptiometry (DXA, Discovery A, Hologic®, MA, USA, coefficient of variation 1 %) for each participant. Z-score or T-score data were interpreted according to the 2013 International Society for Clinical Densitometry consensus [19].
Serum, plasma, and spot urine samples were collected in the morning after at least 8 h of fasting, and were immediately centrifuged at 500×g and 1500×g, respectively. Plasma-free calcium was detected within 2 h after sampling, and all the other samples were stored at −80 °C before assay.
Laboratory assays
Plasma free calcium, serum phosphate, creatinine, and urine calcium, phosphate, and creatinine were analyzed using the Clinical Analyzer 7600-310 (Hitachi High Technologies, Tokyo, Japan). The amount of proteinuria (g/day) was surrogated by the spot urine protein/creatinine ratio. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation and represented as milliliters per minute per 1.73 m2 of body surface area [20]. Renal fractional excretion of inorganic phosphate (FEPi) was calculated as previously described [21]. Serum 1,25(OH)2D3 was measured using an I125 radioimmunoassay kit (DiaSorin, MN, USA) according to the manufacturer’s instructions. Serum intact PTH (iPTH) was quantified with an ARCHITEC® iPTH chemiluminescent microparticle assay kit (Abott, Weisbaden, Germany), and calcitonin was measured with a commercial two-site chemiluminescent immunometric assay (IMMULITE 2000 Calcitonin, the Siemens Laboratory Diagnostics, Germany). The following serum assays were performed using ELISA methods: FGF-23 (Millipore, Billerica, MA), C-terminal telopeptide (CTX, Serum CrossLaps® ELISA, Immunodiagnostics Systems, UK), and osteocalcin (N-MID® Osteocalcin ELISA, Immunodiagnostic Systems).
Statistical analysis
Categorical data were presented as number (%) and were compared with the Pearson’s χ 2 test; numerical data were presented as median (interquartile range) and were analyzed by Kruskal-Wallis test or Mann-Whitney U test. To survey the determinant of BMD levels, we performed multiple linear regression analyses by selecting all the clinical parameters into the model 1 and using stepwise method in the model 2. A receiver operating characteristic (ROC) curve analysis was conducted to determine a BMI cutoff that was properly associated with a higher BMD. To investigate the effects on bone metabolism-related markers, further subgroup analyses based on the utility of CsA and the cutoff dose of GC equivalent to 7.5 mg of prednisolone were used. Spearman’s correlation test was used to analyze the relationship between cumulative dose of GC and CsA and markers of bone and mineral metabolism in SLE patients. A p value of <0.05 was considered statistically significant. All tests were two-tailed and adjusted for multiple comparisons. All statistical analyses were performed using SPSS for Windows, version 19.0 (Chicago, IL, USA).
Results
Characteristics of the study population
In all, 72 SLE patients (97.2 % female; median age, 43.6 years) and 10 healthy volunteers (80 % female; median age, 33.2 years) were enrolled in this study. We classified the SLE patients into three different subgroups: those without GC or CsA treatment, those treated with GC, and those treated with both GC and CsA (as an add-on therapy to GC). Except for higher age, higher rates of postmenopausal woman and rheumatoid arthritis (RA) in SLE patients without GC or CsA treatment, and higher rates of peptic ulcer disease (PUD) or gastroesophageal reflux disease (GERD) in SLE patients with GC and CsA treatment, no other statistical difference was found in gender, BMI, smoking status, comorbidity with endocrine diseases, prior history of nontraumatic fracture, treatment with calcium, vitamin D, or bisphosphonates, and lumbar spine BMD (Table 1).
Compared to the other subgroups, SLE patients treated with both GC and CsA had a higher amount of proteinuria, higher daily dose and treatment duration of GC, higher rate of prior pulse methylprednisolone therapy, and lower rate of medication with hydroxychloroquine (all p < 0.05, Table 1).
