Abstract
Introduction and hypothesis
A multiple-component intensive pelvic floor muscle training (MCI-PFMT) protocol was developed as a neurophysiological-based rehabilitation model to improve neuroplasticity. This study aimed to investigate the effects of the MCI-PFMT protocol on muscle fatigue and symptoms in women with urinary incontinence.
Methods
This randomized controlled trial included 49 female patients with mixed urinary incontinence. Participants were divided into the MCI-PFMT group and the control group. The MCI-PFMT group performed supervised intensive pelvic floor muscle training, while the control group received bladder training and standard pelvic floor muscle training as a home program. Both training sessions were conducted 5 days a week for a single week. Participants' symptoms were evaluated with questionnaires, bladder diary, and pad tests. Superficial electromyography, ultrasonography, and the PERFECT scale were used to evaluate pelvic floor and abdominal muscle functions.
Results
In the post-treatment evaluation, symptoms were decreased in both groups, with a significant decrease in the MCI-PFMT group (p < 0.05). While average and peak work values of pelvic floor muscles, transversus abdominus, and internal oblique muscles increased in both groups, maximum voluntary contraction values of these muscles decreased (p < 0.05). A 12.7% decrease was observed in the maximum voluntary contraction values of pelvic floor muscles in the control group, while a 9.6% decrease was observed in the MCI-PFMT group.
Conclusions
The MCI-PFMT protocol can lead to pelvic floor and abdominal muscle fatigue. However, it may be effective at decreasing symptoms in women with urinary incontinence. Additional studies on this issue are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary incontinence (UI) is defined by the International Urogynecological Association and the International Continence Society as involuntary urinary loss [1]. It is a common problem affecting women of all ages, with varying symptoms. These symptoms can be related to urine storage and discharge [2]. There are different types of UI according to these symptoms: stress UI (complaint of involuntary loss of urine on effort or physical exertion), urgency UI (complaint of involuntary loss of urine associated with urgency), and mixed UI (complaint of involuntary loss of urine associated with urgency and also with effort or physical exertion or on sneezing or coughing) [1]. For almost two decades, pelvic floor muscle (PFM) training has been recommended at grade A as a first-line treatment for mild to moderate UI [3].
A meta-analysis indicated that regular PFM training improves the symptoms of UI, while long-term, high-intensity PFM training may provide positive benefits [4]. Various PFM training protocols exist in the literature. These protocols vary in terms of session duration (20 minutes, 45 minutes, or longer), number of weekly sessions (two, three, or more per week), the total number of sessions (24 sessions, less or more), combination with other types of exercise, biofeedback, or electrical stimulation. However, the intensity, frequency, duration, and type of the most effective PFM training for UI have not yet been reported [5].
The central and peripheral nervous systems may be affected in UI [6]. PFM training has been demonstrated to increase neuroplasticity in the nervous system, and this is reflected in symptoms [7]. In neurological diseases, constraint-induced motor therapy has been developed to increase neuroplasticity through intensive and repeated use of the affected limb [8]. Similarly, an intensive PFM training approach including the whole day can be performed to increase neuroplasticity in UI. This can result in faster neural adaptation and improve symptoms such as UI, fecal incontinence, and pelvic pain.
Studies have focused on acute PFM fatigue following high-impact sports, high-intensity physical activity, or short-term PFM training [9, 10]. In these studies, PFM activity and symptoms were investigated immediately after performing activity that would cause fatigue. However, this activity was not continued for a long duration. PFM fatigue has been reported to increase the symptoms of stress UI, fecal incontinence, and pelvic prolapse [9, 10]. Although there are warnings that symptoms may increase when fatigue occurs after PFM training, there is no study in the literature investigating the effects of an intensive PFM training protocol performed routinely for a whole day or whole week. Therefore, the question of whether such routine training increases symptoms remains unanswered.
This study aims to determine whether a multiple-component intensive PFM training (MCI-PFMT) protocol that we developed increases PFM and abdominal muscle fatigue. Our secondary aim is to compare the MCI-PFMT protocol with standard PFM training to investigate its short-term effects on the symptoms of women with UI.
Material and methods
Study design and participants
This is a randomized controlled trial. Forty-nine women who were diagnosed with mixed UI in the Department of Obstetrics and Gynecology, Dokuz Eylül University Medical Faculty Hospital, were included.
The inclusion criteria were as follows: having been diagnosed with mixed UI by a gynecology and obstetrics specialist, signing a consent form, and being 18 years of age or older. Exclusion criteria were as follows: being pregnant, being in the postpartum or menstrual period, presence of active urinary tract infection, having undergone pelvic or abdominal surgery, presence of concomitant neurological, orthopedic, or psychiatric disease, or having previously received PFM training.
To diagnose mixed UI, a detailed history, gynecological examination, neurological examination, Q-type test, stress test, bladder neck mobility with ultrasound (US), pelvic examination, urinalysis, blood test, bladder diary, and questionnaires were applied to the participants.
