Abstract
Additives such as ozone have been shown both experimentally and numerically to enhance detonation properties, but the effects of ozone on one of the most common fuels used in detonation engines, ethylene, have not been sufficiently investigated. In the present study, the impact of ozonolysis reactions between ethylene and ozone on detonations is numerically examined in detail. Specifically, a combined flow reactor and detonation simulation is performed with residence times within the flow reactor corresponding to timescales relevant to detonation engines. The simulations are carried out over a range of equivalence ratios, ethylene–methane fuel blends, temperatures, and pressures. The results show that ozone addition to ethylene–methane–air mixtures leads to the formation of multiple peaks in thermicity. In some cases, particularly for lean mixtures or high ozone concentrations, the first peak in thermicity caused by the consumption of ozone can surpass the second peak caused by high-temperature fuel oxidation. It is also found that the maximum pressure of ozonolysis-assisted detonations decreases and the induction length increases for higher residence times. Comparisons are made between ozonolysis reactions and parasitic combustion due to the tendency of each to raise the pre-shock temperature but decrease the post-shock pressure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Although the vast majority of combustion engines rely on deflagration, detonative propulsion systems such as pulse detonation engines (PDEs) and rotating detonation engines (RDEs) are capable of even higher theoretical efficiencies [1]. Because of this, research on both fundamental aspects [2, 3] and applications [4,5,6] of detonation waves has accelerated over the past 20 years. While detonation waves are three-dimensional and unstable, perhaps their most important parameter is a simple length scale, the cell size, which is formed by the paths of the triple points in space [7]. The detonation cell size, \(\lambda \), has been shown to correlate well with many properties of detonation waves. It has a linear relationship with the induction length, \(\Delta _{\mathrm{I}}\), [8,9,10] (although the ratio \(\lambda {/}\Delta _{\mathrm{I}}\) tends to be dependent on the mixture) and the critical tube diameter, \(d_{\mathrm{c}}\), [7, 11, 12] (for which \(d_{\mathrm{c}}\)/\(\lambda \) is typically 13 for fuel–air mixtures). Additionally, the cube of the cell size has been shown to scale with the critical initiation energy [7, 8, 13], and correlations have been made between the cell size and the deflagration-to-detonation transition (DDT) distance for certain mixtures [14].
Since detonation engines have a wider range of operability for smaller detonation cell sizes [12, 15, 16], parameters which can decrease the cell size are of great interest. Recently, Crane et al. [10] experimentally revealed that the addition of ozone (\(\hbox {O}_{3})\) leads to a reduction in the cell size. Other studies [17,18,19,20] have shown that \(\Delta _{\mathrm{I}}\) likewise decreases with \(\hbox {O}_{3}\) addition. The DDT distance can also be smaller with the addition of \(\hbox {O}_{3}\) [21, 22].
However, the introduction of \(\hbox {O}_{3}\) in mixtures with unsaturated hydrocarbons such as ethylene (\(\hbox {C}_{2}\hbox {H}_{4})\) leads to ozonolysis reactions [23,24,25]. \(\hbox {C}_{2}\hbox {H}_{4}\) is one of the primary hydrocarbons used in small-scale detonation experiments [22, 26,27,28] and in detonation engines [29,30,31,32], but only a few investigations have examined the detonation properties of mixtures containing both \(\hbox {C}_{2}\hbox {H}_{4}\) and \(\hbox {O}_{3}\). Wang et al. [22] performed \(\hbox {C}_{2}\hbox {H}_{4}\)–\(\hbox {O}_{2}\)–\(\hbox {O}_{3}\) detonation experiments at reduced pressures (\(p \le 30\) kPa \(\approx \) 0.3 atm) and found that a small amount of \(\hbox {O}_{3}\) promoted the onset time of DDT, but that excessive \(\hbox {O}_{3}\) delayed DDT. They attributed this phenomenon to ozonolysis reactions between \(\hbox {C}_{2}\hbox {H}_{4}\) and \(\hbox {O}_{3}\) but did not perform any detailed analysis. In a different study, Kumar et al. [20] numerically simulated the impact of \(\hbox {O}_{3}\) addition on \(\hbox {C}_{2}\hbox {H}_{4}\)–air mixtures and determined that the induction length decreases with \(\hbox {O}_{3}\) addition in all instances; however, they did not include ozonolysis reactions in their chemical kinetic model. Therefore, it is still not known to what extent ozonolysis reactions can modify detonation properties or how their effects can change with mixture composition, temperature, or pressure.
This investigation aims to clarify the impact of ozonolysis reactions between \(\hbox {C}_{2}\hbox {H}_{4}\) and \(\hbox {O}_{3}\) on detonations. One-dimensional numerical simulations of detonation wave structure based on the Zel’dovich–von Neumann–Döring (ZND) model are performed for ethylene–methane–air mixtures with various levels of ozone addition. Due to its importance in determining the extent of ozonolysis reactions, the residence time prior to the detonation wave is considered for ranges that are relevant for PDEs and RDEs. The effects of different ethylene/methane ratios, equivalence ratios, pressures, and temperatures are also examined.
2 Simulation
The numerical simulations in this study are performed with a combination of Cantera 2.4.0 [33] and the Caltech Shock and Detonation Toolbox [34]. Specifically, the output from a FlowReactor module in Cantera is fed into a ZND computation in the Shock and Detonation Toolbox, which is similar to the approach of Romano et al. [35] in which a heated fuel–air mixture was allowed to undergo low-temperature oxidation before transitioning to a detonation. The flow reactor calculation is solved with an adiabatic boundary condition, an absolute tolerance of 10\(^{-15}\), a relative tolerance of 10\(^{-9}\), and a maximum timestep of 10\(^{-5}\) s. The residence time within the flow reactor (\(t_{\mathrm{res}})\), representing the time prior to the detonation wave, varies from 1 to 100 ms. Although PDEs can operate with frequencies between 1 and 100 Hz, \(t_{\mathrm{res}}\) more directly corresponds to the sum of the fill and spark delay times, which is usually in the range of 15–40 ms [32, 36, 37]. RDEs have much higher frequencies of 1–10 kHz [5, 15]; however, some RDEs run in a premixed mode, which can have extended residence times within the plenum [30]. The ZND calculation is performed with an absolute tolerance of 10\(^{-8}\), a relative tolerance of 10\(^{-5}\), and a maximum timestep of 10\(^{-4}\) s.