BMD in SLE patients
Although no statistical significance was reached, 8.5 % of the premenopausal female and male SLE patients aged <50 years had a Z-score ≤ −2.0, compared to the age-matched healthy controls, who all presented with normal BMD (Fig. 1a). In the postmenopausal female and male SLE patients aged ≥50 years, the frequency of osteopenia and osteoporosis was higher than in the matched controls (52 vs. 50 % and 12 vs. 0 %, respectively, Fig. 1b). BMD as related to the age and the disease duration of the SLE patients is shown in Fig. 1c, d, respectively. Adjusted for postmenopausal status, presence of RA, PUD/GERD, SLE duration, SLEDAI score, and cumulative dose of GC and CsA in model 1, the SLE patients exhibited a steady and significant decrement in the BMD level with aging (β = −0.004, p = 0.013). The BMD was also significantly associated with BMI (β = −0.013, p = 0.040). Subsequent ROC analysis for a cutoff of BMI associated with higher BMD indicated a value of 23, which was associated with a BMD above its median, with sensitivity of 65.6 %, specificity of 63.2 %, and area under the curve of 0.626. Thus, BMD was significantly higher in patients with a BMI ≥ 23 than in those with a BMI < 23 (0.996 [0.932–1.069] vs. 0.918 [0.806–1.003] g/cm2, p = 0.014). In model 2, a significantly lower BMD was observed in SLE patients with overlap with RA and in those with a longer disease duration (β = −0.120, p = 0.031; β = −0.004, p = 0.045, respectively in Table 2).
Bone mineral density (BMD) of lumbar spine in SLE patients and healthy controls. a Distribution of Z-score in premenopausal women or men aged below 50 years. Four of the 47 (8.5 %) SLE patients had a Z-score ≤ −2.0. b Distribution of T-score in postmenopausal women or men aged of 50 years or more. Thirteen and 3 of the 25 SLE patients (52 and 12 %) had osteopenia and osteoporosis, respectively. Only two of the four healthy controls had osteopenia. Scattered plot of the data of lumbar spine BMD by age (c) and SLE duration (d). Dash and dotted lines indicate the regression lines of the patients with body weight ≥23 and <23 kg, respectively
GC, CsA, and bone metabolism
Regarding the effect of GC on bone metabolism in SLE patients, significantly lower serum CTX (57.06 vs. 59.95 pg/mL, p < 0.001, Fig. 2f) and osteocalcin (38.69 vs. 564.95 pg/mL, p < 0.001) levels were found in SLE patients treated with GC than in those without GC or CsA treatment. The cumulative dose of GC was inversely correlated with serum CTX (r = −0.581, p < 0.001) and osteocalcin (r = −0.648, p < 0.001, Table 3). Furthermore, a lower serum iPTH level was found in SLE patients treated with GC than in those without GC or CsA treatment (38.0 [16.4–53.4] vs. 50.0 [36.9–65.9] pg/mL, Fig. 2c). The cumulative dose of GC was negatively correlated with the serum iPTH (r = −0.299, p = 0.011) and spot urine calcium/creatinine ratio (r = −0.251, p = 0.033, Table 3). The serum-free calcium did not differ in SLE patients treated with and without GC (Fig. 2d and Table 3).
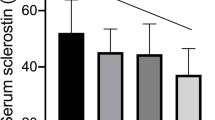
Box and whisker plot of the markers of bone and mineral metabolism in the healthy controls and SLE patients according to GC and CsA treatment. FGF-23 fibroblast growth factor-23, iPTH intact parathyroid hormone, Ca calcium, Cr creatinine, CTX C-terminal telopeptide of type I collagen, GC glucocorticoid, CsA cyclosporine A
To analyze the effect of CsA on bone metabolism, we minimized the effect of GC in SLE patients by inspecting patients who received prednisolone equivalent to ≤7.5 mg/day and neglecting those who received prednisolone equivalent to >7.5 mg/day. After this adjustment, CsA therapy was found to be associated with higher CTX and iPTH (58.3 [57.7–59.7] vs. 56.9 [56.0–57.6] pg/mL in Fig. 3f and 64.8 [46.7–94.0] vs. 38.0 [10.7–51.4] pg/mL in Fig. 3c, respectively). The cumulative dose of CsA was inversely correlated with osteocalcin (r = −0.274, p = 0.020, Table 3). In addition, CsA therapy was significantly associated with an increased serum FGF-23 level (56.2 [46.4–67.4] vs. 25.4 [14.0–43.1] pg/mL, p = 0.033 in Fig. 3a) and was also positively correlated with serum FGF-23 and renal FEPi (r = 0.380, p = 0.001; r = 0.234, p = 0.048, respectively, Table 3). On the other hand, CsA treatment was significantly associated with a lower serum 1,25(OH)2D3 (21.0 [16.3–38.7] vs. 70.1 [56.5–85.0] pg/mL, p = 0.011 in Fig. 3b) and spot urine calcium/creatinine ratio (0.012 [0.003–0.028] vs. 0.073 [0.025–0.137], p = 0.008 in Fig. 3e). Individual values of both of these parameters were also negatively correlated with the cumulative dose of CsA (r = −0.550, p < 0.001 and r = −0.360, p = 0.002, respectively). The serum-free calcium was not associated with CsA treatment (Fig. 3d and Table 3).