Ethics statement
The study was conducted according to the ethical standards of the Helsinki Declaration and was approved by Dokuz Eylul University Institutional Noninvasive Research Ethics Board (number: 4398-GOA). All the individuals gave written consent to participate in the study after receiving appropriate verbal and written information. No participation fees, travel fees, or similar fees were paid to the participants in the study.
Randomization
Volunteer participants who met the inclusion criteria were enrolled in the order they applied to the clinic. Patients were randomized into two groups via a free website (https://www.randomizer.org/): MCI-PFMT group (n = 25) and control group (n = 24).
Intervention
Participants were educated in pelvic floor anatomy, how to find the pelvic floor in their body, the physiological responses of the PFM to exercise, the role of the PFM in UI, the strategies to protect PFM, and correct posture. During the first session, participants were taught the correct contraction and relaxation of the PFM using digital palpation. In both groups, the treatment program was conducted by physiotherapists specialized in this field.
MCI-PFMT group (n = 25)
The MCI-PFMT protocol was developed as a supervised, whole-day rehabilitation model based on neurophysiological principles to improve neuroplasticity. It included approaches for sensory, motor, and reflex effects in the PFM. In this approach, the PFM can be considered a part of the body with sensory, motor, and reflex dysfunction in patients with neurological disorders. PFM is likened to an impaired hemiplegic arm's neurological function in stroke patients. Therefore, the MCI-PFMT protocol was created based on the treatments used in PFM dysfunction and the constraint-induced motor therapy approach.
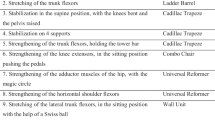
The MCI-PFMT protocol includes education and exercise phases. The exercise phase consists of stretching exercises, PFM exercises on the mat, PFM exercises on the ball, PFM exercises during aerobic training, PFM exercises during functional activities, and relaxation exercises (Figs. 2 and 3). This protocol was performed for a single week, 5 days a week, and 6 hours a day (see Appendix).
Control group (n = 24)
The standard PFM training protocol was performed in the control group every day for a single week as a home program. This protocol was performed in four different positions (supine, bridge, prone, and crawling) with three sets of 10 repetitions in each position. In addition, bladder training was given to the control group.
Type: Isometric, concentric, and eccentric strengthening exercises were performed in various positions. They were performed in isometric, concentric, and eccentric positions, respectively. Exercises included both fast muscle fibers and slow muscle fibers.
Intensity: The exercises were performed as a maximum voluntary contraction (MVC).
Frequency: The home program took place 5 days a week, with three sets a day.
Duration: The exercise program continued uninterrupted for a week, with 1 day of supervision and 5 days of the home program. Relaxation time was 4–10 seconds, contraction time was 10 seconds, and time between sets was 1–2 minutes.
Outcome measurements
The following practices were performed in both groups: pre-treatment evaluation in the first week (5th, 6th, 7th days), treatment in the second week (1st, 2nd, 3rd, 4th, 5th days), and post-treatment evaluation at the end of the second week (5th, 6th, 7th days). All practices were carried out on standard days for 2 weeks. Both groups were asked the following questions on the fifth-day post-treatment:
-
Was there more urine loss during physical activity at the end of the day compared to early morning?
-
Was there more urine loss in the first 30 minutes after the PFM training session?
-
Was urine loss worse at night than in the morning?
-
Was the feeling of intravaginal bloating increased at the end of the day compared to early morning?
Evaluation of urinary symptoms
Questionnaires with documented validity and reliability in Turkish (International Consultation on Incontinence Questionnaire–Urinary Incontinence Short Form [11], Urinary Distress Inventory-6 [12], Incontinence Impact Questionnaire-7 [12], Overactive Bladder-Validated 8-question [13], Female Sexual Function Index (FSFI) [14]), bladder diary [15], and 1-hour pad test [16] were used. One-hour pad test (5th day), questionnaires (6th day), and bladder diary (6th and 7th days) were applied in the pre-treatment and post-treatment evaluation, respectively.
Evaluation of pelvic floor and abdominal muscle functions
The PFM function was evaluated by palpation with two fingers according to the PERFECT scale, which includes assessments of the power (P), endurance (E), number of repetitions (R), and number of fast contractions (F). Additionally, every (E) contraction (C) was timed (T). The PFM strength was graded from 0 to 5, according to the Oxford grading system. This evaluation was adapted from the PERFECT method described by Laycock et al. [17].
PFM, rectus abdominus (RA), transversus abdominus (TrA), internal oblique (IO), and external oblique (EO) muscle activity was evaluated by superficial electromyography and muscle thicknesses were evaluated by ultrasonography. Superficial electromyography and the PERFECT scale were used to evaluate PFM fatigue [9].
