Abstract
Objective
Sclerostin is an osteocyte-derived glycoprotein which inhibits the canonical Wnt pathway essential for osteoblastic activity decreasing bone formation. Its potential role in rheumatoid arthritis (RA) pathogenesis was highlighted by experimental studies. Here we measured the serum sclerostin in RA patients and evaluated its relationship with disease activity and damage.
Methods
One hundred RA patients and 80 age and sex-matched healthy controls were enrolled in the study. Bone biomarkers were evaluated for all participants including total calcium, phosphorus, alkaline phosphatase, 25-hydroxy vitamin D, and intact parathyroid hormone, in addition to fibroblast growth factor-23 (FGF23) and serum sclerostin. For RA patients, carotid intima-media thickness, brachial artery flow dilatation, and musculoskeletal ultrasonography using ultrasonography-7 joint score were done, and DAS28-ESR was calculated.
Results
Median serum sclerostin in our patients was 186.5 ± 22.7 pg/ml which was significantly higher than in controls 60.6 ± 7.1 pg/ml (p < 0.002). Serum sclerostin showed no correlation with disease activity, bone erosions, carotid intima-media thickness, brachial flow dilatation, and the examined bone biomarkers. However, it had a strong correlation with FGF23 (r coefficient 0.988, p < 0.000).
Conclusion
Although serum sclerostin was elevated in RA patients, it could not be used as a prognostic marker for disease activity, bone erosions or atherosclerosis.
Key Points • Serum sclerostin may not reflect changes in the joint microenvironment being not correlated with ultrasonography-detected synovitis or erosions. • Serum sclerostin was elevated in RA patients irrespective to their age or gender. • The positive correlation with FGF23 may provide evidence for sclerostin contribution in bone demineralization in RA patients. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease that predominantly affects the synovial joints. It is characterized by regional and systemic bone loss [1]; the former results in peri-articular osteopenia, bone erosions, and joint damage, while the systemic bone loss increased the risk of osteoporotic fractures [2,3,4]. Both may occur early in the disease, and are likely to share the same pathophysiological mechanisms that enhance osteoclast-mediated bone resorption and inhibit osteoblast-mediated bone formation [5,6,7].
The bone formation and mineralization are under the restrict control of the Wnt signaling pathway [3]. The Wnt signals through canonical (β-catenin-dependent) and non-canonical (β-catenin-independent) pathways [8]. Canonical Wnt/β-catenin pathway is activated by binding of Wnt proteins to receptor complexes composed of frizzled receptors and co-receptors of the low-density lipoprotein receptor-related protein (LRP) family, LRP5 and 6. This event stabilizes the cytosolic β-catenin, allowing its translocation to the nucleus to activate gene transcription promoting osteoblast differentiation and bone formation [9]. An overactive Wnt/β-catenin pathway is found in the synovial membrane of RA patients [8, 10]. However, it may have different roles in different types of cells. Both fibroblast-like synoviocytes (FLS) and osteoblasts are derived from the mesenchymal cells. While the Wnt signaling pathway results in proliferation of FLS, its effect on osteoblast precursors seems to be blocked by several inhibitors, which also upregulated in the inflamed synovium and secreted by osteocytes, leading to suppression of bone formation [11].
Among the numerous inhibitors, Dickkopf homolog (DKK-1) and sclerostin have been most studied in animal models and adult populations [12]. In addition to their anti-anabolic effect, they promote the catabolic activity of osteoclast through expression of receptor activator of nuclear factor kappa-Β ligand (RANKL), and decreasing the expression of osteoprotegerin (OPG) [13, 14]. DKK-1 was found to be raised in serum RA patients [15] with positive correlation with disease activity, bone erosions, and bone mineral density (BMD), reflecting its role in RA pathogenesis [16,17,18]. Compared with DKK-1 that is produced by many cell types that address a concern about its specificity, sclerostin in particular gained attention being secreted by limited cell types. It is secreted almost exclusively by osteocytes. To lesser extent, it may be produced by vascular cells [13].
In addition to bone loss, RA is characterized by accelerated atherosclerosis that so far has been linked to pro-inflammatory cytokines together with the traditional risk factors. Interestingly, individuals with mutations in LRP6, the co-receptor target of sclerostin, exhibit premature coronary artery disease as well as severe osteoporosis [12]. In consequence, sclerostin may qualify as a candidate for the bone-vascular axis and so a potential player in RA pathogenesis.
The serum levels of sclerostin in RA as well as its exact contribution in bone destruction and inflammation were inconsistent among studies [19,20,21,22]. In this study, we explored the potential role of sclerostin in RA, evaluating the relationship between serum sclerostin levels and RA disease activity, disease damage, biomarkers of bone profile including alkaline phosphatase (ALP), 25-hydroxy vitamin D, intact parathyroid hormone (iPTH), and fibroblast growth factor-23 (FGF23) and co-morbidity, namely accelerated atherosclerosis.