FGF-23, bone metabolism, and FGF-23 determinants
To understand the general effect of FGF-23 on bone metabolism, its levels in sera from SLE patients and normal individuals were analyzed as a whole. FGF-23 in serum was inversely correlated with osteocalcin (r = −0.234, p = 0.034) but not CTX (r = −0.112, p = 0.317), regardless of what treatment group, patients or normal controls. It was also inversely correlated with serum 1,25(OH)2D3 (r = −0.266, p = 0.016) but not iPTH (r = −0.018, p = 0.871). To analyze the determinants of FGF-23, we performed a stepwise multiple linear regression by adjusting age, postmenopausal status, presence of RA, PUD/GERD, hemoglobin level, eGFR, urine protein, serum iPTH, 1,25(OH)2D3, and cumulative dose of GC and found that the cumulative dose of CsA per kilogram of body weight was significantly associated with FGF-23 (β = −0.005, p = 0.008).
Discussion
This cross-sectional, case-control study demonstrated that premenopausal female and male SLE patients aged <50 years more frequently had a lumbar spine Z-score ≤ −2.0, while postmenopausal female SLE and male SLE patients aged ≥50 years had higher rates of osteopenia and osteoporosis than the age-matched healthy individuals. SLE patients exhibited a conspicuous decreasing in BMD parallel with aging and prolonged disease duration. A low BMD is also significantly associated with a low BMI or overlap with RA in SLE patients. A cumulative dose of GC was negatively correlated with serum iPTH and CTX. A cumulative dose of CsA was positively correlated with serum FGF-23, but negatively with serum 1,25(OH)2D3. Both cumulative doses of GC and CsA were inversely correlated with serum osteocalcin. These results imply that sufficient nutrition to improved BMI and regular check of osteoporosis after the diagnosis of SLE is a feasible strategy to ameliorate the bone loss effect of GC in SLE patients undergoing regular GC therapy. The osteoporotic effect of GC may originate partly from the inhibition of PTH and osteocalcin, and that of CsA originates instead from increasing FGF-23 and decreasing 1,25(OH)2D3 as well as osteocalcin production.
Up to now, the exact role of FGF-23 in bone metabolism and GC- or CsA-induced osteoporosis has not been well documented. The present investigation is probably the first to evaluate the effect of GC and CsA on FGF-23, together with its interaction in the bone metabolism of patients with SLE. Our results have provided a more comprehensive view of the changes in bone and mineral metabolism exerted by drug treatments and autoimmune process per se in SLE patients. Most of the potential confounding factors affecting osteoporosis showed no statistical differences between subgroups. Although the advanced age, postmenopausal status, and overlap syndrome of SLE and RA appeared to be most frequent in SLE patients without GC or CsA treatment, compared to those occurred in other subgroups, our data are still qualified, without exclusion, for the analyses to determine the differences of BMD, FGF-23, and the effect of GC and CsA on bone metabolism in SLE, rendering our deduction more straightforward.