A superficial electromyography (EMG) device (NeuroTrac MyoPlus 4 Pro Verity Medical Ltd., UK) was used to evaluate the electromyographic activity of the PFM and abdominal muscles. The technical specifications of the device are as described in our previous study [18]. PFM activity was evaluated with a cylindrical endovaginal probe (Verity Medical Ltd., UK) 8.7 cm long and 2.6 cm in diameter. After applying the anti-allergy gel, the probe's metal sensors were inserted into the vagina at 3–9 o’clock. The activity of all abdominal muscles was evaluated using disposable, superficial, self-adhesive, silver-silver chloride (Ag/Ag CI), circular electrodes with a diameter of 3.2 cm. The skin area was cleaned with an alcohol swab to decrease skin impedance. Surface electrodes were placed on the abdominal muscles as described in our previous study [18]. The electrodes were fixed with adhesive hypoallergenic medical tape to maintain contact and avoid artifact formation.
The participants were required to empty their bladders. They were informed of the definition, location, and function of the PFM using anatomical models. Also, participants were instructed on the correct contraction of the PFM with digital palpation to prevent straining and contraction of different muscles. In the relax command, participants were asked to completely relax their PFM. At contract command, they were asked to pull their PFM into/up by squeezing tightly as if they were holding urine or stool. Meanwhile, they were prevented from tightening their abdominal muscles, hips, and thighs, drawing their abdomen, and holding their breath. In the supine position, measurements were performed during MVC of the PFM for 6 seconds and maximum relaxation of 6 seconds between contractions [19]. Measurements were repeated three times. Rest intervals of at least 2 minutes were provided between measurements to prevent muscle fatigue. After three measurements, the device automatically recorded the minimum, maximum, average, standard deviation, MVC%, and the onset of contraction and relaxation. The results were expressed in percent (%). A measurement was made to check that the electrodes were in the correct positions.
A two-dimensional ultrasound imaging unit set in B-mode (LOGIQ e, GE Healthcare, Milwaukee, WI, USA) with a 2.5–7.5 MHz linear probe was used to measure the changes in the thickness of the RA, TrA, IO, and EO muscles during PFM contraction and relaxation. The measurements were made on the right side of the abdominal wall. US measurements were taken during expiration following normal breathing. The participants were asked to perform a maximum voluntary PFM contraction; the instructions were “draw in and lift the PFM” and the cursor points measured the muscle thickness between the fascial bands. The image was frozen on the screen and the muscle thickness was measured in millimeters. The ultrasound transducer was not displaced during the testing procedure. To ensure reliability, all measurements were performed by one examiner. In addition, the same procedure as during EMG measurement was applied during pelvic floor contractions and relaxations. For US measurements of the TrA, IO, and EO muscles, the abdominal wall was exposed. The ultrasound transducer was transversely located across the abdominal wall over the anterior axillary line midway between the 12th rib and the iliac crest to obtain a clear image of the three deep abdominal layers [20].
Evaluation of pelvic muscle exercise self-efficacy
Perceived self-efficacy was measured with the Broome Pelvic Muscle Self-Efficacy Scale. The scale is composed of two sub-dimensions, effectiveness and outcome expectations, and 23 items in total. The efficacy expectation sub-dimension consists of items that assess the person's perception of their ability to perform PFM exercise in various positions and situations. In the outcome expectation sub-dimension, the person is asked to imagine doing the exercise in various situations and how much they think the exercise will impede UI. The total score is calculated by taking the average of the scores obtained from all items in the scale and varies between 0 and 100 (≤ 32 points: low self-efficacy, 33–66 points: moderate self-efficacy, ≥ 66 points: high self-efficacy) [21].
Statistical analysis
IBM SPSS Statistics 25.0 software (SPSS Inc., USA) was used for statistical analysis. Non-parametric tests were used because the number of people in the groups was small. Data were expressed as median (IQR) or number (%). Within-group change was analyzed using the Wilcoxon signed-rank test, and the difference between groups was analyzed using the Mann–Whitney U test. Statistical significance was accepted at p < 0.05.
Prior to the study, a power analysis was performed using the G*Power 3.0 program, according to the results of the study by Orhan et al. [22]. It was determined that a minimum of 20 individuals should be included for each group, taking into account the 0.05 type I error level and 80% power.
Results
Each group started with 49 participants, but a total of 12 women did not complete the study. Eight women dropped out due to the pandemic, and the other four stated no reason. Twenty-one individuals in the MCI-PFMT group and 16 individuals in the control group completed the study (Fig. 1).
Sociodemographic characteristics and symptoms were similar between the two groups in the pre-treatment evaluation (Table 1) (p > 0.05). No women in the MCI-PFMT group reported increased UI or other symptoms at post-treatment evaluation.
In the post-treatment evaluation, symptoms were significantly decreased in both groups (p < 0.05). Although the decrease in symptoms was greater in the MCI-PFMT group, there was no significant difference between the two groups (p > 0.05). Additionally, FSFI scores were decreased in both groups post-treatment. However, only the decrease in the control group was statistically significant (p = 0.009) (Table 2).
The PERFECT values and the average and peak work values for the PFM increased significantly in both groups (p < 0.05). The PFM MVC% values decreased in both groups, and the difference was significant in the control group (p < 0.05). Although a 12.7% decrease in the PFM MVC% values was seen in the control group, a 9.6% decrease was observed in the MCI-PFMT group (Tables 2 and 3).