Patient and methods
Patients were recruited from the outpatient Rheumatology and Clinical Immunology clinic and Internal Medicine Departments of Kasr Al-Ainy hospital; that is considered a tertiary referral center serving patients from all governorates of Egypt, during June to December 2017. A total of 100 RA patients fulfilling the 2010 ACR/EULAR classification criteria [23] plus 80 healthy volunteers of comparable age and sex to the patients’ group were included in our cross-sectional, case-control study.
With regard to control, care was taken in selecting healthy volunteers who were not on supplemental therapy at least 1 month before the study.
The main exclusion criteria include RA patients with manifestations overlapped with other autoimmune diseases, except for Sjögren’s syndrome. All the participants provided signed informed consent and the study was approved by the local ethics committee.
All participants were subjected to thorough history and comprehensive physical examination. The disease duration, medication history, functional assessment using modified health assessment questionnaire (mHAQ), and 28 tender and swollen joint counts were recorded for all patients. Disease activity was determined using the Disease Activity Score with a 28 joint count and ESR (DAS 28-ESR); it was graded as follows: DAS28 ≤ 2.6 = clinical remission, ≤ 3.2 = low disease activity, ≤ 5.1 = moderate disease activity, and > 5.1 = high disease activity [24]. mHAQ was calculated and scored as mild (MHAQ < 1.3), moderate (1.3 < MHAQ < 1.8), and severe (MHAQ > 1.8) functional losses [25].
Serum sclerostin was obtained from all participants in addition to serum, creatinine, urea, and bone biomarkers including total calcium, phosphorus, ALP, 25-hydroxy vitamin D, iPTH, and FGF23. The complete blood count (CBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), lipid profile, rheumatoid factor (RF), and anti-cyclic citrullinated peptide (anti-CCP) were done for RA patients.
The serum level of 25-hydroxy vitamin D was measured using high-performance liquid chromatography (HPLC). The serum level of iPTH was evaluated using enzyme amplified sensitivity immunoassay (Roche Diagnostics, Indianapolis, IN). The serum level of intact FGF23 was determined using a two-site (NH2-terminal/C-terminal) enzyme-linked immunosorbent assay (ELISA) (Immutopics, San Clemente, CA). According to the instructions of the manufacturer, samples were collected in the morning after 12-h fasting. The collected samples were centrifuged and the plasma was separated from the cells. Samples were assayed immediately or stored at – 70 °C or below. Serum sclerostin was assayed using ELISA assay (Quantikine ELIZA) according to the manufacturer’s instructional protocol. RF and anti-CCP were considered positive when their concentrations were higher than the cut-off value of the kit (15 IU/ml for RF and 20 U/ml for anti-CCP); high titer was considered if the level is 3 times upper the cut-off value.
The patients only were subjected to musculoskeletal ultrasonography (MSUS) and Doppler studies for carotids and brachial arteries. Both were performed using a (LOGIQ-P6), on the same day of clinical and laboratory assessment, by two examiners (one examiner for each study) who were blinded to clinical data.
Musculoskeletal ultrasonographic assessment
Gray scale ultrasonography (GS) and power Doppler ultrasonography (PD) were performed using a high-frequency broadband linear array transducer; at 10–13 MHz. Power Doppler (PD) settings were adjusted to low wall filters, and a pulse repetition frequency of 500 Hz that enhance the sensitivity for detecting synovial vessels without or with minimal artifact. Standardized methodology according to German ultrasonography-7 score (US-7) was applied [26]. The patients were examined in the following seven joint areas: wrist, second and third metacarpophalangeal (MCP), second and third proximal interphalangeal (PIP), and second and fifth metatarsophalangeal joints of the clinically more affected hand and foot. The GS and PD synovitis as well as PD tenosynovitis were scored on 0–3 semiquantitative scales [27]. The GS Tenosynovitis and erosions were scored for presence or absence. Sum scores of GS, PDS, and erosions will be calculated as the sum of GS synovitis, PD synovitis, GS tenosynovitis, PD tenosynovitis, and erosions.
Carotids and brachial artery Doppler
Doppler studies were performed for evaluation of the carotid intema media thickness (CIMT) and brachial flow-mediated dilatation using a linear 10–13 MHz transducer.
The CIMT was evaluated in a supine position. The gain settings were adjusted optimally to facilitate edge detection. A single measurement was taken manually for both sides in a longitudinal view just before the bifurcation of common carotid artery [28]. Atherosclerosis is defined as CIMT score > 0.9 mm [29].