Decreased BMD is a common comorbidity of SLE. As shown in the present investigation, the prevalence rate of Z-score ≤ −2.0 is 8.5 % among the young SLE patients, which is comparable with that reported (8.8 %) by Lee et al. [22]. Also, the prevalence rates of osteopenia (52 %; 39.7 to 54 %) and osteoporosis (12 %; 5 to 18 %) among the elderly SLE patients were compatible with those reported recently [15, 23–27]. Advanced age and low BMI, but not SLE disease activity, were significantly associated with low BMD and were consistent with those of previous investigations [23, 24]. The previous studies of association between low BMD and SLE disease duration were variable and inconsistent due to differences in age and races [28–30]. Herein, we demonstrate the association between disease duration and low BMD in adult SLE patients, showing the cumulative effect of long-term inflammation on BMD, which was in accordance with a study of juvenile SLE patients [30]. Furthermore, we show that overlap with RA is associated with a significantly low BMD in SLE patients and strengthen the evidence for RA as a well-known clinical risk factor for osteoporosis and fracture [31].
Alterations of bone turnover markers have been observed in SLE patients, especially in those taking GC or CsA [32]. GC treatment is associated with the decreased bone formation [2]. The present investigation also demonstrated that the cumulative GC dose was negatively correlated with serum osteocalcin in SLE patients, which was consistent with a recent study [33]. However, the effect of GC treatment on bone resorption in SLE patients remains uncertain. Baker-LePain et al. [33] reported that lupus activity, but not GC treatment, was a negative predictor of bone resorption. Banno et al. [34] found that there was no difference in bone resorption markers between osteoporotic and non-osteoporotic SLE patients. We have shown that a marked reduction of serum CTX is associated with GC treatment even in the absence of a different BMD. On the other hand, CsA treatment has been found to be associated with increased bone formation and bone resorption in vivo [1, 35]. Although it was difficult to segregate the contributions of CsA and GC treatment to bone turnover in our SLE patients, we have provided evidence for higher bone turnover (increased CTX in serum) as a result of CsA treatment combined with a low-dose GC. However, the effect of CsA on bone formation may have been abolished by concurrent GC administration.
GC and CsA treatments may lead to fluctuations in the vitamin D level in SLE patients. GC exposure has been reported to be associated with high [12], equivocal [13], or low [14, 36] vitamin D levels. An animal study revealed that CsA treatment is associated with vitamin D resistance through downregulation of vitamin D receptors in spite of an increase in 1,25(OH)2D3 level [37]. On the contrary, the present investigation has shown that CsA therapy is inversely correlated with the level of 1,25(OH)2D3 and is positively correlated with FGF-23 in SLE patients. Since FGF-23 inhibits 1α-hydroxylase, it is conceivable that CsA may inhibit 1,25(OH)2D3 via the stimulation of FGF-23 excretion. In addition, a higher serum iPTH level was found in CsA users who also received low-dose GC. These results were consistent with those reported by Kovalik et al. [38], who showed a significantly increased serum iPTH level in atopic dogs undergoing CsA therapy. Despite this consistency, we have found an inverse correlation between serum iPTH and the cumulative dose of GC (r = −0.299, p = 0.011), suggesting that GC and CsA are reciprocally antagonizing each other in their interaction with iPTH. Trends toward an increased renal calcium excretion and a decreased iPTH were observed among CsA users who treated with prednisolone equivalent to >7.5 mg/day than those with ≤7.5 mg/day (Fig. 3c–e), suggesting PTH keeps normocalcemia by stimulating renal calcium reabsorption [39].
The production of FGF-23 is increased in conditions such as chronic renal failure, high-phosphate diet, elevated iPTH and 1,25(OH)2D3, and iron-deficiency anemia [40]. Lundberg et al. [41] have reported that high FGF-23 is associated with albuminuria and progression of chronic renal failure in patients with IgA nephropathy. As shown in the present investigation, cumulative dose of CsA was still significantly associated with high FGF-23 even after adjusting these factors. Additionally, a significantly decreased renal calcium excretion and steady serum-free calcium was observed in CsA users than in non-CsA users (Fig. 3a, d, and e), suggesting that FGF-23 maintains calcium homeostasis by stimulating renal calcium reabsorption [42]. Elevated FGF-23 was also associated with suppressed 1,25(OH)2D3 and osteocalcin particularly in SLE patients with the highest degree of proteinuria treated with both GC and CsA. Thus, it can be inferred that the increased FGF-23 parallel to the CsA treatment may synergistically act with GC to result in decreased bone turnover in SLE patients, which in turn leads to osteoporosis in the long run [10, 11].