Although average and peak work values for the TrA and IO muscles increased in both groups, the MVC% values decreased (p < 0.05). Moreover, the rates of increase and decrease in the two groups were similar (p > 0.05). These muscle thicknesses also increased significantly by post-treatment in both groups. Work onset values increased in all muscles (p < 0.05) (Tables 2 and 3).
Discussion
This study showed that symptoms decreased in the short term in both groups. However, the decrease in symptoms was greater in the MCI-PFMT group than in the control group. Although the changes in PFM and abdominal muscles were similar in the post-treatment evaluation, they were significant in the control group. These findings show that the MCI-PFMT protocol can lead to PFM and abdominal muscle fatigue, but it is not significant enough to increase symptoms. Additionally, no side effects or adverse events of MCI-PFMT were observed.
Considering that the nervous system is affected by UI, our study showed that neurophysiological approaches similar to constraint-induced motor therapy can be developed to increase correct cortical reorganization and neuroplasticity. It has been used primarily in stroke patients and has continued to be used in many nervous system diseases. It aims to use the patient's affected body areas throughout the waking hours of the day. After long hours of practice in the rehabilitation center, the patient is given exercises that must be performed at home, and these are checked. Each treatment session lasts 3–6 hours, and treatment sessions are completed within 10–15 days, based on the level of deficit [8]. Developed for this purpose, the MCI-PFMT protocol can lead to neural adaptations and neuroplasticity, resulting in faster recovery of symptoms such as UI, fecal incontinence, or pelvic pain. Moreover, this protocol may not increase PFM fatigue. Therefore, various rehabilitation protocols can be developed by adding this protocol to training programs for athletes suffering from UI who train the whole day. It can also be used in different functional states of PFM and different types of UI. Additional studies should be conducted on this issue. Therefore, our study will shed light on the development of intensive PFM training protocols in the future.
The literature reports a significant reduction in symptoms due to changes in neural adaptations a week after the onset of PFM training, but it takes longer for muscle hypertrophy to occur (> 8 weeks) [23]. Therefore, the MCI-PFMT protocol was conducted for a single week in this study. Another reason for performing this protocol for a single week was to prevent worsening of symptoms or unexpected effects.
MVC is recommended as the gold standard to measure muscle fatigue. It is an individual's effort to recruit as many muscle fibers as possible to develop strength. Increased or decreased EMG activity should be observed during the MVC to indicate that muscle fatigue occurred under experimental conditions [24]. While most studies expressed muscle fatigue as an increase or decrease in symptoms, some reported a decrease in MVC% values. In our study, the PERFECT, average, and peak values of PFM increased and MVC% values decreased in both groups post-treatment evaluation. These results may indicate that PFM fatigue occurred in both groups. While PFM fatigue was more pronounced in the control group with less exercise, fatigue was not reflected in symptoms in the MCI-PFMT group despite more exercise. This can be explained by the occurrence of more intense neural adaptations.
If muscle fatigue is evaluated with EMG, it is recommended to consider MVC% and relaxation times [10]. In this study, we did not note this because no changes were observed in the relaxation values of PFM and abdominal muscles in either group. Since the relaxation time was maintained constant at 6 seconds, the relaxation-related outcomes may have been impacted.
Muscle fatigue can be interpreted in terms of physiological, instrumental, sensory, or psychological measures. However, it is generally defined as any decrease in the MVC's ability to produce force or power. Several processes contribute to the onset of fatigue, such as voluntary command, excitation, muscle fiber, and contractile response characterized by cycles of contraction and relaxation [24]. The contraction and relaxation times in the MCI-PFMT protocol may have been at levels that would have decreased PFM fatigue.
Muscle fatigue is defined as decreased strength caused by acute exercise and is a sign that overload has occurred during exercise. Because PFM consists largely of type-I slow fibers, they are considered to be fatigue-resistant muscles. Activities such as jumping, high-impact landing, and running cause more urine loss [9]. However, in our study, participants were evaluated in semi-lithotomy positions in the laboratory. Therefore, the results may not reflect the PFM overload that occurs during daily activities. Furthermore, the MCI-PFMT protocol may have included exercises that would not lead to muscle overload.
Muscle fatigue was defined in a previous study as a 10% decrease from the initial reference force of the PFM [25]. Based on this definition, in our study, MVC% declined further in the control group, while values close to this percentage were obtained in both groups. For this reason, it seems that the MCI-PFMT protocol that we developed can cause less fatigue and increase neuroplasticity.
Perineal fatigue can play a role in the pathophysiology of female stress UI. However, it has not been investigated in other types of UI or pelvic floor dysfunction, as the heterogeneous nature, anatomy, biomechanical properties, and cellular metabolism of the PFM make it difficult to investigate. Although the concept of perineal fatigue is widely accepted in practice in women with stress UI, studies on this subject are limited. The findings of most studies are disappointing or difficult to interpret [24]. Additionally, there are no studies in the literature investigating the relationship between PFM training and muscle fatigue.
