Abstract
Introduction and hypothesis
Stress urinary incontinence (SUI) is the most common urinary complaint among women and is defined by the International Continence Society as any involuntary loss of urine due to physical effort, sneezing or coughing. Many women with SUI state that the loss of urine occurs after performing repetitive movements, which may suggest that it is the result of fatigue of the pelvic floor muscles (PFM). Thus, we performed the systematic review of the literature on the influence of PFM fatigue on the development or worsening of the symptoms of SUI in women.
Methods
The PubMed, Scopus, EMBASE, PEDro, LILACS, SciELO, Cochrane Library, Google Scholar, CINAHL and Periódicos CAPES databases were searched for articles using the keywords “fatigue”, “pelvic floor”, “stress urinary incontinence” and “women”, in Portuguese and in English. Methodological quality was assessed using the Downs and Black scale, and the data collected from the studies were analyzed descriptively.
Results
Of the 2,010 articles found, five met the inclusion criteria and were analyzed. They were published between 2004 and 2015, and included a total of 30,320 women with ages ranging from 24 to 53.6 years. Of the studies analyzed, three showed an association between fatigue and SUI, and two did not show such an association.
Conclusions
This study confirmed that PFM fatigue can influence the development and/or worsening of SUI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary incontinence (UI), defined by the International Continence Society as any involuntary loss of urine due to physical effort, sneezing or coughing, can be classified as stress UI (SUI), urge UI (UUI) and mixed UI (MUI) [1]. According to Dedicação et al. [2], UI affects more than 50 million people in the world, and affects more women than men at a ratio of 2 to 1. SUI affects people’s quality of life, with physical, psychological, sexual and social implications. Generally, women with SUI report physical limitations, for example, when engaging in sport and when carrying objects, as well as changes in social, occupational and domestic activities that negatively influence their emotional state [3]. The most common type of UI is SUI [1, 3]. This type of UI affects not only the elderly, but has a prevalence of 30% among middle-aged women, reaching 47% in women who exercise regularly [4].
The risk factors for SUI include advancing age, being white, obesity, vaginal delivery, instrumental delivery, multiparity and pregnancy in advanced age, estrogen deficiency, conditions associated with increased intraabdominal pressure, smoking, diabetes, collagen disease, neuropathies and previous hysterectomy [5]. Physical activity can also be a risk factor for SUI in both athletes and non-athletes [6]. One explanation for the development of SUI in people who practice physical activity is that strenuous exercise leads to increased intraabdominal pressure that overloads the pelvic floor muscle (PFM) during exercise. Another explanation is that the determining factor for SUI associated with physical activity is muscle fatigue. During physical activity type II fibers are recruited, which have a low capacity for maintaining the muscle tone of the pelvic floor, one of the factors that can compromise the urinary continence mechanism [4, 7].
There is only one previous systematic review [8] carried out in 2006 that assessed the involvement of muscle fatigue in UI. This indicates the importance of this systematic review, the purpose of which was to determine the influence of muscle fatigue on SUI and its relationship with the symptoms of SUI in women. The results of this study will be useful for the planning of evidence-based SUI prevention and treatment strategies.
Materials and methods
This study followed the recommendations of the Cochrane Collaboration and the PRISMA statement [9] for the reporting of systematic reviews.
Eligibility criteria
This systematic review included observational studies and randomized controlled trials (RCTs) analyzing the relationship between PFM fatigue and the development or worsening of SUI symptoms in women. The inclusion criteria were: (1) studies in adult women with SUI symptoms, (2) studies that addressed the subject of fatigue of the PFM, and (3) studies published from 2000 onwards. Systematic reviews, literature reviews, theses, dissertations and proceedings of scientific meetings on the subject studied, as well as studies investigating other types of voiding, or fecal or pelvic floor dysfunction related to PFM fatigue, were excluded.
Search strategy
The studies were identified by searching the PubMed, Scopus, EMBASE, PEDro, LILACS, SciELO, Cochrane Library, Google Scholar, CINAHL and Periódicos CAPES electronic databases, and the references in studies that had already been published on the subject were also searched manually. The complete strategy used to search PubMed was as follows:
-
1.