Flow-mediated dilatation (FMD) of the brachial artery was assessed in the right arm as described in a technique report by the International Brachial Artery Reactivity Task Force [30]. The initial measurement of the brachial artery diameter was done at rest (Di). Then, ischemia was induced by inflating the pneumatic cuff to a pressure 50 mmHg above a systolic one [30], for 10 min, in order to obliterate the brachial artery and induce ischemia. A 10-min occlusion period was used as prolonged period of ischemia was shown to induce greater reperfusion [31]. Subsequently, the cuff was deflated and the artery diameter was re-measured once 60 s post deflation (Df), being maximal vasodilation was previously observed after this period. FMD was calculated with the formula: FMD = [(Df –Di)\ DI] × 100 [32]. Zero % indicates failure of dilatation reflecting vascular stiffness.
Statistical analysis
Data were coded and entered using the statistical package SPSS (Statistical Package for the Social Sciences) version 12. Data was summarized using mean and standard deviation in quantitative data and using frequency (count) and relative frequency (percentage) for categorical data. For comparing categorical data, chi square (χ2) test was performed to compare qualitative variables between groups. Fisher exact test was used instead when one expected cells are less than 5. Unpaired t test was used to compare quantitative variables of parametric data. Mann-Whitney U test was used instead of unpaired t test in independent samples. One-way ANOVA test was used to compare quantitative variables between more than two groups in normally distributed variables. p values less than 0.05 were considered statistically significant.
Results
The demography, drug profile, laboratory, ultrasonography, and Doppler characteristics of our patients are demonstrated in Table 1. The enrolled RA patients were predominantly females (84%). They had a mean age of 45 ± 13 years and mean disease duration of 7 ± 7.58 years. Most patients were seropositive for either RF (67%) or anti-CCP (52%), and 20% was sero-negative.
The studied group had generally mild levels of functional impairment, as measured by the mHAQ (mean 0.912 ± 0.866), while they showed different degrees of disease activity as defined by DAS28-ESR (mean was 4.66 ± 1.38). Eleven of our patients were in remission.
The majority of our patients (58%) had erosions in at least one site of the scanned joints as assessed using US-7 score. Other US values are illustrated in Table 1. Carotid Doppler assessment revealed 52 patients with evidence of atherosclerosis as defined as CIMT > 0.9 mm. Eleven patients had failure of brachial artery FMD reflecting endothelial dysfunction.
25-OH-Vitamin D were significantly low in our patients with mean of 17.1 ± 5.5 ng/ml, in comparison with the control with mean of 36.6 ± 3.3 ng/ml (p value < 0.001).
It should be noted that at the time of the study, none of our patients were receiving vitamin D replacement therapy. Only those who received steroid treatment took prophylactic doses of vitamin D (Table 2).
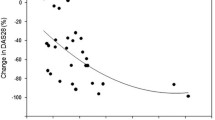
On the other hand, iPTH, FGF23, and sclerostin levels were significantly higher in our patients in comparison with the control (p value 0.043, < 0.001, and < 0.002 respectively), as illustrated in Fig. 1a–d.
The serum concentrations of a Sclerostin, b FGF-23, c iPTH, and d 25-OH vitamin D in RA patients and healthy individuals. e Distribution of sclerostin levels in RA patients according to the category of disease activity as defined by DAS 28-ESR. FGF-23, fibroblast growth factor-23; iPTH, intact parathyroid hormone. The asterisk indicates that is is significant; double asterisks indicate that it is highly significant
Relationships of serum sclerostin with demographic, clinical, laboratory, and radiological parameters of our patients are demonstrated in Tables 3 and 4. Regarding the patients’ demography, sclerostin levels showed no relationship with the patients’ serology, gender (Table 3), age, or disease duration (Table 4). Regarding the disease-modifying antirheumatic drugs (DMARDs) used in the treatment of our patients, the sclerostin levels did not show a relationship with any of the synthetic DMARDs (Table 3). In addition, they showed no correlation with the doses of prednisone and methotrexate (Table 4). This could not be studied in respect to the biological DMARDS as only ten patients of the cohort received such treatment at the time of study.
The serum sclerostin showed no correlation with disease activity measured by clinical score DAS-28-ESR. Moreover, serum sclerostin levels showed no statistical significant difference between the different categories of disease activity (Fig. 1e). Furthermore, no correlation was found with different US-7 scores including GS synovitis (p 0.977), PD synovitis (p 0.783), GS tenosynovitis (p 0.975), and PD tenosynovitis (p 0.998) as well as the ultrasonography-detected erosions (Table 4).
Sclerostin levels also showed no significant correlation with cholesterol profile, carotid intema media thickness, and brachial flow dilatation (Table 4). Finally, its levels showed no correlation with serum calcium, phosphorus, ALP, iPTH, or 25-OH-Vitamin D (Table 4). A significant correlation was found with FGF-23 (r 0.988, p value 0.00) as shown in Table 4.