The effects of intensive physical activity can be explained by two hypotheses: physical activity can strengthen PFM, or overload, stretch, and weaken them. It has also been proven that there is the more frequent urinary loss associated with muscle fatigue after intensive exercise [26]. This suggests that intensively performed PFM training may increase symptoms. Contrary to this, no participants in our study reported an increase in symptoms the day after the session. Completing the program with relaxation exercises and beginning the program with stretching the muscles surrounding the pelvis may have led to this outcome.
Marques et al. developed an exercise protocol with an exercise ball, focused on the abdominal cavity. This protocol not only supports PFM but also provides benefits for body posture and respiratory strength [27]. Alves et al. performed a multiple-component fitness program that included PFM and global muscle stretching, strengthening, and relaxation in postmenopausal women with UI. Participants performed PFM exercises with four sets of 10 repetitions of rapid contraction and four sets of 10 repetitions of slow contractions in five different positions (supine, sitting, on a pilates ball, squatting, and standing) in each session. Exercises were completed twice a week for 6 weeks, for a total of 12 sessions of 30 minutes. The control group was given a 60-minute fitness program without any PFM exercises. A multiple-component fitness program was found to be effective in reducing UI and pelvic organ prolapse symptoms and increasing contractility of the PFM [28]. These studies showed that multiple-component protocols decreased symptoms. These protocols were performed 2–3 times per week for 30–60 minutes and did not cover the whole day. However, we observed that the intensive, multiple-component and full-day MCI-PFMT protocol decreased symptoms.
The effects of different intensities of PFM training in women with UI were investigated. For 12 weeks, one group received 30 minutes of high-intensity PFM training 5 days a week, while the other group received 15 minutes of low-intensity PFM training 2 days a week. High-intensity PFM training has been found to decrease incontinence attacks, narrow the levator hiatus area, and increase PFM strength and endurance. The PFM training is high-intensity; however, it does not include multiple components such as sensory, motor and reflex training [29]. Different components should be added to PFM training programs, and their effects on symptoms should be studied. In our study, we aimed to investigate the change in symptoms by adding various components to high-intensity exercises.
The literature recommends short-term (10–45 minutes) PFM training for 24 sessions or 12 weeks, 3–7 days a week, to achieve significant changes in women with UI [5]. It is also suggested that a larger number of shorter sessions is preferable to fewer longer sessions [4]. However, no studies comparing the two protocols exist. In our study, it was observed that longer sessions decreased symptoms in the MCI-PFMT group.
Although PFM training is recommended as the gold standard in the treatment of pelvic floor dysfunction such as UI, interest in other exercise programs is increasing. A recent systematic review reported that pilates, Paula method, and hypopressive exercises were ineffective in increasing PFM strength unless combined with PFM training [30]. The strength of our study is that the MCI-PFMT protocol, which includes combined exercises, will bring a different perspective to the literature.
Sensory, motor, and reflex effects of PFM may occur in pelvic floor dysfunction. Healthy PFM lacks sensory feedback compared to other muscles [31]. Because both the central and peripheral nervous systems are affected in UI [6], not only are strength and coordination required for normal PFM function, but sensory and reflex components are required as well. For this purpose, these components were given importance when creating the MCI-PFMT. In sensory training, digital palpation, biofeedback, posture training, patient education, and bladder training were included in the program, and visual, auditory, and tactile stimuli were used. Motor training included correct contraction and relaxation, coordination, symmetry, strength, endurance, stretching, aerobics, mat and ball exercises using motor learning, motor control strategies, and co-contraction mechanisms. Reflex training included functional training, aerobics with PFM contractions, and jumping on the ball. There is no other example in the literature that has associated the PFM of patients with UI to the hemiplegic arm of stroke patients who have sensory, motor, and reflex disorders. The results of our study confirm our view of associating the PFM of individuals with UI with a neurologically affected body part. However, longer-term studies are needed to support this view.
Conclusion
The MCI-PFMT protocol was developed as a supervised rehabilitation model that includes neurophysiological-based approaches for sensory, motor, and reflex dysfunction in PFM. This study demonstrated that the MCI-PFMT protocol decreases symptoms of UI more than standard PFM training in a short period. Additionally, both training protocols cause a small amount of PFM and abdominal muscle fatigue that does not affect the symptoms. Therefore, this protocol that we developed can be used to improve neuroplasticity.
Data availability
Data is available upon reasonable request from the corresponding author.
References
Haylen BT, Freeman RM, Swift SE, Cosson M, Davila GW, Deprest J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint terminology and classification of the complications related directly to the insertion of prostheses (meshes, implants, tapes) & grafts in female pelvic floor surgery. Neurourol Urodyn. 2011;22(1):3–15. https://doi.org/10.1002/nau.21036.