“Pelvic Floor” [MeSH] OR “pelvic floor” OR “Floor, Pelvic” OR “Pelvic Diaphragm” OR “Diaphragm, Pelvic” OR “Diaphragms, Pelvic” OR “Pelvic Diaphragms” OR “Pelvic Floor Muscles” OR “abdomino-pelvic musculature” OR “perineal musculature” OR “Perineum” [MeSH] OR “perineum” OR “perineums” OR “Pelvis” [MeSH] OR “pelvis” OR “Pelvic Region” OR “Region, Pelvic” OR “perineal function” OR “pelvic floor contraction”
-
2.
“Muscle Fatigue” [MeSH] OR “Muscular Fatigue” OR “Fatigue, Muscular” OR “Fatigue, Muscle” OR “muscle fatigue”
-
3.
“Women” [MeSH] OR “women” OR “woman” OR “Women’s Groups” OR “Group, Women’s” OR “Groups, Women’s” OR “Women Groups” OR “Women’s Group”
-
4.
“Urinary Incontinence” [MeSH] OR “Urinary Incontinence” OR “Incontinence, Urinary” OR “Urinary Incontinence, Stress” [MeSH] OR “Urinary Incontinence, Stress” OR “Urinary Stress Incontinence” OR “Incontinence, Urinary Stress” OR “Stress Incontinence, Urinary”
-
5.
Items 1 AND 2 AND 3 AND 4
The search was carried in January 2016 and updated in September 2016, using the keywords “fatigue”, “pelvic floor”, “stress urinary incontinence” and “women”, in Portuguese and English. Only articles indexed and published in Portuguese and English since 2000 were selected.
Selection of studies and data extraction
In the first phase of the selection, two reviewers independently assessed the titles and abstracts of the studies identified by the search strategy. All the abstracts that did not give sufficient information regarding the inclusion and exclusion criteria were selected for assessment of the complete article. In the second stage, the same reviewers independently assessed the complete articles and selected the studies in accordance with the eligibility criteria. The data were extracted by the same reviewers, independently, using a standardized and previously prepared form containing the following information to characterize the studies: author, year of publication, study type and purpose, population and sample size, variables, assessment tools, methods of observation, and outcomes. Disagreements between reviewers during the study selection and data extraction stages were resolved by a third reviewer. The outcome assessed was the development of SUI due to PFM fatigue.
Assessment of methodological quality
The methodological quality of the selected articles was assessed by two reviewers, independently, using the Downs and Black scale [10], that was developed and validated for the assessment of the quality and risk of bias of randomized and observational studies. This tool is composed of 27 items that assess the domains “Reporting”, “External validity”, “Bias”, “Confounding/selection bias” and “Power of the study”. The full version of the scale was used for the assessment of the randomized clinical trials. In order to assess the observational studies, an adaptation was suggested by the Cochrane Collaboration according to the Methodological Guidelines of the Ministry of Health for the elaboration of a systematic review of observational studies [11], excluding items related to experimental studies and cohort and case-control studies (items 4, 7, 8, 12, 13, 14, 15, 17, 19, 21, 22, 23, 24 and 27), because they did not fit the methodological design of the studies analyzed.
In this systematic review, the studies were classified as having high methodological quality when their scores were equal to or greater than 70% on the scale (about 19 points for RCTs and 9 points for observational studies) following the criteria adopted by other authors for systematic reviews [12]. Disagreements between the reviewers were resolved by consensus.
Data analysis
The heterogeneity identified in the designs and sample numbers of the studies made it impossible to carry out a meta-analysis. Therefore, a descriptive analysis of the studies included in the systematic review was performed.
Results
Description of the studies
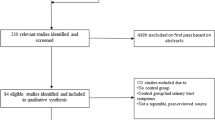
The search strategy revealed 2,010 studies, of which 81 were excluded because they were duplicated in the databases. No new studies were identified through manual searches of the references of published articles on the subject. Analysis of the titles and abstracts led to the exclusion of 1,923 studies because they did not meet the previously established eligibility criteria, and six studies remained for detailed analysis. Of the six studies analyzed, one was excluded because it was published only in the proceedings of a scientific meeting [13]. Therefore, five studies [14,15,16,17,18] were included in this systematic review. Figure 1 shows a flow chart of the selection of studies and Table 1 presents the characteristics of the studies and their quality scores according to the Downs and Black scale [10].