Discussion
In this current study, the sclerostin levels were significantly elevated in RA patients compared with controls. This was also reported by El-Bakry et al.; they demonstrated 96.8% sclerostin sensitivity to discriminate RA patients from control at serum level of 267 ng/dl using ROC curve analysis [20].
Other studies had demonstrated no significant difference in the serum sclerostin levels between RA patients and controls [19, 21, 22], although Paccou et al. assumed that was attributed to the disease status of their RA cohort, being most of them were in clinical remission or presented low disease activity [19]. This was not the case in study conducted by Mehaney et al., as 95% of their RA population had moderate and high disease activity [21].
Reasons for these seemingly discrepant results are unclear. One potential explanation is that levels of sclerostin have a broad range even between normal individuals, so it is difficult to define normal levels and distinguish them from abnormal ones. The differences in the sclerostin values may also be related to different commercially available assays for circulating sclerostin [33].
In our study, we found no correlation of sclerostin level and the patients’ age, their gender, disease duration, or serology, which was in harmony with previous studies [19,20,21].
Despite the elevated serum sclerostin levels in our patients, they were not statistically correlated with inflammatory markers (ESR and CRP), disease activity score (DAS-28 ESR), and mHAQ. In addition, serum sclerostin levels showed no statistical significant difference between the different categories of disease activity. Moreover, they were not correlated with ultrasonography-detected synovial hypertrophy, hyperemia, or bone erosions. These findings are consistent with many clinical studies that failed to establish a link between serum sclerostin and disease activity [19,20,21,22] on one hand as well as X-ray radiographic erosions on the other hand [21, 22]. By contrary, El-Bakry et al. found a significant correlation between the serum sclerostin level and the radiographic damage as assessed by the Larsen score [20].
These data should be interpreted carefully as serum levels of many biomarkers change depending on many circumstances. This has been demonstrated with sclerostin levels, which have been shown to be altered in response to hormonal stimuli and across a variety of normal physiological and pathophysiological conditions. Furthermore, current evidence indicates that sclerostin likely functions as a local/paracrine regulator of bone metabolism rather than as an endocrine hormone [33]. Accordingly, the sclerostin levels may not always reflect the tissue concentration, so they do not necessarily indicate local activity and damage. These aspects can explain why other similar studies done regarding serum levels of sclerostin in RA also had inconsistent results.
The role of sclerostin in developing bone erosions has been demonstrated in animal models. In TNF-α transgenic mice model of RA, inhibition of sclerostin with a monoclonal antibody arrested the progression of bone erosions, as well as peri-articular and systemic bone loss [34]. Interestingly, in rat adjuvant-induced arthritis model, Courbon et al. demonstrated early expression of sclerostin before the onset of synovial inflammation, which was associated with inhibited regional bone formation. They assumed that before arthritis, onset osteocytes were the primary source for sclerostin rather than synovium [35]. The early expressed sclerostin may be contributed to the peri-articular osteopenia, a common finding in RA patients, that frequently precedes the development of marginal joint erosions and may have high predictive value with respect to the subsequent development of marginal joint erosions in the hand [2]. Notably, Vincent et al. revealed TNF-α-enhanced sclerostin synthesis by osteoblast and synovial fibroblast, confirming the role of inflammation in enhancing sclerostin production [36].
In the current study, our patients were significantly having low vitamin D levels and elevated iPTH compared with the control. In addition, the vitamin D deficiency was only detected in our RA patients (73%). These results to some extent correspond to a meta-analysis conducted on fifteen studies and included a total of 1143 RA patients and 963 controls. This meta-analysis showed that the serum vitamin D level was significantly lower in the RA patients who were more prevalent for vitamin D deficiency compared with the control group (55.2% vs. 33.2) [37].
Despite low levels of vitamin D, the serum phosphorus in RA group was essentially normal and even was significantly higher compared with control. This was also reported by other studies [38, 39].
We hypothesized that increased bone turnover as evidenced by the presence of erosion in 58% in the RA group may explain this. The FGF-23, which was elevated in our patients, normalized the serum phosphorus by increasing renal excretion.
FGF-23 is another osteocytes-derived hormone that handles vitamin D metabolism and renal phosphate to match bone mineralization and remodeling. The accelerated bone remodeling increased phosphate efflux, enhancing FGF-23 that is in turn increases renal phosphate excretion and suppresses 1,25(OH)2D, with subsequent osteomalacia [40].
The association of FGF23 and bone demineralization in RA patients was evaluated by Sato et al. They found that the serum FGF23 levels in patients with RA were positively correlated with bone resorption markers, namely the serum matrix metalloproteinase-3 (r = 0.331, p = 0.015) and type I collagen cross-linked N-telopeptide (r = 0.272, p = 0.034) [41].