Woodley SJ, Lawrenson P, Boyle R, Cody JD, Mørkved S, Kernohan A, et al. (2020). Pelvic floor muscle training for preventing and treating urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev. 5(5). https://doi.org/10.1002/14651858.CD007471.pub4
Charette M, Bérubé MÈ, Brooks K, O'Neil J, Brosseau L, McLean L. How well do published randomized controlled trials on pelvic floor muscle training interventions for urinary incontinence describe the details of the intervention? A review Neurourol Urodyn. 2020;39(1):35–44. https://doi.org/10.1002/nau.24208.
Nie X-F, Ouyang Y-Q, Wang L, Redding SR. A meta-analysis of pelvic floor muscle training for the treatment of urinary incontinence. Int J Gynaecol Obstet: the official organ of the International Federation of Gynaecology and Obstetrics. 2017;138(3):250–5. https://doi.org/10.1002/ijgo.12232.
García-Sánchez E, Ávila-Gandía V, López-Román J, Martínez-Rodríguez A, Rubio-Arias JÁ. What pelvic floor muscle training load is optimal in minimizing urine loss in women with stress urinary incontinence? A systematic review and meta-analysis. Int J Environ Res Public Health. 2019;16(22):4358. https://doi.org/10.3390/ijerph16224358.
Andersson K-E, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev. 2004;56(4):581–631. https://doi.org/10.1124/pr.56.4.4.
Herms ADG, Veit R, Reisenauer C, Herms A, Grodd W, Enck P, et al. Functional imaging of stress urinary incontinence. Neuroimage. 2006;29(1):267–75. https://doi.org/10.1016/j.neuroimage.2005.07.018.
Etoom M, Hawamdeh M, Hawamdeh Z, Alwardat M, Giordani L, Bacciu S, et al. Constraint-induced movement therapy as a rehabilitation intervention for upper extremity in stroke patients: systematic review and meta-analysis. Int J Rehabil Res. 2016;39(3):197–210. https://doi.org/10.1097/MRR.0000000000000169.
Teng M, Kervinio F, Moutounaïck M, Miget G, Charlanes A, Chesnel C, et al. Review of pelvic and perineal neuromuscular fatigue: Evaluation and impact on therapeutic strategies. Ann Phys Rehabil Med. 2018;61(5):345–51. https://doi.org/10.1016/j.rehab.2018.06.006.
Thomaz RP, Colla C, Darski C, Paiva LL. Influence of pelvic floor muscle fatigue on stress urinary incontinence: a systematic review. Int Urogynecol J. 2018;29(2):197–204. https://doi.org/10.1007/s00192-017-3538-6.
Cetinel B, Ozkan B, Can G. The validation study of ICIQ-SF Turkish version. Turkish Journal of Urology. 2004;30(3):332–8.
Cam C, Sakalli M, Ay P, Cam M, Karateke A. Validation of the short forms of the incontinence impact questionnaire (IIQ-7) and the urogenital distress inventory (UDI-6) in a Turkish population. Neurourology and Urodynamics: Official Journal of the International Continence Society. 2007;26(1):129–33. https://doi.org/10.1002/nau.20292.
Tarcan T, Mangir N, Ozgur MO, Akbal C. OAB-V8 Overactive Bladder Questionnaire Validation Study. Bulletin of Urooncology. 2012;21(21):113–6.
Aygin D, Eti AF. The Turkish Adaptation of The Female Sexual Function Index. Turkiye Klinikleri J Med Sci. 2005;25(3):393–9.
Bright E, Cotterill N, Drake M, Abrams P. Developing and validating the International Consultation on Incontinence Questionnaire bladder diary. Eur Urol. 2014;66(2):294–300. https://doi.org/10.1016/j.eururo.2014.02.057.
Jørgensen L, Lose G, Andersen JT. One-hour pad-weighing test for objective assessment of female urinary incontinence. Obstet Gynecol. 1987;69(1):39–42.
Laycock J, Jerwood D. Pelvic floor muscle assessment: the PERFECT scheme. Physiother. 2001;87(12):631–42.
Tosun OC, Dayican DK, Keser I, Kurt S, Yildirim M, Tosun G. (2022). Are clinically recommended pelvic floor muscle relaxation positions really efficient for muscle relaxation? Int Urogynecol J. 1–10. https://doi.org/10.1007/s00192-022-05119-3.
Halski T, Słupska L, Dymarek R, Bartnicki J, Halska U, Król A, et al. Evaluation of bioelectrical activity of pelvic floor muscles and synergistic muscles depending on orientation of pelvis in menopausal women with symptoms of stress urinary incontinence: a preliminary observational study. Biomed Res Int. 2014. https://doi.org/10.1155/2014/274938.
Arab AM, Chehrehrazi M. The response of the abdominal muscles to pelvic floor muscle contraction in women with and without stress urinary incontinence using ultrasound imaging. Neurourol Urodyn. 2011;30(1):117–20. https://doi.org/10.1002/nau.20959.
Broome B. Development and testing of a scale to measure self-efficacy for pelvic muscle exercises in women with urinary incontinence. Urol Nurs. 1999;19(4):258–68.