There are several clinical and research implications from the present study. Since low vitamin D level is common, especially in the SLE patient treated with GC and CsA, adequate vitamin D supplementation has been advocated in recent clinical trials [43, 44]. Vitamin D can offset the phosphate diabetes caused by FGF-23 and hence preclude further bone resorption. Therefore, in patients with advanced age and low body weight, a more important strategy to deter osteoporosis is adequate nutrition rather than a reduced GC or CsA dose.
Although we have demonstrated the role of FGF-23 in the development of drug-induced osteoporosis, especially the CsA-induced osteoporosis in patients with SLE, there are several drawbacks to the present investigation. Since data concerning medications, including GC and CsA, were collected retrospectively from the medical records, the dosing and combination therapy as well as compliance with the prescription could not be controlled. Despite these concerns, a decrease in bone turnover was still observed. On the other hand, the lack of significant differences in BMD among subgroups and data on microarchitectural deterioration impeded our ability to analyze the fracture risk. The independent effects of CsA and GC on bone and mineral metabolism were difficult to assess because calcineurin inhibitors such as CsA seldom serve as monotherapy in lupus nephritis patients [45]. Nevertheless, the present investigation has still shown the alterations and interrelationship of 1,25(OH)2D3, FGF-23, and iPTH in SLE patients treated with GC and CsA. In conclusion, careful screening for osteoporosis is of paramount importance in SLE patients with advanced age, prolonged disease, and low BMI who take various immunosuppressants. The association found between FGF-23 and CsA administration warrants further research.
References
Movsowitz C, Epstein S, Ismail F, Fallon M, Thomas S (1989) Cyclosporin A in the oophorectomized rat: unexpected severe bone resorption. J Bone Miner Res 4:393–398
Lane NE, Lukert B (1998) The science and therapy of glucocorticoid-induced bone loss. Endocrinol Metab Clin North Am 27:465–483
Koga T, Matsui Y, Asagiri M, Kodama T, de Crombrugghe B, Nakashima K, Takayanagi H (2005) NFAT and Osterix cooperatively regulate bone formation. Nat Med 11:880–885
van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C (2000) Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology (Oxford) 39:1383–1389
Bultink IE, Harvey NC, Lalmohamed A, Cooper C, Lems WF, van Staa TP, de Vries F (2014) Elevated risk of clinical fractures and associated risk factors in patients with systemic lupus erythematosus versus matched controls: a population-based study in the United Kingdom. Osteoporos Int 25:1275–1283
Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T (2004) Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113:561–568
Baum M, Schiavi S, Dwarakanath V, Quigley R (2005) Effect of fibroblast growth factor-23 on phosphate transport in proximal tubules. Kidney Int 68:1148–1153
Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T (2004) FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19:429–435
Krajisnik T, Björklund P, Marsell R, Ljunggren O, Akerström G, Jonsson KB, Westin G, Larsson TE (2007) Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol 195:125–131
Celik E, Guzel S, Abali R, Guzelant AY, Celik Guzel E, Kuçukyalcin V (2013) The relationship between fibroblast growth factor 23 and osteoporosis in postmenopausal women. Minerva Med 104:497–504
Mirza MA, Karlsson MK, Mellström D, Orwoll E, Ohlsson C, Ljunggren O, Larsson TE (2011) Serum fibroblast growth factor-23 (FGF-23) and fracture risk in elderly men. J Bone Miner Res 26:857–864