The five included studies [14,15,16,17,18] were published between 2004 and 2015 and included a total of 708 women, with an average age ranging from 24 to 53.6 years. Two of the studies were performed in Norway [15, 16], and the other three were performed in three different countries: Brazil [17], USA [14] and France [18]. Three studies [15, 17, 18] included women with and without SUI for comparison, one [16] included women with symptoms of SUI, and one [14] included female nurses without specifying the type of UI. Thus, four studies [15,16,17,18] assessed the relationship between physical activity and SUI specifically in relation to muscle fatigue, while the study by Townsend et al. [14] analyzed the risk of developing UI in general, including SUI, UUI and MUI.
Regarding instruments and strategies for assessing outcomes, two studies [14, 18] used questionnaires to identify the risk of developing UI related to physical activity/PFM fatigue. Townsend et al. [14] investigated the relationship between physical activity and the risk of UI and the frequency and type of urinary loss using the Nurses’ Health Study II questionnaire. Deffieux et al. [18] assessed urinary loss related to physical fatigue using a specific questionnaire, and the impact of UI symptoms on the patients’ quality of life using the Bristol Female Lower Urinary Tract Symptoms questionnaire, and also determined maximum urethral closure pressures. In the other three studies electromyography was used for evaluation: Verelst et al. [15] assessed PFM fatigue and preactivation times between the PFM and abdominal muscles on coughing; Ree et al. [16] determined maximum voluntary contraction (MVC), resting vaginal pressure and sustained contraction time of the PFM; and Burti et al. [17] assessed MVC using electromyography, the level of effort in terms of the Borg exertion scale, and heart rate.
Assessment of the quality of the studies
Regarding the methodological quality of the studies, as assessed using the Downs and Black scale [10], the five included studies [14,15,16,17,18] were considered of high quality, with scores equal to or greater than 70%. The only RCT [16] had a total score of 19 (70%), while the other observational studies [14, 15, 17, 18] had scores of 10 (71.42%).
Relationship between the risk of UI and PFM fatigue
Townsend et al. [14] demonstrated that in middle-aged women moderate physical activity does not increase the risk of UI development in general, since it does not have a detrimental effect on PFM function. They also found a significant decrease in the risk of SUI in the most active women, and attributed this result to the fact that exercise plays an important role in maintaining body weight. Verelst et al. [15] concluded that muscle fatigue does not appear to be associated with SUI. The authors found no significant difference in time to PFM fatigue among women with and without SUI, but in those with SUI PFM strength was lower than in those without.
Ree et al. [16] found a 20% decrease in MVC after strenuous physical activity, and suggested that PFM fatigue from strenuous physical activity may increase the risk of developing SUI. Burti et al. [17] found that women with SUI had worse performance in the endurance test, with shorter time to fatigue. In addition, during and after the PFM resistance test, women with SUI had worse performance and functional status of the muscles. Deffieux et al. [18] found a strong association between the symptom of fatigue-related loss of urine reported in the questionnaires and a marked decrease in maximal urethral closure pressure after repeated coughs.
Discussion
Overall, this systematic review shows that PFM fatigue may be associated with SUI, although the included studies were quite heterogeneous, making it difficult to compare the outcomes analyzed. Deffieux et al. [8], in a previous systematic review, also concluded that PFM fatigue may play an important role in the pathophysiology of SUI in women. The population of these studies was very diverse, in terms of both the number of participants, that ranged from 12 women in one study [16] to approximately 30,000 (523 with SUI) in another [14], and the profile of the participants, who included nulliparous, multiparous and premenopausal and postmenopausal women, which may have influenced the development or not of fatigue. Therefore, the heterogeneity and the difference in the number of participants may be a bias of the studies analyzed.
Three studies [15,16,17] used electromyography to assess muscle fatigue, while the other two [14, 18] used questionnaires to assess muscle fatigue and the risk of developing UI related to fatigue. According to Cifrek et al. [19], electromyography is the most commonly used technique to investigate the effects of localized muscle fatigue, since it produces information about both the quantity and the quality of the electrical activation of the muscle. The protocols used to generate fatigue were diverse, and some studies did not report this aspect clearly. This is the case for Ree et al. [16], who used high-impact physical exercises without specifying the number of sets and repetitions, or the time between sets and exercises, and is also the case for Burti et al. [17], who determined the time to fatigue (level 10 on the Borg scale), but did not report the average fast contractions required to cause fatigue in women with and without SUI, data that are important for clinical practice.