In our study, we did not reveal significant correlations between sclerostin levels and bone biochemical markers, namely iPTH, 25(OH) vitamin D, ALP, calcium, and phosphorus. This is in harmony with Paccou et al. who could not find significant correlations between sclerostin on one hand and C-telopeptide, iPTH, calcium, and phosphorus on the other hand [19].
Interestingly, the serum sclerostin was strongly correlated with FGF-23. Accordingly, we assumed that sclerostin may have indirect influence on the mineralization and vitamin D status through regulation of FGF-23.
It is revealed that sclerostin may directly regulate FGF-23 in experimental models, through inhibiting PHEX (a membrane-bound endopeptidase) [42], that was found to degrade FGF-23 [43]. This was supported by studies done in sclerostin knockout mice, which showed reduced concentrations of FGF-23 together with increased concentrations of 1α,25(OH)2D compared with those found in wild mice. These changes were not associated with altered levels of PTH reflecting that the increase of 1α,25(OH)2D was likely to be due to the decrement in serum FGF-23; the latter was likely downregulated as a result of deficient sclerostin [44].
The increased atherosclerotic risk is well established in RA patients increasing the cardiovascular co-morbidity in these patients [45]. In our study, 52 patients had increased carotid intima-media thickness, and 11 had failure of FMD which reflect atherosclerotic changes. However, no correlation has been found between sclerostin levels and CIMT, as well as with FMD.
Although emerging evidence suggests implication of Wnt pathway in vascular calcification and atherosclerosis [46], positive association of sclerostin, a Wnt pathway inhibitor, with vascular calcification was found in diabetic [47] and CKD patients [48]. Whether this indicates a direct implication in the development of vascular calcifications or simply reflects a phenotypic change of the vascular wall cells towards a bone-like phenotype is not fully understood [47].
This study has strengths and limitations. The strengths are the relatively large sample size of both patients and control. The variety of RA patients who presented the different types of disease activity added to the power of this current study.
It is noteworthy to mention that the composite clinical scores to assess disease activity and plain radiography to assess joint damage were the tools used in previous clinical studies. By contrast, we used the ultrasonography in our assessment. The ultrasonography has recently emerged as a valid tool for accurate assessment of synovial hypertrophy and hyperemia that were found to be more superior to clinical assessment and not inferior to MRI. Furthermore, the ultrasonography is more sensitive to detect bone erosions in comparison with plain X-ray [49].
The number and distribution of joints that should be evaluated by ultrasonography to reflect a good assessment of RA status are still under debate. Many studies showed comparable results of comprehensive and restricted joint count [50, 51]. In this study, we preferred using the reduced US-7 score that allowed assessment of joints and tendons as well as erosions, in locations that are frequently affected by RA, in three separate scores [26].
The main limitations include the cross-sectional design of our study; longitudinal studies are more favorable in studying the biomarkers. They allow to evaluate the changes in serum biomarkers regarding to the disease activity status; also they are more able to detect the variations of biomarkers’ levels according to different circumstances [52].
Another limitation was the assessment of bone erosions on the clinically affected side, which may underestimate the erosive status of our patients. Finally we did not assess the BMD as a valid tool to assess the risk of osteoporosis; however, instead we evaluated bone biomarkers like ALP, iPTH, 25 (OH) vitamin D, and FGF-23.
Conclusion
In the era of using anti-sclerostin monoclonal antibodies in treatment of postmenopausal osteoporosis and possibility to use such medications in RA patients who have significant risk of osteoporosis, it is important to explore the role of sclerostin in RA patients. This study was conducted on a large sample size and found significant elevation of serum sclerostin in RA patients in comparison with control.
The serum sclerostin was positively correlated with FGF23 that may provide evidence for its contribution in bone demineralization in RA patients. Serum sclerostin may not reflect changes in the joint microenvironment; it was not correlated with the disease activity or ultrasonography-detected activity and marginal erosions. Thus, future studies evaluating sclerostin in synovial fluid and biopsy may provide deep insights in its function in joint microenvironment of RA patients. This may provide the link between experimental and clinical studies; in addition, it could explain the contradictory results between the clinical studies themselves.
Finally, serum sclerostin did not reflect the atherosclerotic status and vascular stiffness in the RA patients. In conclusion, although serum sclerostin may be elevated in RA patients, its measurement cannot be used as a marker for clinical activity and damage.