Toloza SM, Cole DE, Gladman DD, Ibañez D, Urowitz MB (2010) Vitamin D insufficiency in a large female SLE cohort. Lupus 19:13–19
Souto M, Coelho A, Guo C, Mendonca L, Argolo S, Papi J, Farias M (2011) Vitamin D insufficiency in Brazilian patients with SLE: prevalence, associated factors, and relationship with activity. Lupus 20:1019–1026
Chaiamnuay S, Chailurkit LO, Narongroeknawin P, Asavatanabodee P, Laohajaroensombat S, Chaiamnuay P (2013) Current daily glucocorticoid use and serum creatinine levels are associated with lower 25(OH) vitamin D levels in Thai patients with systemic lupus erythematosus. J Clin Rheumatol 19:121–125
Jacobs J, Korswagen LA, Schilder AM, van Tuyl LH, Dijkmans BA, Lems WF, Voskuyl AE, Bultink IE (2013) Six-year follow-up study of bone mineral density in patients with systemic lupus erythematosus. Osteoporos Int 24:1827–1833
Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ (1982) The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25:1271–1277
Hochberg MC (1997) Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40:1725
Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH (1992) Derivation of the SLEDAI. A disease activity index for lupus patients. The committee on prognosis studies in SLE. Arthritis Rheum 35:630–640
Schousboe JT, Shepherd JA, Bilezikian JP, Baim S (2013) Executive summary of the 2013 International Society for Clinical Densitometry Position Development Conference on bone densitometry. J Clin Densitom 16:455–466
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Young A, Hodsman AB, Boudville N, Geddes C, Gill J, Goltzman D, Jassal SV, Klarenbach S, Knoll G, Muirhead N et al (2012) Bone and mineral metabolism and fibroblast growth factor 23 levels after kidney donation. Am J Kidney Dis 59:761–769
Lee JJ, Aghdassi E, Cheung AM, Morrison S, Cymet A, Peeva V, Neville C, Hewitt S, DaCosta D, Pineau C et al (2012) Ten-year absolute fracture risk and hip bone strength in Canadian women with systemic lupus erythematosus. J Rheumatol 39:1378–1384
Pineau CA, Urowitz MB, Fortin PJ, Ibanez D, Gladman DD (2004) Osteoporosis in systemic lupus erythematosus: factors associated with referral for bone mineral density studies, prevalence of osteoporosis and factors associated with reduced bone density. Lupus 13:436–441
Mendoza-Pinto C, García-Carrasco M, Sandoval-Cruz H, Escárcega RO, Jiménez-Hernández M, Etchegaray-Morales I, Soto-Vega E, Muñoz-Guarneros M, López-Colombo A, Delezé-Hinojosa M et al (2009) Risks factors for low bone mineral density in pre-menopausal Mexican women with systemic lupus erythematosus. Clin Rheumatol 28:65–70
Yeap SS, Fauzi AR, Kong NC, Halim AG, Soehardy Z, Rahimah S, Chow SK, Goh EM (2009) Influences on bone mineral density in Malaysian premenopausal systemic lupus erythematosus patients on corticosteroids. Lupus 18:178–181
Furukawa M, Kiyohara C, Tsukamoto H, Mitoma H, Kimoto Y, Uchino A, Nakagawa M, Oryoji K, Shimoda T, Akashi K et al (2011) Prevalence of and risk factors for low bone mineral density in Japanese female patients with systemic lupus erythematosus. Rheumatol Int 31:365–376
Souto MI, Coelho A, Guo C, Mendonca LM, Pinheiro MF, Papi JA, Farias ML (2012) The prevalence of low bone mineral density in Brazilian patients with systemic lupus erythematosus and its relationship with the disease damage index and other associated factors. J Clin Densitom 15:320–327
Li EK, Tam LS, Young RP, Ko GT, Li M, Lau EM (1998) Loss of bone mineral density in Chinese pre-menopausal women with systemic lupus erythematosus treated with corticosteroids. Br J Rheumatol 37:405–410
Lakshminarayanan S, Walsh S, Mohanraj M, Rothfield N (2001) Factors associated with low bone mineral density in female patients with systemic lupus erythematosus. J Rheumatol 28:102–108