The chemical kinetic model (FFCM-ozonolysis) is identical to the one used in previous studies of ozonolysis-assisted combustion [38, 39] and consists of a combination of the Foundational Fuel Chemistry Model version 1.0 (FFCM-1) [40] for the base hydrocarbon chemistry, HP-Mech [41] for the ozone chemistry, and four reactions from Rousso et al. [24] for describing ozonolysis between \(\hbox {C}_{2}\hbox {H}_{4}\) and \(\hbox {O}_{3}\). Specifically, the four reactions are the following:
Since most commercial ozone generators require pure \(\hbox {O}_{2}\) to produce \(\hbox {O}_{3}\), the ozone concentration is defined as the mole fraction of \(\hbox {O}_{3}\) in the \(\hbox {O}_{2}\)–\(\hbox {O}_{3}\) mixture. Ozone concentrations of up to 3% \(\hbox {O}_{3}\) are examined in this study, which correspond to \(\hbox {O}_{3}\) mole fractions of 5600–6000 ppm in the overall \(\hbox {C}_{2}\hbox {H}_{4}\)–\(\hbox {CH}_{4}\)–\(\hbox {N}_{2}\)–\(\hbox {O}_{2}\)–\(\hbox {O}_{3}\) mixture, depending on the equivalence ratio and fuel blending. The equivalence ratio (\(\phi )\) considers both \(\hbox {O}_{2}\) and \(\hbox {O}_{3}\) in determining the fuel/oxidizer ratio, specifically, \(\phi = (3\hbox {X}_{\mathrm{C2H4}} + 2\hbox {X}_{\mathrm{CH4}}){/}(\hbox {X}_{\mathrm{O2}} + 1.5\hbox {X}_{\mathrm{O3}})\). The ethylene content refers to the mole fraction of \(\hbox {C}_{2}\hbox {H}_{4}\) in the \(\hbox {C}_{2}\hbox {H}_{4}\)–\(\hbox {CH}_{4}\) fuel mixture. The N/O ratio is fixed at 3.762 so that a mixture with 0% \(\hbox {O}_{3}\) addition is “air.” Finally, \(\Delta _{\mathrm{I}}\) is defined as the distance (x) from the shock wave (at \(x = 0\)) to the location of the maximum thermicity.
3 Results and discussion
3.1 Effects of ozonolysis on detonation structure
The impact of \(\hbox {O}_{3}\) addition on \(\hbox {C}_{2}\hbox {H}_{4}\)–\(\hbox {CH}_{4}\)–air detonations is first examined without the upstream flow reactor computation (\(t_{\mathrm{res}}= 0\) ms), i.e., only including the ZND computation. The initial (pre-shock) conditions are \(p = 1\) atm (101.3 kPa) and \(T = 300\) K. Figure 1 shows that \(\hbox {O}_{3}\) addition results in a local maximum in the thermicity (\(\sigma \)), an increase in the peak \(\sigma \), and a decrease in \(\Delta _{\mathrm{I}}\). The local maximum in the spatial profile of \(\sigma \) becomes more pronounced with higher levels of \(\hbox {O}_{3}\) addition. With 3% \(\hbox {O}_{3}\) addition, for example, the first peak in \(\sigma \) near \(x = 0.014\) mm is more than half of the second peak near \(x = 0.76\) mm.
Figure 2 shows the spatial profiles of temperature, pressure, thermicity, and mole fraction for the 3% \(\hbox {O}_{3}\) case. The consumption of \(\hbox {O}_{3}\), as shown by the blue line in Fig. 2b, is partially responsible for the local peak in \(\sigma \) near \(x = 0.014\). Specifically, the reactions most contributing to the first peak in thermicity are \(\hbox {H} + \hbox {O}_{3} =\hbox {O}_{2} + \hbox {OH}\), \(\hbox {C}_{2}\hbox {H}_{3} +\) \(\hbox {O}_{2} = \hbox {CH}_{2}\)O \(+\) HCO, and HCO \(+\) \(\hbox {O}_{2} = \hbox {CO} +\) \(\hbox {HO}_{2}\). The main peak in \(\sigma \) near \(x = 0.76\) mm is caused by the oxidation of even smaller intermediate species by reaction pathways typically associated with high-temperature combustion chemistry such as \(\hbox {CH}_{3} +\) O \(=\) \(\hbox {CH}_{2}\)O \(+\) H, \(\hbox {H}_{2} +\) OH \(=\) H \(+\) \(\hbox {H}_{2}\)O, H \(+\) \(\hbox {O}_{2} +\) M \(=\) \(\hbox {HO}_{2} +\) M, and HCO \(+\) OH \(=\) CO \(+\) \(\hbox {H}_{2}\)O.
3.2 Effects of residence time
When a flow reactor simulation is performed prior to the ZND computation, ozonolysis reactions produce a rise in temperature, even under atmospheric pressure (\(p = 1\) atm \(=\) 101.3 kPa) and room temperature (\(T = 300\) K) conditions. The temperature rise at the end of the flow reactor (\(T_{\mathrm{end}})\) increases for longer \(t_{\mathrm{res}}\) and higher \(\hbox {O}_{3}\) concentrations (Fig. 3). At the highest \(T_{\mathrm{end}}\) of 411 K occurring with 3% \(\hbox {O}_{3}\) addition, 90% and 11% of the initial \(\hbox {O}_{3}\) and \(\hbox {C}_{2}\hbox {H}_{4}\) are consumed, respectively. The amount of \(\hbox {O}_{3}\) and \(\hbox {C}_{2}\hbox {H}_{4}\) consumed, moreover, is directly proportional to \(T_{\mathrm{end}}\) for a given \(\hbox {O}_{3}\) concentration, confirming that the pre-shock temperature rise is primarily due to ozonolysis reactions (\(\sim \) 70% of the overall heat release rate).