Of the five studies analyzed [14,15,16,17,18], two [14, 15] showed no association between PFM fatigue and SUI, and three [16,17,18] did show such a relationship. Townsend et al. [14] found that moderate physical activity does not increase the risk of UI, but they assessed average activity, where the participants were asked to report the average time spent exercising during the week; thus, they did not assess loss of urine at the time of physical activity, which may be related to fatigue. In addition, they specifically investigated women who performed low to moderate impact activities, excluding those who performed high-impact activities, which, according to some authors [20, 21], increases the risk of UI by up to nine times due to increased abdominal pressure and consequent pelvic floor overload.
Verelst et al. [15] also found no association between fatigue and SUI, but the fatigue test demanded only three MVCs and a long relaxation time, which may have influenced the results. According to Yeung et al. [22], fatigue is common in endurance sports and daily activities. However, the test in this study [15] was performed in a laboratory environment with the subjects in the semilithotomy position, and the results may not reflect the PFM overload that occurs during daily activities. Ree et al. [16] found an association between SUI and fatigue after strenuous activity in nulliparous women, corroborating the findings of a study by Soares et al. [23], who found that activities that led to the greatest urinary loss included jumps, high-impact landings and running. According to Da Roza et al. [24], UI is generally found in women in the climacteric period and in multiparous women, but pelvic floor dysfunction may also occur in young nulliparous women during physical activity [16]. An explanation for UI in nulliparous and physically active young women who do not have any potential risk factors may be the type of physical activity they perform, which can cause a repeatedly high intra-abdominal pressure on the pelvic floor [24].
According to Reis et al. [4], the increase in intra-abdominal pressure is not the only risk factor for the development of SUI in athletes, since PFM fatigue caused by long periods of training, with high weekly frequency and no time for the muscles to recover may increase the predisposition to such dysfunction. According to Araujo et al. [25], UI my occur during resistance exercises due to muscle fatigue, since the majority of PFM fibers, which are type I and resistant to fatigue, have their contractile capacity diminished in situations of oxygen compromise (when activated repeatedly) and are replaced by type II fibers, which are fast-twitch fibers and are not as efficient in maintaining PFM tone, and thus the continence mechanism is compromised. This corroborates the findings of Burti et al. [17], who found that women with SUI had worse performance in the endurance test, with shorter time to fatigue than women without SUI. According to Hodges et al. [13], PFM are more rapidly fatigued than limb muscles due to central fatigue, which can be demonstrated as a reduction in the ability to fully activate a muscle during MVC. This is important for physiotherapists and patients since it may help prevent PFM fatigue and thus preserve the PFM for continence control.
The results of this study indicate that, although PFM are considered fatigue-resistant muscles because they are largely composed of slow fibers, the majority of women suffering from SUI show loss of urine after repetitive efforts, which may suggest, among other things, that PFM fatigue affects UI.
Limitations of the study
The small number of articles identified on the topic which met the eligibility criteria stipulated for the present study limited the number of articles selected. This factor, together with the methodological differences found, such as the large differences in sample numbers, made it impossible to carry out a meta-analysis and, therefore, there is uncertainty in the degree to which PFM fatigue affects UI.
Conclusions
This systematic review confirmed that PFM fatigue can influence the development and/or worsening of SUI. However, due to the scarcity and heterogeneity of the studies on the subject, the conclusions are limited. Therefore, we suggest that new studies be performed to provide data to guide the clinical practice of professionals based on evidence.