Compeyrot-Lacassagne S, Tyrrell PN, Atenafu E, Doria AS, Stephens D, Gilday D, Silverman ED (2007) Prevalence and etiology of low bone mineral density in juvenile systemic lupus erythematosus. Arthritis Rheum 56:1966–1973
Sandhu SK, Hampson G (2011) The pathogenesis, diagnosis, investigation and management of osteoporosis. J Clin Pathol 64:1042–1050
Mok CC (2013) Vitamin D and systemic lupus erythematosus: an update. Expert Rev Clin Immunol 9:453–463
Baker-LePain JC, Nakamura MC, Shepherd J, von Scheven E (2011) Assessment of bone remodelling in childhood-onset systemic lupus erythematosus. Rheumatology (Oxford) 50:611–619
Banno S, Matsumoto Y, Naniwa T, Hayami Y, Sugiura Y, Yoshinouchi T, Ueda R (2002) Reduced bone mineral density in Japanese premenopausal women with systemic lupus erythematosus treated with glucocorticoids. Mod Rheumatol 12:323–328
Yeo H, McDonald JM, Zayzafoon M (2006) NFATc1: a novel anabolic therapeutic target for osteoporosis. Ann N Y Acad Sci 1068:564–567
O’Regan S, Chesney RW, Hamstra A, Eisman JA, O’Gorman AM, Deluca HF (1979) Reduced serum 1,25-(OH)2 vitamin D3 levels in prednisone-treated adolescents with systemic lupus erythematosus. Acta Paediatr Scand 68:109–111
Lee CT, Ng HY, Lien YH, Lai LW, Wu MS, Lin CR, Chen HC (2011) Effects of cyclosporine, tacrolimus and rapamycin on renal calcium transport and vitamin D metabolism. Am J Nephrol 34:87–94
Kovalik M, Mellanby RJ, Evans H, Berry J, van den Broek AH, Thoday KL (2012) Ciclosporin therapy is associated with minimal changes in calcium metabolism in dogs with atopic dermatitis. Vet Dermatol 23:481–e491
Gesek FA, Friedman PA (1992) On the mechanism of parathyroid hormone stimulation of calcium uptake by mouse distal convoluted tubule cells. J Clin Invest 90:749–758
Wolf M, Koch TA, Bregman DB (2013) Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res 28:1793–1803
Lundberg S, Qureshi AR, Olivecrona S, Gunnarsson I, Jacobson SH, Larsson TE (2012) FGF23, albuminuria, and disease progression in patients with chronic IgA nephropathy. Clin J Am Soc Nephrol 7:727–734
Andrukhova O, Smorodchenko A, Egerbacher M, Streicher C, Zeitz U, Goetz R, Shalhoub V, Mohammadi M, Pohl EE, Lanske B et al (2014) FGF23 promotes renal calcium reabsorption through the TRPV5 channel. EMBO J 33:229–246
Terrier B, Derian N, Schoindre Y, Chaara W, Geri G, Zahr N, Mariampillai K, Rosenzwajg M, Carpentier W, Musset L et al (2012) Restoration of regulatory and effector T cell balance and B cell homeostasis in systemic lupus erythematosus patients through vitamin D supplementation. Arthritis Res Ther 14:R221
Abou-Raya A, Abou-Raya S, Helmii M (2013) The effect of vitamin D supplementation on inflammatory and hemostatic markers and disease activity in patients with systemic lupus erythematosus: a randomized placebo-controlled trial. J Rheumatol 40:265–272
Austin HA 3rd, Illei GG, Braun MJ, Balow JE (2009) Randomized, controlled trial of prednisone, cyclophosphamide, and cyclosporine in lupus membranous nephropathy. J Am Soc Nephrol 20:901–911
Acknowledgments
This study was supported by intramural grants from the Taipei Veterans General Hospital (V101A-025, V102A-028, and V103A-002) and Ministry of Science & Technology (NSC 101-2314-B-075-030-MY3, NSC102-2314-B-075-067-MY3), Taiwan.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lai, CC., Chen, WS., Chang, DM. et al. Increased serum fibroblast growth factor-23 and decreased bone turnover in patients with systemic lupus erythematosus under treatment with cyclosporine and steroid but not steroid only. Osteoporos Int 26, 601–610 (2015). https://doi.org/10.1007/s00198-014-2910-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-014-2910-3