Figure 4 shows the differences in the spatial profiles of \(\sigma \) and \(\hbox {O}_{3}\) mole fraction for three different \(t_{\mathrm{res}}\). The \(t_{\mathrm{res}} = 0\) ms and \(t_{\mathrm{res}} = 3\) ms cases have nearly the same \(\Delta _{I}\) and maximum \(\sigma \) since less than 3% of the initial \(\hbox {O}_{3}\) is consumed in the flow reactor for the \(t_{\mathrm{res}} = 3\) ms case. However, for the \(t_{\mathrm{res}}= 50\) ms case, the post-shock \(\hbox {O}_{3}\) mole fraction is only 45% of that of the \(t_{\mathrm{res}} = 0\) ms case. As a result, the local maximum in \(\sigma \) is reduced significantly (below even that of the 0% \(\hbox {O}_{3}\) case), and \(\Delta _{\mathrm{I}}\) is 24% larger than that of the \(t_{\mathrm{res}} = 0\) ms case. Therefore, for cases with higher \(t_{\mathrm{res}}\), the extended time for ozonolysis reactions to proceed results in a smaller amount of post-shock \(\hbox {O}_{3}\) in the mixture, which leads to a reduction in both peaks in \(\sigma \) and a longer \(\Delta _{I}\) for the detonation, despite the rise in pre-shock temperature (Fig. 3).
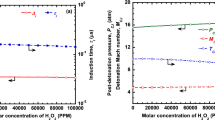
Other effects of extended \(t_{\mathrm{res}}\) are shown in Fig. 5. The Chapman–Jouguet velocity (\(U_{\mathrm{CJ}})\) slightly rises with \(\hbox {O}_{3}\) addition at short \(t_{\mathrm{res}}\) (Fig. 5a); for example, the addition of 3% \(\hbox {O}_{3}\) produces an increase of 5 m/s in \(U_{\mathrm{CJ}}\) at \(t_{\mathrm{res}} = 0\) ms. However, \(U_{\mathrm{CJ}}\) decreases more substantially for long \(t_{\mathrm{res}}\), as the same addition of 3% \(\hbox {O}_{3}\) results in a drop of more than 13 m/s at \(t_{\mathrm{res}}= 50\) ms. The crossover point at which \(\hbox {O}_{3}\) addition negatively impacts \(U_{\mathrm{CJ}}\) is approximately at \(t_{\mathrm{res}} = 15\) ms for all \(\hbox {O}_{3}\) concentrations examined. Similarly, the pressure at the von Neumann state (\(p_{\mathrm{VN}})\) marginally increases from \(p = 32.7\) atm with 0% \(\hbox {O}_{3}\) to \(p = 33.0\) atm with 3% \(\hbox {O}_{3}\) at \(t_{\mathrm{res}} = 0\) ms (Fig. 5b); however, at \(t_{\mathrm{res}} = 50\) ms, 3% \(\hbox {O}_{3}\) produces a 19% decrease in \(p_{\mathrm{VN}}\). Moreover, the crossover point for \(p_{\mathrm{VN}}\) occurs much earlier (\(\sim \) 3 ms). The weakened detonation at high \(\hbox {O}_{3}\) concentration and long \(t_{\mathrm{res}}\) is reminiscent of “parasitic combustion” or “preburning” that can occur in detonation engines [42,43,44,45]. In both cases, subsonic fuel consumption leads to a pre-shock temperature rise, and the detonation that follows has a reduced wave speed and peak pressure. Additionally, since the interaction between shocks and flames can be an important precursor to DDT [46], the reduction in \(p_{\mathrm{VN}}\) as ozonolysis reactions progress (Fig. 5b) may also possibly explain the results of Wang et al. [22], in which excess \(\hbox {O}_{3}\) addition delayed the DDT process in \(\hbox {C}_{2}\hbox {H}_{4}\)–\(\hbox {O}_{2}\)–\(\hbox {O}_{3}\) mixtures.
Figure 6 reveals the impact of \(t_{\mathrm{res}}\) on \(\Delta _{\mathrm{I}}\). Unlike \(U_{\mathrm{CJ}}\) and \(p_{\mathrm{VN}}\), \(\Delta _{\mathrm{I}}\) is monotonically dependent on \(\hbox {O}_{3}\) concentration for all \(t_{\mathrm{res}}\) (i.e., there is no crossover point). Another difference is that the relationship between \(\Delta _{I}\) and \(\hbox {O}_{3}\) addition is highly nonlinear, even at short \(t_{\mathrm{res}}\). A small amount of \(\hbox {O}_{3}\) can produce a large change in \(\Delta _{\mathrm{I}}\). For example, the addition of 0.2% \(\hbox {O}_{3}\) leads to an almost 30% reduction in \(\Delta _{I}\); in contrast, 0.2% \(\hbox {O}_{3}\) addition changes \(p_{\mathrm{VN}}\) by 1–2% at most. However, \(\hbox {O}_{3}\) addition is also less effective on \(\Delta _{\mathrm{I}}\) at long \(t_{\mathrm{res}}\), particularly for higher \(\hbox {O}_{3}\) concentrations. As shown in Fig. 7, 3% \(\hbox {O}_{3}\) addition goes from decreasing \(\Delta _{I}\) by 59% at \(t_{\mathrm{res}}= 0\) ms to 49% at \(t_{\mathrm{res}} = 50\) ms. At \(t_{\mathrm{res}} = 100\) ms, in fact, 3% \(\hbox {O}_{3}\) addition is only slightly more impactful than 1% \(\hbox {O}_{3}\) addition. Overall, the effects of ozonolysis reactions on detonation properties at extended \(t_{\mathrm{res}}\) are complicated—while \(p_{\mathrm{VN}}\) is considerably lower compared to that of the detonation without \(\hbox {O}_{3}\) addition (indicative of a weaker detonation), \(\Delta _{\mathrm{I}}\) is much shorter (indicative of a stronger detonation).