References
Haylen BT, de Ridder D, Freeman RM et al (2010) An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 29:4–20
Dedicação AC, Haddad M, Saldanha MES, Driusso P (2009) Comparação da qualidade de vida nos diferentes tipos de incontinência urinária feminina. Rev Bras Fisioter 13(2):116–122
Papanicolaou S, Hunskaar S, Lose G, Sykes D (2005) Assessment of bothersomeness and impact on quality of life of urinary incontinence in women in France, Germany, Spain and UK. BJU Int 96(6):831–838
Reis AO, Câmara CNS, Santos SG, Dias TS (2011) Estudo comparativo da capacidade de contração do assoalho pélvico em atletas de voleibol e basquetebol. Rev Bras Med Esporte 17(2):97–101
Oliveira E, Zuliani LMM, Ishicava J et al (2010) Avaliação dos fatores relacionados à ocorrência da incontinência urinária feminina. Rev Assoc Med Bras 56(6):688–690
Bo K (2004) Urinary incontinence, pelvic floor dysfunction, exercise and sport. Sports Med 34:451–464
Araújo MP, Oliveira E, Zucchi EVM et al (2008) Relação entre incontinência urinária em mulheres atletas corredoras de longa distância e distúrbio alimentar. Rev Assoc Med Bras 54(2):146–149
Deffieux X, Hubeaux K, Lapeyre E et al (2006) Perineal neuromuscular fatigue. Ann Readapt Med Phys 49(6):413–417
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4):264–269
Downs SH, Black N (1998) The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. Epidemiol Community Health 52(6):377–384
Ministério da Saúde. Secretaria de Ciência, Tecnologia e Insumos Estratégicos (2014) Diretrizes metodológicas: elaboração de revisão sistemática e metanálise de estudos observacionais comparativos sobre fatores de risco e prognóstico. Ministério da Saúde, Departamento de Ciência e Tecnologia. Brasília. p 41. ISBN 978-85-334-2171-4
Silva FF, Carvalho JF (2015) Intensity of anticoagulation in the treatment of thrombosis in the antiphospholipid syndrome: a meta-analysis. Rev Bras Reumatol 55(2):159–166
Hodges P, Schabrun S, Stafford R Pelvic floor muscles have greater central fatigue during voluntary contractions than muscles of the limbs. Proceedings of the 2010 Joint Meeting of the International Continence Society and the International Urogynecological Association (ICS-IUGA 2010), 23–27 August, Toronto, Canada. Wiley, Hoboken, pp 1010–1011
Townsend MK, Danforth KN, Rosner B et al (2008) Physical activity and incident urinary incontinence in middle-aged women. J Urol 179(3):1012–1017
Verelst M, Leivseth G (2004) Are fatigue and disturbances in pre-programmed activity of pelvic floor muscles associated with female stress urinary incontinence? Neurourol Urodyn 23(2):143–147
Ree ML, Nygaard I, Bø K (2007) Muscular fatigue in the pelvic floor muscles after strenuous physical activity. Acta Obstet Gynecol Scand 86(7):870–876
Burti JS, Hacad CR, Zambon JP et al (2015) Is there any difference in pelvic floor muscles performance between continent and incontinent women? Neurourol Urodyn 34(6):544–548
Deffieux X, Hubeaux K, Dick J et al (2009) Urine leakage related to physical fatigue in women with urinary stress incontinence. J Obstet Gynaecol Res 35(4):738–745
Cifrek M, Medved V, Tonković S, Ostojić S (2009) Surface EMG based muscle fatigue evaluation in biomechanics. Clin Biomech 24(4):327–340
Bo K, Borgen JS (2001) Prevalence of stress and urge urinary incontinence in elite athletes and controls. Med Sci Sports Exerc 33(11):1797–1802
Vitton V, Baumstarck-Barrau K, Brardjanian S et al (2011) Impact of high-level sport practice on anal incontinence in a healthy young female population. J Womens Health 20(5):757–763
Yeung SS, Au AL, Chow CC (1999) Effects of fatigue on the temporal neuromuscular control of vastus medialis muscle in humans. Eur J Appl Physiol 80:379–385
Soares MC, Galvão TR, Schuindt da Silva V (2013) Incontinência urinária em atletas: uma revisão de literatura. EFDeportes.com, Revista DIgital, Buenos Aires. Vol. 18, no. 179. http://www.efdeportes.com/efd179/incontinencia-urinaria-em-atletas.htm. Accessed 12 December 2017
Da Roza T, Brandão S, Mascarenhas T et al (2015) Urinary incontinence and levels of regular physical exercise in young women. Int J Sports Med 36(9):776–780
Araujo MP, Parmigiano TR, Della Negra LG et al (2015) Avaliação do assoalho pélvico de atletas: existe relação com a incontinência urinária? Rev Bras Med Esporte 21(6):442–446
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Thomaz, R.P., Colla, C., Darski, C. et al. Influence of pelvic floor muscle fatigue on stress urinary incontinence: a systematic review. Int Urogynecol J 29, 197–204 (2018). https://doi.org/10.1007/s00192-017-3538-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-017-3538-6