References
Firestein GS, McInnes IB (2017) Immunopathogenesis of rheumatoid arthritis. Immunity 46(2):183–196. https://doi.org/10.1016/j.immuni.2017.02.006
Goldring SR (2015) Inflammatory signaling induced bone loss. Bone 80:143–149. https://doi.org/10.1016/j.bone.2015.05.024
Corrado A, Maruotti N, Cantatore FP (2017) Osteoblast role in rheumatic diseases. Int J Mol Sci 18(6):1272. https://doi.org/10.3390/ijms18061272
Bosello S, Fedele AL, Peluso G, Gremese E, Tolusso B, Ferraccioli G (2011) Very early rheumatoid arthritis is the major predictor of major outcomes: clinical ACR remission and radiographic non-progression. Ann Rheum Dis 70(7):1292–1295. https://doi.org/10.1136/ard.2010.142729
Rossini M, Fassio A, Idolazzi L, Viapiana O, Fracassi E, Adami G et al (2015) Pathogenesis of bone erosions in rheumatoid arthritis: not only inflammation. J Rheum Dis Treat 1(2):1–5
Baum R, Gravallese EM (2016) Bone as a target organ in rheumatic disease: impact on osteoclasts and osteoblasts. Clin Rev Allergy Immunol 51(1):1–15. https://doi.org/10.1007/s12016-015-8515-6.Bone
Wazen RM, Kuroda S, Nishio C, Sellin K, Brunski JB, Nanci A (2014) Resolution of inflammation induces osteoblast function and regulates the Wnt signaling pathway. Arthritis Rheum 8(9):1385–1395. https://doi.org/10.2217/nnm.12.167.Gene
Rabelo FDS, da Mota LMH, Lima RAC, Lima FAC, Barra GB, de Carvalho JF, Amato AA (2010) The Wnt signaling pathway and rheumatoid arthritis. Autoimmun Rev 9(4):207–210. https://doi.org/10.1016/j.autrev.2009.08.003
Delgado-Calle J, Sato AY, Bellido T (2017) Role and mechanism of action of sclerostin in bone. Bone 96:29–37. https://doi.org/10.1016/j.bone.2016.10.007
Daoussis D, Andonopoulos AP, Liossis SNC (2010) Wnt pathway and IL-17: novel regulators of joint remodeling in rheumatic diseases. Looking beyond the RANK-RANKL-OPG Axis. Semin Arthritis Rheum 39(5):369–383. https://doi.org/10.1016/j.semarthrit.2008.10.008
Miao CG, Yang YY, He X, Li XF, Huang C, Huang Y et al (2013) Wnt signaling pathway in rheumatoid arthritis, with special emphasis on the different roles in synovial inflammation and bone remodeling. Cell Signal 25(10):2069–2078. https://doi.org/10.1016/j.cellsig.2013.04.002
Brandenburg VM, D’Haese P, Deck A, Mekahli D, Meijers B, Neven E, Evenepoel P (2016) From skeletal to cardiovascular disease in 12 steps—the evolution of sclerostin as a major player in CKD-MBD. Pediatr Nephrol 31(2):195–206. https://doi.org/10.1007/s00467-015-3069-7
Pietrzyk B, Smertka M, Chudek J (2017) Sclerostin: intracellular mechanisms of action and its role in the pathogenesis of skeletal and vascular disorders. Adv Clin Exp Med 26(8):1283–1291. https://doi.org/10.17219/acem/68739
Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ (2011) Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One 6(10). https://doi.org/10.1371/journal.pone.0025900
Suen PK, Qin L (2016) Sclerostin, an emerging therapeutic target for treating osteoporosis and osteoporotic fracture: a general review. J Orthopaed Transl 4:1–13. https://doi.org/10.1016/j.jot.2015.08.004
Ma Y, Zhang X, Wang M, Xia Q, Yang J, Wu M et al (2018) The serum level of Dickkopf-1 in patients with rheumatoid arthritis: a systematic review and meta-analysis. Int Immunopharmacol 59(April):227–232. https://doi.org/10.1016/j.intimp.2018.04.019
Sma A, Mg Z (2015) Role of Dickkopf-1 and musculoskeletal ultrasound in bone loss in rheumatoid arthritis. J Med Diagnos Methods 04(04). https://doi.org/10.4172/2168-9784.1000185
Gómez-Vaquero C, Martín I, Loza E, Carmona L, Ivorra J, Narváez JA et al (2016) Effect of osteoprotegerin and dickkopf-related protein 1 on radiological progression in tightly controlled rheumatoid arthritis. PLoS One 11(12):1–11. https://doi.org/10.1371/journal.pone.0166691
Paccou J, Mentaverri R, Renard C, Liabeuf S, Fardellone P, Massy Z a et al (2014) The relationships between serum sclerostin, bone mineral density, and vascular calcification in rheumatoid arthritis. J Clin Endocrinol Metab 99(12):4740–4748. https://doi.org/10.1210/jc.2014-2327
El-Bakry S, Saber N, Zidan H, Samaha D (2016) Sclerostin as an innovative insight towards understanding rheumatoid arthritis. Egyptian Rheumatol 38(2):71–75. https://doi.org/10.1016/j.ejr.2015.05.001