3.3 Effects of changes in ethylene content
In order to examine the interplay between \(\hbox {O}_{3}\) addition and fuel reactivity on detonation structure, Fig. 8 displays \(\Delta _{\mathrm{I}}\) as a function of ethylene content at \(t_{\mathrm{res}} = 30\) ms. It is clear that \(\Delta _{I}\) is quite sensitive to the \(\hbox {C}_{2}\hbox {H}_{4}\)/\(\hbox {CH}_{4}\) ratio. Without \(\hbox {O}_{3}\) addition, a 0% \(\hbox {C}_{2}\hbox {H}_{4}\) mixture (i.e., 100% \(\hbox {CH}_{4})\) has a \(\Delta _{\mathrm{I}}\) more than 30 times larger than that of a 100% \(\hbox {C}_{2}\hbox {H}_{4}\) mixture, which is why methane–air mixtures have rarely been successfully used in detonation engines [6]. At \(t_{\mathrm{res}} = 30\) ms, however, increasing amounts of \(\hbox {O}_{3}\) result in decreasing \(\Delta _{\mathrm{I}}\) across all levels of ethylene content. For example, the 50% \(\hbox {C}_{2}\hbox {H}_{4}\) case with 1% \(\hbox {O}_{3}\) addition (\(\Delta _{\mathrm{I}}=\) 1.07) has nearly the same \(\Delta _{\mathrm{I}}\) as the 75% \(\hbox {C}_{2}\hbox {H}_{4}\) case without \(\hbox {O}_{3}\) addition (\(\Delta _{\mathrm{I}}=\) 1.01).
The change in \(\Delta _{\mathrm{I}}\) with \(\hbox {O}_{3}\) addition for different levels of ethylene content is shown in Fig. 9. For both residence times (\(t_{\mathrm{res}} = 1\) ms and 30 ms), the relationship between ethylene content and the change in \(\Delta _{\mathrm{I}}\) is modified by the amount of \(\hbox {O}_{3}\) addition. Specifically, the 0.2% \(\hbox {O}_{3}\) cases display larger relative decreases in \(\Delta _{I}\) with increasing ethylene content, but the 3% \(\hbox {O}_{3}\) cases have the opposite trend. The impact of ozonolysis reactions also becomes clearer at higher amounts of \(\hbox {O}_{3}\) addition. While there is not much difference between the \(t_{\mathrm{res}} = 1\) ms and \(t_{\mathrm{res}} = 30\) ms cases at 0.2% \(\hbox {O}_{3}\), a distinct separation is apparent at 3% \(\hbox {O}_{3}\). At 100% \(\hbox {C}_{2}\hbox {H}_{4}\), in fact, the difference between the two residence times at 3% \(\hbox {O}_{3}\) is 10% (i.e., a 46% decrease in \(\Delta _{\mathrm{I}}\) versus a 56% decrease). Note that \(t_{\mathrm{res}}\) has no influence on \(\Delta _{\mathrm{I}}\) for the 0% \(\hbox {C}_{2}\hbox {H}_{4}\) case since ozonolysis reactions do not occur for pure methane.
3.4 Effects of changes in equivalence ratio
When \(\hbox {O}_{3}\) is added to a mixture at lean equivalence ratios, it is possible for the first peak in \(\sigma \) to exceed the second peak in \(\sigma \). Figure 10 reveals an example of such an occurrence. At \(t_{\mathrm{res}} = 30\) ms, the second peak in \(\sigma \) at \(x = 4.44\) mm is slightly lower than the first peak in \(\sigma \) at \(x = 0.032\) mm for the \(\phi = 0.55\) mixture with 3% \(\hbox {O}_{3}\); therefore, \(\Delta _{\mathrm{I}}\) is shorter by approximately two orders of magnitude compared to the same case with \(t_{\mathrm{res}} = 50\) ms in which \(\Delta _{\mathrm{I}} = 4.69\) mm. For ozone-assisted detonations, however, it is unclear whether parameters such as the critical ignition energy or the critical tube diameter would scale with this first peak in \(\sigma \) within the ozone decomposition zone, as implied by Kumar et al. [20] for mixtures with large amounts of \(\hbox {O}_{3}\) addition. For example, the authors in [20] computed that \(\Delta _{\mathrm{I}} = 0.010\) mm (which is within the ozone decomposition zone) for their stoichiometric \(\hbox {C}_{2}\hbox {H}_{4}\)–air case with 15,000 ppm \(\hbox {O}_{3}\), but the second peak in \(\sigma \) for this condition is at \(x \approx 0.180\) mm in the middle of the high-temperature reaction zone. As has been stated previously by Ng et al. [47], experimental measurements are needed in the future to determine how detonation properties (e.g., critical tube diameter) scale for detonations with multiple peaks in thermicity.
Figure 11 shows \(\Delta _{\mathrm{I}}\) as a function of \(t_{\mathrm{res}}\) for four different equivalence ratios. Note that the second peak in \(\Delta _{I}\) of the \(\phi = 0.55\) case is also included for reference. Even with 3% \(\hbox {O}_{3}\), \(\Delta _{I}\) is still strongly dependent on the equivalence ratio. For example, at \(t_{\mathrm{res}} \le 30\) ms, \(\Delta _{I}= 1.0\)–1.1 mm for the \(\phi = 0.85\) case and 4.2–4.4 mm for the \(\phi = 0.55\) case. The effect of increased \(t_{\mathrm{res}}\) is also slightly lower at decreased equivalence ratios, shifting from a 24% difference in \(\Delta _{\mathrm{I}}\) between the \(t_{\mathrm{res}} = 0\) ms and \(t_{\mathrm{res}} = 50\) ms cases at \(\phi = 1\) to a 17% difference over the same range at \(\phi = 0.7\). The reduced consumption of \(\hbox {O}_{3}\) in the lean case (39% at \(\phi = 0.7\) versus 55% at \(\phi = 1\)) is thought to be responsible for this discrepancy. Therefore, for a given \(t_{\mathrm{res}}\), the progress of ozonolysis reactions impacts the detonation structure to a slightly lesser degree at lower equivalence ratios.