Orhan C, Akbayrak T, Ozgul S, Baran E, Uzelpasaci E, Nakip G, et al. Effects of vaginal tampon training added to pelvic floor muscle training in women with stress urinary incontinence: randomized controlled trial. Int Urogynecol J. 2019;30(2):219–29. https://doi.org/10.1007/s00192-018-3585-7.
Bø, K. Overview of physical therapy for pelvic floor dysfunction. Evidence-based physical therapy for the pelvic floor: Bridging science and clinical practice. Edinburgh, Scotland: Churchill Livingstone; 2007.
Deffieux X, Hubeaux K, Lapeyre E, Jousse M, Ismael SS, Thoumie P, et al. Perineal neuromuscular fatigue. Ann Readapt Med Phys. 2006. https://doi.org/10.1016/j.annrmp.2006.03.009.
Verelst M, Leivseth G. Are fatigue and disturbances in pre-programmed activity of pelvic floor muscles associated with female stress urinary incontinence? Neurourol Urodyn: Official Journal of the International Continence Society. 2004;23(2):143–7. https://doi.org/10.1002/nau.20004.
Khowailed IA, Pinjuv-Turney J, Lu C, Lee H. Stress incontinence during different high-impact exercises in women: a pilot survey. Int J Environ Res Public Health. 2020;17(22):8372. https://doi.org/10.3390/ijerph17228372.
Marques J, Botelho S, Pereira LC, Lanza AH, Amorim CF, Palma P, et al. Pelvic floor muscle training program increases muscular contractility during first pregnancy and postpartum: electromyographic study. Neurourol Urodyn. 2013;32(7):998–1003. https://doi.org/10.1002/nau.22346.
Alves FK, Riccetto C, Adami DB, Marques J, Pereira LC, Palma P, et al. A pelvic floor muscle training program in postmenopausal women: A randomized controlled trial. Maturitas. 2015;81(2):300–5. https://doi.org/10.1016/j.maturitas.2015.03.006.
Hagovská M, Urdzík P, Švihra J. (2020). A randomized interventional parallel study to evaluate the effect of pelvic floor muscle training with stabilization exercises of high and low intensity in women with stress urinary incontinence: The PELSTAB study. Med. 99(29). https://doi.org/10.1097/MD.0000000000021264.
Jacomo RH, Nascimento TR, da Siva ML, Salata MC, Alves AT, da Cruz PRC, et al. Exercise regimens other than pelvic floor muscle training cannot increase pelvic muscle strength-a systematic review. J Bodyw Mov Ther. 2020;24(4):568–74. https://doi.org/10.1016/j.jbmt.2020.08.005.
Bo K, Frawley HC, Haylen BT, Abramov Y, Almeida FG, Berghmans B, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and nonpharmacological management of female pelvic floor dysfunction. Int Urogynecol J. 2017;28(2):191–213. https://doi.org/10.1007/s00192-016-3123-4.
Acknowledgements
The authors declare no acknowledgments.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Celiker Tosun O: Protocol/project development, Data collection and management, Data analysis, Manuscript writing/editing.
Keser I: Protocol/project development, Data collection, Data analysis
Korkmaz Dayican D: Protocol/project development, Data collection, Data analysis
Yavuz O: Protocol/project development, Data collection
Tosun G: Manuscript writing/editing
Kurt S: Protocol/project development, Data collection
Corresponding author
Ethics declarations
This work has not been presented at a prior conference or meeting congress.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Informed consent
Written informed consent was obtained from the patient to publish these Images in the International Urogynecology Journal and any accompanying images. The person in the figures is one the authors of the current manuscript, I.K. They are barefaced as the reproduction of the pictures has been approved by the legal representative.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Multiple-Component Intensive Pelvic Floor Muscle Training (MCI-PFMT)
The MCI-PFMT protocol was developed as a supervised, whole-day performed rehabilitation model based on neurophysiological principles to improve neuroplasticity. It included approaches for sensory, motor, and reflex effects in the pelvic floor muscles (PFM). PFM are divided into four types according to their functional status: normal, overactive, underactive, and non-functional. Physiotherapy approaches to treating these four conditions differ from one another. Another aim of this protocol is to create a protocol that can be used in all functional states of the PFM and in all types of incontinence. The MCI-PFMT protocol includes the following: teaching the correct PFM function, gaining the relaxation and contraction function of the PFM, training the strength, coordination, endurance, and symmetry of the PFM, motor learning and control, dynamic lumbopelvic stability, PFM and abdominal co-contraction, and the reflex activation of PFM. This program consists of education and exercise phases.
Education phase
This phase included the pelvic floor anatomy, finding the pelvic floor in the individual's own body, physiological responses of the PFM to exercise, the role of PFM in UI, strategies to protect the PFM, the importance of correct posture training, and lifestyle changes. Additionally, bladder training was provided to patients based on the results obtained in the bladder diary. Individual adjustments to education were made. Information provided in education was continually repeated during treatment sessions. The education took place on the day of the first evaluation, Monday morning, at the beginning of the treatment program.