Mehaney D, M, E., S, A, Fakhr El-din S (2015) Serum sclerostin level among Egyptian rheumatoid arthritis patients: relation to disease activity , bone mineral density and radiological grading. Acta Reumatol Port 40:268–274
Seror R, Boudaoud S, Pavy S, Nocturne G, Schaeverbeke T, Saraux A et al (2016) Increased Dickkopf-1 in recent-onset rheumatoid arthritis is a new biomarker of structural severity. Data from the ESPOIR cohort. Sci Rep 6:1–11. https://doi.org/10.1038/srep18421
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO et al (2010) 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum 62(9):2569–2581. https://doi.org/10.1002/art.27584
Smolen JS, Aletaha D (2008) Activity assessments in rheumatoid arthritis. Curr Opin Rheumatol 20(3):306–313. https://doi.org/10.1097/BOR.0b013e3282fbd382
Wolfe F, Pincus T (1999) Listening to the patient: a practical guide to self-report questionnaires in clinical care. Arthritis Rheum 42(9):1797–1808. https://doi.org/10.1002/1529-0131(199909)42:9<1797::AID-ANR2>3.0.CO;2-Q
Backhaus M, Ohrndorf S, Kellner H, Strunk J, Backhaus TM, Hartung W et al (2009) Evaluation of a novel 7-joint ultrasound score in daily rheumatologic practice: a pilot project. Arthritis Care Res 61(9):1194–1201. https://doi.org/10.1002/art.24646
Naredo E, Wakefield RJ, Iagnocco A, Terslev L, Filippucci E, Gandjbakhch F et al (2011) The OMERACT ultrasound task force - status and perspectives. J Rheumatol 38(9):2063–2067. https://doi.org/10.3899/jrheum.110425
Wikstrand JCM (2012) Methodological considerations of ultrasound measurement of carotid artery intima-media thickness and lumen diameter. Ultrasound Carotid Bifurcation Atherosclerosis 9781848826:165–176. https://doi.org/10.1007/978-1-84882-688-5_10
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M et al (2013) 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 34(28):2159–2219. https://doi.org/10.1093/eurheartj/eht151
Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager M a et al (2002) Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the international brachial artery reactivity task force. J Am Coll Cardiol 39(2):257–265. https://doi.org/10.1016/S0735-1097(01)01746-6
Rodriguez-Miguelez P, Seigler N, Harris R a (2016) Ultrasound assessment of endothelial function: a technical guideline of the flow-mediated dilation test. J Vis Exp 2016(110):1–10. https://doi.org/10.3791/54011
Luo C, Li Y, Liu D, Hu C, Du Z (2012) The association of brachial flow-mediated dilation and high-sensitivity C-reactive protein levels with Duke treadmill score in patients with suspected microvascular angina. Exp Clin Cardiol 17(4):197–201
Drake MT, Khosla S (2017) Hormonal and systemic regulation of sclerostin. Bone 96:8–17. https://doi.org/10.1016/j.bone.2016.12.004
Chen XX, Baum W, Dwyer D, Stock M, Schwabe K, Ke HZ et al (2013) Sclerostin inhibition reverses systemic, periarticular and local bone loss in arthritis. Ann Rheum Dis 72(10):1732–1736. https://doi.org/10.1136/annrheumdis-2013-203345
Courbon G, Lamarque R, Gerbaix M, Caire R, Linossier MT, Laroche N et al (2018) Early sclerostin expression explains bone formation inhibition before arthritis onset in the rat adjuvant-induced arthritis model. Sci Rep 8(1):2–11. https://doi.org/10.1038/s41598-018-21886-w
Vincent C, Findlay DM, Welldon KJ, Wijenayaka AR, Zheng TS, Haynes DR et al (2009) Pro-inflammatory cytokines TNF-related weak inducer of apoptosis (TWEAK) and TNFα induce the mitogen-activated protein kinase (MAPK)-dependent expression of sclerostin in human osteoblasts. J Bone Miner Res 24(8):1434–1449. https://doi.org/10.1359/jbmr.090305
Lee YH, Bae SC (2016) Vitamin D level in rheumatoid arthritis and its correlation with the disease activity: a meta-analysis. Clin Exp Rheumatol 34(5):827–833
Walwadkar SD, Suryakar AN, Katkam RV, Kumbar KM, Ankush RD (2006) Oxidative stress and calcium-phosphorus levels in rheumatoid arthritis. Indian J Clin Biochem 21(2):134–137. https://doi.org/10.1007/BF02912928
Mahmoud A, Ismail M (2012) Anemia, iron status and calcium-phosphorus levels in rheumatoid arthritis patients. Nat Sci 10(7):110–114