The occurrence of detonations with two-step heat release has been investigated previously for nitromethane-\(\hbox {O}_{2}\) [48], \(\hbox {H}_{2}\)–\(\hbox {NO}_{2}\) [49], and dimethyl ether-\(\hbox {O}_{2}\) mixtures [47], which can result in experimental observation of “double cellular detonations” under certain conditions [50]. Likewise, detonations with two peaks in thermicity for \(\hbox {C}_{2}\hbox {H}_{4}\)–\(\hbox {CH}_{4}\)–air mixtures with \(\hbox {O}_{3}\) addition are shown in Fig. 12. The first and second induction lengths (Fig. 12a) tend to decrease with increasing \(\phi \), and the first and second peaks in thermicity (Fig. 12b) monotonically increase with increasing \(\phi \). As seen previously in Fig. 11, the first peak in thermicity surpasses the second peak in thermicity for very lean (\(\phi > 0.6\)) equivalence ratios. This unusual characteristic (most of the previous studies observed two peaks in thermicity in rich mixtures [48], and none had a crossover point in the maximum thermicity) is due to the simple fact that there is more \(\hbox {O}_{3}\) relative to fuel for leaner mixtures. In other words, \(\hbox {O}_{3}\) consumption increases as fuel oxidation weakens. Additionally, the ratio between the first and second peaks in thermicity ranges from 2.8 at \(\phi = 1\) to 0.23 at \(\phi = 0.4\), which is a wide variation compared to other mixtures [51]. The ratio between the first and second induction lengths, likewise, spans approximately an order of magnitude from 43 to 450. Of course, these ratios are significantly modified when either \(t_{\mathrm{res}}\) or the \(\hbox {O}_{3}\) concentration changes.
The relative change in \(\Delta _{\mathrm{I}}\) from 3% \(\hbox {O}_{3}\) addition is shown in Fig. 13 for both the 50% \(\hbox {C}_{2}\hbox {H}_{4}\) case and the 100% \(\hbox {C}_{2}\hbox {H}_{4}\) case. For both fuel blends, the relative decrease in \(\Delta _{I}\) is more prominent for lower equivalence ratios; additionally, the impact of ozonolysis reactions (i.e., the sensitivity to \(t_{\mathrm{res}})\) at a given equivalence ratio is similar between the two blends. Interestingly, the 50% \(\hbox {C}_{2}\hbox {H}_{4}\) case has a larger relative decrease in \(\Delta _{I}\) than the 100% \(\hbox {C}_{2}\hbox {H}_{4}\) case for \(\phi = 1\) and \(\phi = 0.85\) but a smaller decrease for \(\phi = 0.55\). This trend is consistent across the entire range of \(t_{\mathrm{res}}\) examined. It should be noted, however, that for 0.2% \(\hbox {O}_{3}\) addition (not shown) the relative decrease in \(\Delta _{\mathrm{I}}\) is greater for the 100% \(\hbox {C}_{2}\hbox {H}_{4}\) case across all lean equivalence ratios, which is not altogether surprising considering the trends from Fig. 9.
3.5 Effects of changes in pressure and temperature
Since the wall temperatures in detonation engines can reach 800 K or higher over the span of a few seconds [5, 52], the temperature of the fresh gas mixture can be modified by heat transfer from the walls. Figure 14a shows \(\Delta _{\mathrm{I}}\) for initial temperatures of 300 K, 400 K, and 500 K. With 0% \(\hbox {O}_{3}\) addition, \(\Delta _{\mathrm{I}}\) is a strict function of temperature; for example, \(\Delta _{I}\) of the \(T = 500\) K case is \(\sim \) 30% smaller than that of the \(T = 300\) K case. However, with 3% \(\hbox {O}_{3}\) addition, higher temperatures result in faster ozonolysis reaction rates for a given \(t_{\mathrm{res}}\), which leads to some interesting trends in \(\Delta _{\mathrm{I}}\). At \(t_{\mathrm{res}} = 0\) ms, \(\Delta _{\mathrm{I}}\) of the \(T = 500\) K case (\(\Delta _{I}= 0.69\) mm) is slightly smaller than those of the \(T = 400\) K (\(\Delta _{\mathrm{I}} = 0.73\) mm) and \(T = 300\) K (\(\Delta _{\mathrm{I}} = 0.76\) mm) cases. At moderate residence times such as \(t_{\mathrm{res}} = 20\) ms, the order becomes jumbled (\(\Delta _{\mathrm{I,300\,K}}< \Delta _{\mathrm{I,500\,K}} < \Delta _{\mathrm{I,400\,K}})\). Finally, at \(t_{\mathrm{res}} = 100\) ms, at which point almost all of the \(\hbox {O}_{3}\) is consumed before the shock, \(\Delta _{I}\) regains the original trend (\(\Delta _{\mathrm{I,500~K}}<\Delta _{\mathrm{I,400K}} < \Delta _{\mathrm{I,300~K}})\).