Bladder training
Prior to treatment, patients were asked to fill out a bladder diary, and individual bladder training was provided according to that diary. The most frequent urination times were determined in the patients' completed bladder diaries, and they were asked to progressively extend these periods. Patients who had difficulty in doing this were instructed to contract their PFM to delay the need to urinate, to sit if standing, and to stop if they were dealing with water. They were also asked to encourage themselves by telling themselves that they could succeed.
Exercise phase
This phase consisted of two parts. In the first part, the participants performed supervised PFM training, and in the second part they repeated the PFM training taught during activities of daily living at home. The first part lasts approximately 6 hours. All participants started the exercises on Monday at 9 am and finished on Friday.
Initially, the correct PFM function was taught to all participants (correct contraction, relaxation, coordination, symmetry, etc.) using digital palpation and biofeedback training. These practices were performed during the evaluation week even if the participants contracted their PFM correctly at the first examination. They increase the sensory stimuli and provide the correct motor responses (voluntary contraction and voluntary relaxation) of the PFM. Biofeedback training began in a single session and continued until the correct function was learned. Then, the participants were instructed to “contract for 2 seconds, relax for 2 seconds” for fast contractions and “contract, hold for 10 seconds, and relax” for slow contractions, and the terms “elevator going up and down” were used for better understanding. After a set of 10 repetitions of slow contraction, a set of 10 repetitions of fast contractions was performed. The exercises were conducted under the supervision of a physiotherapist.
The MCI-PFMT protocol was conducted 5 days a week for a single week. In this protocol, stretching exercises, PFM exercises on the mat, PFM exercises on the ball, PFM exercises during aerobic training, PFM exercises during functional activities, and relaxation exercises were performed, respectively. Participants were asked to perform PFM exercises for the remainder of the day during all functional activities. The following day, the participants were checked to see whether they were performing PFM exercises during functional activities and asked whether there was an increase in symptoms.
Stretching exercises
The program began by stretching the PFM and muscles around the pelvis. The aim was to provide the normal length–tension relationship of muscles, eliminate muscle strength imbalances, achieve maximum function by bringing the muscles to their optimal length. This was also to teach about voluntary relaxation with regard to PFM. Thirty-second static stretching exercises with three repetitions were performed on each of the hip flexors, hamstrings, gluteus maximus, piriformis, adductors, abdominal muscles, and lumbar extensors. A 10-second rest period was provided between each stretching activity (Fig. 2). PFM stretched in reverse Kegel in yoga position
PFM exercises on the mat
PFM was performed in four different positions: supine hook, bridge, prone, and crawling positions. A total of 40 slow contractions and 40 rapid contractions were performed at each position, a set of 10 repetitive slow contractions and a set of 10 repetitive fast contractions. The aim was to increase strength, coordination, endurance, and symmetry of PFM, motor training and control, dynamic lumbopelvic stability, and co-contraction between PFM and abdominal muscles (Fig. 3).
PFM exercises on the ball
With the knees flexed at 90 degrees, hands on the knees and the trunk in neutral position on the exercise ball, four different exercises were performed with the ball as forward–backward, right–left, circle drawing, and jumping, without the knees moving. A total of 40 slow contractions and 40 fast contractions were performed, a set of 10 repetitive slow contractions and a set of 10 repetitive fast contractions for each. The aim was to increase the endurance of voluntary relaxation and contraction of the PFM. Furthermore, it was to increase lumbopelvic stability and ensure the correct functioning of the co-contraction mechanism between the PFM and the abdominal muscles (Fig. 3).
PFM exercises during aerobic training
Aerobic training consisted of the first 3 minutes of warm-up and the last 3-minute cool-down periods and 30 minutes of walking training. Slow and fast PFM contractions were performed between certain distances during walking. The intensity of aerobic training was determined using the Borg scale and was conducted at an intensity of 12 to 14. To stimulate the reflex activity, the PFM was contracted during heel strike and ankle dorsiflexion, and the PFM was relaxed during toe lift. The physiotherapist verbally instructed the patient about PFM throughout the aerobic training.
PFM exercises during functional activities
PFM exercises consisting of a set of fast and a set of slow contractions during sitting up, lifting weights, washing hands, climbing stairs, and carrying weights were performed for 20 minutes. The aim was to stimulate reflex PFM contraction during activities.
Relaxation exercises
The autogenic relaxation technique and diaphragmatic breathing were applied to increase the patient's body awareness, relax the PFM, and reduce stress. Patients were asked to think of a peaceful environment accompanied by relaxing music and controlled breathing. The aim was to increase the relaxation ability of whole body muscles and PFM.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tosun, O.C., Keser, I., Dayican, D.K. et al. Does multiple-component intensive pelvic floor muscle training decrease muscle fatigue and symptoms in women with urinary incontinence?. Int Urogynecol J 34, 2067–2080 (2023). https://doi.org/10.1007/s00192-023-05499-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-023-05499-0