Jaskiewicz F (2000) Role of FGF23 in vitamin D and phosphate metabolism: implications in chronic kidney disease. Exp Cell Res 318(9):14. https://doi.org/10.1016/j.yexcr.2012.02.027.Role
Sato H, James Kazama J, Murasawa A, Otani H, Abe A, Ito S et al (2016) Serum fibroblast growth factor 23 (FGF23) in patients with rheumatoid arthritis. Intern Med 55(2):121–126. https://doi.org/10.2169/internalmedicine.55.5507
Atkins G, Rowe P, Lim H, Welldon K, Ormsby R (2012) Sclerostin is a locally acting regulator of late-osteoblast/preosteocyte differentiation and regulates mineralization through a MEPE-ASARM-dependent mechanism. J Bone Miner Res 26(7):1425–1436. https://doi.org/10.1002/jbmr.345.Sclerostin
Bowe AE, Finnegan R, Jan de Beur SM, Cho J, Levine M a, Kumar R, Schiavi SC (2001) FGF-23 inhibits renal tubular phosphate transport and is a PHEX substrate. Biochem Biophys Res Commun 284(4):977–981. https://doi.org/10.1006/bbrc.2001.5084
Ryan ZC, Ketha H, McNulty MS, McGee-Lawrence M, Craig T a, Grande JP et al (2013) Sclerostin alters serum vitamin D metabolite and fibroblast growth factor 23 concentrations and the urinary excretion of calcium. Proc Natl Acad Sci 110(15):6199–6204. https://doi.org/10.1073/pnas.1221255110
Im CH, Kim NR, Kang JW, Kim JH, Kang JY, Bae GB et al (2014) Inflammatory burden interacts with conventional cardiovascular risk factors for carotid plaque formation in rheumatoid arthritis. Rheumatology (United Kingdom) 54(5):808–815. https://doi.org/10.1093/rheumatology/keu376
Marinou K, Christodoulides C, Antoniades C, Koutsilieris M (2012) Wnt signaling in cardiovascular physiology. Trends Endocrinol Metab 23(12):628–636. https://doi.org/10.1016/j.tem.2012.06.001
Novo-Rodríguez C, García-Fontana B, De Dios Luna-Del Castillo J, Andújar-Vera F, Avila-Rubio V, García-Fontana C et al (2018) Circulating levels of sclerostin are associated with cardiovascular mortality. PLoS One 13(6):1–14. https://doi.org/10.1371/journal.pone.0199504
Brandenburg VM, Kramann R, Koos R, Krüger T, Schurgers L, Mühlenbruch G et al (2013) Relationship between sclerostin and cardiovascular calcification in hemodialysis patients: a cross-sectional study. BMC Nephrol 14(1). https://doi.org/10.1186/1471-2369-14-219
Szkudlarek M, Narvestad E, Klarlund M, Court-Payen M, Thomsen HS, Østergaard M (2004) Ultrasonography of the metatarsophalangeal joints in rheumatoid arthritis: comparison with magnetic resonance imaging, conventional radiography, and clinical examination. Arthritis Rheum 50(7):2103–2112. https://doi.org/10.1002/art.20333
El-Gohary RM, Ahmed Mahmoud AAM, Khalil A, El-Gendy H, Gado KH (2019) Validity of 7-joint versus simplified 12-joint ultrasonography scoring systems in assessment of rheumatoid arthritis activity. J Clin Rheumatol 25(6):264–271. https://doi.org/10.1097/RHU.0000000000000847
Naredo E, Rodríguez M, Campos C, Rodríguez-Heredia JM, Medina J a, Giner E et al (2008) Validity, reproducibility, and responsiveness of a twelve-joint simplified power Doppler ultrasonographic assessment of joint inflammation in rheumatoid arthritis. Arthritis Care Res 59(4):515–522. https://doi.org/10.1002/art.23529
Verheul MK, Fearon U, Trouw L a, Veale DJ (2015) Biomarkers for rheumatoid and psoriatic arthritis. Clin Immunol 161(1):2–10. https://doi.org/10.1016/j.clim.2015.04.005
Author information
Authors and Affiliations
Contributions
All authors have contributed significantly and equally in the design of this work, data acquisition, analysis, and interpretation. In addition to the writing and revising of this manuscript, all authors approved the final version before submission.
Corresponding author
Ethics declarations
Disclosures
None.
Human rights
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Ethical committee approval
The ethical committee of the Internal Medicine Department, Kasr Al-Ainy School of Medicine, Cairo University approved this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fayed, A., Elgohary, R. & Fawzy, M. Evaluating the role of serum sclerostin as an indicator of activity and damage in Egyptian patients with rheumatoid arthritis: university hospital experience. Clin Rheumatol 39, 1121–1130 (2020). https://doi.org/10.1007/s10067-019-04878-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-019-04878-7