The relative change in \(\Delta _{\mathrm{I}}\), as shown in Fig. 14b, is consistently less pronounced for higher temperatures. For \(T = 300\) K, the decrease in \(\Delta _{\mathrm{I}}\) is nearly linear with \(t_{\mathrm{res}}\) due the progress of ozonolysis reactions. However, the trend for \(T = 500\) K case is much less linear as a result of the increased ozonolysis reaction rates at higher temperatures; in fact, the decrease in \(\Delta _{\mathrm{I}}\) plateaus at 32% for \(t_{\mathrm{res}} \ge 20\) ms due to all of the \(\hbox {O}_{3}\) being consumed. This, of course, is due to the changes in pre-shock \(\hbox {O}_{3}\) consumption with temperature. Specifically, at \(t_{\mathrm{res}} = 20\) ms in Fig. 14, 20%, 82%, and 99% of \(\hbox {O}_{3}\) have been consumed prior to the shock for \(T = 300\) K, 400 K, and 500 K, respectively. Ozonolysis reactions consume 71%, 76%, and 71% of this pre-shock \(\hbox {O}_{3}\), respectively. Therefore, increasing the temperature is not the most effective way to reduce \(\Delta _{I}\) for ozonolysis-assisted detonations because the effects of higher post-shock temperatures and increased ozonolysis reaction rates tend to cancel each other out. Additionally, the pressure gain (i.e., \(p_{\mathrm{VN}})\) is higher at \(T = 300\) K than at \(T = 500\) K, which offsets the higher initial temperature.
Finally, although changes in temperature have only moderate effects on \(\Delta _{\mathrm{I}}\) with \(\hbox {O}_{3}\) addition, modifying the pressure still considerably impacts \(\Delta _{\mathrm{I}}\). Figure 15 displays how \(\Delta _{\mathrm{I}}\) decreases with increasing pressure for both 0% \(\hbox {O}_{3}\) and 3% \(\hbox {O}_{3}\) addition. Depending on the pressure, the relationship between \(\Delta _{I}\) and \(t_{\mathrm{res}}\) can be nearly constant (\(p~=~0.2\) atm \(~=~\) 20.3 kPa), linear (\(p~=~1\) atm \(~=~\) 101.3 kPa), or nonlinear (\(p = 5\) atm \(=\) 506.6 kPa) with \(\hbox {O}_{3}\) addition. For the lowest pressure case of \(p = 0.2\) atm, only 22% of the initial \(\hbox {O}_{3}\) is consumed before the shock for \(t_{\mathrm{res}} = 100\) ms. Moreover, as previously seen in the higher-temperature cases, higher pressures of \(p = 5\) atm result in increased ozonolysis reaction rates and the nearly complete (\(\sim \) 98%) consumption of \(\hbox {O}_{3}\) by \(t_{\mathrm{res}} = 30\) ms. Additionally, the relative decrease in \(\Delta _{\mathrm{I}}\) (not shown) of the \(p = 5\) atm case is extremely similar for \(t_{\mathrm{res}} = 0\) ms (\(\sim \) 59%) and \(t_{\mathrm{res}} = 100\) ms (\(\sim \) 41%) to the \(p = 1\) atm case but less pronounced at moderate \(t_{\mathrm{res}}\).
4 Conclusions
The answer to the question posed in the title of this paper is a mild “yes.” While ozonolysis reactions are unlikely to affect the general sizing requirements of a detonation engine, they can cause similar effects to parasitic combustion. In particular, for residence times relevant to pulse detonation engines (15–40 ms), this investigation revealed that ozonolysis reactions had appreciable effects on the detonation properties of ethylene–methane–air mixtures with ozone addition. Ozone addition resulted in larger peak pressures, higher maximum thermicities, and shorter induction lengths for short residence times. However, at extended residence times, the detonation weakened due to the consumption of ethylene by ozonolysis reactions. Higher ozone concentrations led to more extreme deviations in the peak pressure.
Ozone addition also had a tremendous effect on the induction length. The largest relative decreases in the induction length occurred for shorter residence times, higher ozone concentrations, leaner mixtures, and lower temperatures. For very lean mixtures or high ozone concentrations, the peak in thermicity in the ozone consumption zone exceeded the peak in thermicity caused by high-temperature fuel oxidation. At extended residence times, ozonolysis reactions resulted in longer induction lengths, especially for elevated pressure and temperature conditions. However, the ozonolysis-affected induction lengths still had values well below those without ozone addition.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Wolanski, P.: Detonative propulsion. Proc. Combust. Inst. 34, 125–158 (2013). https://doi.org/10.1016/j.proci.2012.10.005
Lee, J.H.S.: The Detonation Phenomenon. Cambridge University Press, Cambridge (2018). https://doi.org/10.1017/CBO9781316226926
Shepherd, J.E.: Detonation in gases. Proc. Combust. Inst. 32, 83–98 (2009). https://doi.org/10.1016/j.proci.2008.08.006
Kailasanath, K.: Recent developments in the research on pulse detonation engines. AIAA J. 41, 145–159 (2003). https://doi.org/10.2514/2.1933
Lu, F.K., Braun, E.M.: Rotating detonation wave propulsion: experimental challenges, modeling, and engine concepts. J. Prop. Power 30, 1125–1142 (2014). https://doi.org/10.2514/1.B34802
Anand, V., Gutmark, E.: Rotating detonation combustors and their similarities to rocket instabilities. Prog. Energy Combust. Sci. 73, 182–234 (2019). https://doi.org/10.1016/j.pecs.2019.04.001
Lee, J.H.S.: Dynamic parameters of gaseous detonations. Annu. Rev. Fluid Mech. 16, 311–336 (1984). https://doi.org/10.1146/annurev.fl.16.010184.001523
Westbrook, C.K.: Chemical kinetics of hydrocarbon oxidation in gaseous detonations. Combust. Flame 46, 191–210 (1982). https://doi.org/10.1016/0010-2180(82)90015-3
Gavrikov, A.I., Efimenko, A.A., Dorofeev, S.B.: A model for detonation cell size prediction from chemical kinetics. Combust. Flame 120, 19–33 (2000). https://doi.org/10.1016/S0010-2180(99)00076-0
Crane, J., Shi, X., Singh, A.V., Tao, Y., Wang, H.: Isolating the effect of induction length on detonation structure: hydrogen–oxygen detonation promoted by ozone. Combust. Flame 200, 44–52 (2019). https://doi.org/10.1016/j.combustflame.2018.11.008
Knystautas, R., Lee, J.H., Guirao, C.M.: The critical tube diameter for detonation failure in hydrocarbon-air mixtures. Combust. Flame 48, 63–83 (1982). https://doi.org/10.1016/0010-2180(82)90116-X
Vasil’ev, A.A.: Cell size as the main geometric parameter of a multifront detonation wave. J. Propuls. Power 22, 1245–1260 (2006). https://doi.org/10.2514/1.20348
Bull, D.C., Elsworth, J.E., Shuff, P.J., Metcalfe, E.: Detonation cell structures in fuel/air mixtures. Combust. Flame 45, 7–22 (1982). https://doi.org/10.1016/0010-2180(82)90028-1
Dorofeev, S.B., Sidorov, V.P., Kuznetsov, M.S., Matsukov, I.D., Alekseev, V.I.: Effect of scale on the onset of detonations. Shock Waves 10, 137–149 (2000). https://doi.org/10.1007/s001930050187
Roy, A., Bedick, C.R., Ferguson, D.H., Sidwell, T., Strakey, P.A.: Investigating instabilities in a rotating detonation combustor operating with natural gas-hydrogen fuel blend–effect of air preheat and annulus width. J. Eng. Gas Turbines Power 141, 111017 (2019). https://doi.org/10.1115/1.4044980
Walters, I.V., Journell, C.L., Lemcherfi, A., Gejji, R.M., Heister, S.D., Slabaugh, C.D.: Operability of a natural gas-air rotating detonation engine. J. Propuls. Power 36, 453–464 (2020). https://doi.org/10.2514/1.B37735
Magzumov, A.E., Kirillov, I.A., Rusanov, V.D.: Effect of small additives of ozone and hydrogen peroxide on the induction-zone length of hydrogen-air mixtures in a one-dimensional model of a detonation wave. Combust. Expl. Shock Waves 34, 338–341 (1998). https://doi.org/10.1007/BF02672728
Mével, R., He, Y.Z.: Effect of oxygen atom precursors addition on LTC-affected detonation in DME–\(\text{ O}_{2}\)–\(\text{ CO}_{2}\) mixtures. Shock Waves 30, 799–807 (2020). https://doi.org/10.1007/s00193-020-00953-0
Crane, J., Shi, X., Xu, R., Wang, H.: Natural gas versus methane: Ignition kinetics and detonation limit behavior in small tubes. Combust. Flame. 237, 111719 (2022). https://doi.org/10.1016/j.combustflame.2021.111719
Kumar, D.S., Ivin, K., Singh, A.V.: Sensitizing gaseous detonations for hydrogen/ethylene-air mixtures using ozone and \(\text{ H}_{2}\text{ O}_{2}\) as dopants for application in rotating detonation engines. Proc. Combust. Inst. 38, 3825–3834 (2021). https://doi.org/10.1016/j.proci.2020.08.061
Sepulveda, J., Rousso, A., Ha, H., Chen, T., Cheng, V., Kong, W., Ju, Y.: Kinetic enhancement of microchannel detonation transition by ozone addition to acetylene mixtures. AIAA J. 57, 476–481 (2019). https://doi.org/10.2514/1.J057773
Wang, C., Gu, G.T., Han, W.H., Cai, Y.: Role of \(\text{ O}_{3}\) addition in the deflagration-to-detonation transition of an ethylene–oxygen mixture in a macroscale tube. Shock Waves 30, 781–787 (2020). https://doi.org/10.1007/s00193-020-00981-w
Bailey, P.S.: The reactions of ozone with organic compounds. Chem. Rev. 58, 925–1010 (1958). https://doi.org/10.1021/cr50023a005
Rousso, A.C., Hansen, N., Jasper, A.W., Ju, Y.: Low-temperature oxidation of ethylene by ozone in a jet-stirred reactor. J. Phys. Chem. A 122, 8674–8685 (2018). https://doi.org/10.1021/acs.jpca.8b06556
Sun, W., Gao, X., Wu, B., Ombrello, T.: The effect of ozone addition on combustion: kinetics and dynamics. Prog. Energy Combust. Sci. 73, 1–25 (2019). https://doi.org/10.1016/j.pecs.2019.02.002
Hasson, A., Avinor, M., Burcat, A.: Transition from deflagration to detonation, spark ignition, and detonation characteristics of ethylene–oxygen mixtures in a tube. Combust. Flame 49, 13–26 (1983). https://doi.org/10.1016/0010-2180(83)90147-5
Moen, I.O., Ward, S.A., Thibault, P.A., Lee, J.H., Knystautas, R., Dean, T., Westbrook, C.K.: The influence of diluents and inhibitors on detonations. Proc. Combust. Inst. 20, 1717–1725 (1985). https://doi.org/10.1016/S0082-0784(85)80668-8
Auffret, Y., Desbordes, D., Presles, H.N.: Detonation structure of \(\text{ C}_{2}\text{ H}_{4}\)–\(\text{ O}_{2}\)–Ar mixtures at elevated initial temperature. Shock Waves 9, 107–111 (1999). https://doi.org/10.1007/s001930050145
Brophy, C.M., Hanson, R.K.: Fuel distribution effects on pulse detonation engine operation and performance. J. Propuls. Power 22, 1155–1161 (2006). https://doi.org/10.2514/1.18713
Andrus, I.Q., Polanka, M.D., King, P.I., Schauer, F.R., Hoke, J.L.: Experimentation of premixed rotating detonation engine using variable slot feed plenum. J. Propuls. Power 33, 1448–1458 (2017). https://doi.org/10.2514/1.B36261
Nakagami, S., Matsuoka, K., Kasahara, J., Kumazawa, Y., Fujii, J., Matsuo, A., Funaki, I.: Experimental visualization of the structure of rotating detonation waves in a disk-shaped combustor. J. Propuls. Power 33, 80–88 (2017). https://doi.org/10.2514/1.B36084
Murray, A.P., Smith, T.L., Pittman, E.D., Liu, H., Crisp, E.M., Moore, J.D., Risha, G.A.: Frequency and spark timing effects on thrust for pulse detonation engine. J. Propuls. Power 37, 242–251 (2021). https://doi.org/10.2514/1.B38049
Goodwin, D.G., Speth, R.L., Moffat, H.K., Weber, B.W.: Cantera: an object-oriented software toolkit for chemical kinetics, thermodynamics, and transport processes. http://www.cantera.org (2018). Accessed 25 Jan 2022
Browne, S., Ziegler, J., Shepherd, J.E.: Numerical solution methods for shock and detonation jump conditions. GALCIT Report FM2006.006 (2008)
Romano, M.P., Radulescu, M.I., Higgins, A.J., Lee, J.H.S., Pitz, W.J., Westbrook, C.K.: Sensitization of hydrocarbon–oxygen mixtures to detonation via cool-flame oxidation. Proc. Combust. Inst. 29, 2833–2838 (2002). https://doi.org/10.1016/S1540-7489(02)80346-7
Tucker, K.C., King, P.I., Schauer, F.R.: Hydrocarbon fuel flash vaporization for pulsed detonation combustion. J. Propul. Power 24, 788–796 (2008). https://doi.org/10.2514/1.28412
Stevens, C., King, P., Nagley, E., Schauer, F.: Fuel composition and coking analysis of endothermically heated hydrocarbon fuels for use in a pulsed detonation engine. 47th AIAA Aerospace Sciences Meeting, Orlando, FL, AIAA Paper 2009-501 (2009). https://doi.org/10.2514/6.2009-501
Reuter, C.B., Ombrello, T.M.: Flame enhancement of ethylene/methane mixtures by ozone addition. Proc. Combust. Inst. 38, 2397–2407 (2021). https://doi.org/10.1016/j.proci.2020.06.122
Reuter, C.B., Ombrello, T.M.: Numerical simulations of ozone addition to strained flames. Combust. Sci. Technol. (2022). https://doi.org/10.1080/00102202.2021.1923703
Smith, G.P., Tao, Y., Wang, H.: Foundational fuel chemistry model version 1.0 (FFCM-1). http://nanoenergy.stanford.edu/ffcm1 (2016). Accessed 25 Jan 2022
Zhao, H., Yang, X., Ju, Y.: Kinetic studies of ozone assisted low temperature oxidation of dimethyl ether in a flow reactor using molecular-beam mass spectrometry. Combust. Flame 173, 187–194 (2016). https://doi.org/10.1016/j.combustflame.2016.08.008
Schwer, D.A., Kailasanath, K.: Physics of heat-release in rotating detonation engines. 53rd AIAA Aerospace Sciences Meeting, Kissimmee, FL, AIAA Paper 2015-1602 (2015). https://doi.org/10.2514/6.2015-1602
Chacon, F., Gamba, M. Study of parasitic combustion in an optically accessible continuous wave rotating detonation engine. AIAA SciTech Forum, San Diego, CA, AIAA Paper 2019-0473. (2019). https://doi.org/10.2514/6.2019-0473
Prakash, S., Raman, V.: The effects of mixture preburning on detonation wave propagation. Proc. Combust. Inst. 38, 3749–3758 (2021). https://doi.org/10.1016/j.proci.2020.06.005
Sato, T., Chacon, F., White, W., Raman, V., Gamba, M.: Mixing and detonation structure in a rotating detonation engine with an axial air inlet. Proc. Combust. Inst. 38, 3769–3776 (2021). https://doi.org/10.1016/j.proci.2020.06.283
Khokhlov, A.M., Oran, E.S.: Numerical simulation of detonation initiation in a flame brush: the role of hot spots. Combust. Flame 119, 400–416 (1999). https://doi.org/10.1016/S0010-2180(99)00058-9
Ng, H.D., Chao, J., Yatsufusa, T., Lee, J.H.: Measurement and chemical kinetic prediction of detonation sensitivity and cellular structure characteristics in dimethyl ether–oxygen mixtures. Fuel 88, 124–131 (2009). https://doi.org/10.1016/j.fuel.2008.07.029
Presles, H.N., Desbordes, D., Guirard, M., Guerraud, C.: Gaseous nitromethane and nitromethane–oxygen mixtures: a new detonation structure. Shock Waves 6, 111–114 (1996). https://doi.org/10.1007/BF02515194
Joubert, F., Desbordes, D., Presles, H.N.: Detonation cellular structure in \(\text{ NO}_{2}\)/\(\text{ N}_{2}\text{ O}_{4}\)-fuel gaseous mixtures. Combust. Flame 152, 482–495 (2008). https://doi.org/10.1016/j.combustflame.2007.11.005
Guilly, V., Khasainov, B., Presles, H.N., Desbordes, D.: Simulation numérique des détonations à double structure cellulaire. C. R. Méc. 334, 679–685 (2006). https://doi.org/10.1016/j.crme.2006.05.002
Mével, R., Gallier, S.: Structure of detonation propagating in lean and rich dimethyl ether–oxygen mixtures. Shock Waves 28, 955–966 (2018). https://doi.org/10.1007/s00193-018-0837-x
Ishihara, K., Nishimura, J., Goto, K., Nakagami, S., Matsuoka, K., Kasahara, J., Matsuo, A., Funaki, I., Moriai, H., Mukae, H., Yasuda, K., Nakata, D., Higashino, K.: Study on a long-time operation towards rotating detonation rocket engine flight demonstration. 55th AIAA Aerospace Sciences Meeting, Grapevine, TX, AIAA Paper 2017-1062 (2017). https://doi.org/10.2514/6.2017-1062
Acknowledgements
This research was performed while C. B. Reuter held a National Research Council (NRC) Research Associateship Award at the Air Force Research Laboratory and an American Society for Engineering Education (ASEE) Postdoctoral Fellowship Award at the Naval Research Laboratory.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Ciccarelli.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Reuter, C.B., Ombrello, T.M. & Tuttle, S.G. Can ozonolysis reactions influence detonations?. Shock Waves 32, 363–371 (2022). https://doi.org/10.1007/s00193-022-01082-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00193-022-01082-6