Abstract
Introduction and hypothesis
Ehlers–Danlos Syndrome (EDS) is a group of inherited connective tissue disorders associated with abnormal collagen, and is more prevalent in women than in men. The aim of this cross-sectional study was to characterize pelvic floor symptoms in cisgender women with EDS and to describe their impact on quality of life.
Methods
An online questionnaire on obstetric and gynecological experiences of cisgender women with EDS was disseminated through EDS patient societies and social media. This study was a sub-analysis of the broader questionnaire and focused on pelvic floor disorders, whereby self-reported symptoms and validated questionnaires were used to assess pelvic floor symptom severity (Pelvic Floor Distress Inventory, PFDI-20), impact on quality of life (Pelvic Floor Impact Questionnaire, PFIQ-7), and sexual function (Female Sexual Function Index, FSFI-6). Groups based on age and EDS type were compared using Kruskal–Wallis and Chi-squared tests.
Results
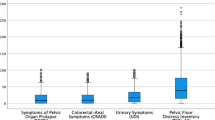
A total of 1,303 participants were included in the analysis. Pelvic floor symptom prevalence included: stress urinary incontinence in 60%, urgency urinary incontinence in 54%, fecal incontinence in 24%, and pelvic organ prolapse in 21%. Bladder symptoms were reported to be the most bothersome. The impact of prolapse symptoms on quality of life was higher in women under age 40 than in older participants (p<0.001). Pelvic pain was reported in 71%. Pain ratings were highest for dysmenorrhea, muscle and joint pain, and backache (median 7 out of 10 for each). Almost half of participants screened positive for possible sexual dysfunction and 36% reported dyspareunia more than half the time.
Conclusions
This large, observational study demonstrated that cisgender women with EDS report a high prevalence of pelvic floor symptoms that appear to be more severe than in the general population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ehlers–Danlos syndrome (EDS) consists of a group of connective tissue disorders that involve abnormal collagen production, resulting in the fragility of tissues, including skin, ligaments, bones, blood vessels, and hollow organs [1]. Thirteen subtypes are recognized in the 2017 International Classification. The subtypes differ in clinical presentation and in the genetic locus involved, with joint hypermobility and skin hyperextensibility being common symptoms across several subtypes. Twelve subtypes have specific associated genetic mutations that affect collagen production and processing pathways [1]. For the most common subtype, hypermobile EDS (hEDS), there is no known genetic mutation.
The overall prevalence of EDS is estimated to be 1 in 5,000, with hEDS being the most prevalent subtype [2]. There appears to be a higher prevalence of EDS in women (73–89%) compared with men, particularly in hEDS [3], as well as other subtypes of the condition [4]. The median age at diagnosis is most commonly the late third or early fourth decade of life [4].
An association between EDS and female pelvic floor disorders has been described and is thought to be mediated by abnormal collagen in the pelvis. Data on pelvic floor disorders in female EDS patients is primarily based on case series [5,6,7] and a single cross-sectional clinical study, primarily in patients with hEDS [2]. More recently, online recruitment has allowed for larger survey studies to be carried out in this population [8,9,10]. From the limited existing evidence, there are wide ranges of reported pelvic floor symptoms. Reports of urinary incontinence (UI) in patients with EDS range from 38 to 60% [5,6,7,8], with daily incontinence reported in 13 to 20% [6, 7]. Fecal incontinence (FI) prevalence ranges widely between 2% and 19% [8, 11,12,13] and rectal prolapse ranges between 2 and 16% [8, 11, 13]. Pelvic organ prolapse (POP) is also broadly reported in 13 to 75% of EDS patients [5, 6, 8, 14]. Pelvic pain is commonly described in this patient population, with reports of dysmenorrhea ranging between 73 to 93% [2, 9, 14] and dyspareunia ranging between 30 to 77% [2, 5, 7, 9, 10, 14]. Despite these published prevalence rates, no studies have systematically addressed pelvic floor symptom severity and impact on quality of life in women diagnosed with EDS.
The primary aim of this study was to characterize self-reported pelvic floor symptoms in a large, anonymous population of cisgender women with EDS. Our hypothesis was that cisgender women with EDS will report a higher prevalence of pelvic floor symptoms than those described in the general population, with a significant impact on quality of life.
Materials and methods
Study design and setting
This study used an observational cross-sectional design to conduct an online survey of cisgender women with a diagnosis of EDS between June 2020 and February 2021. The survey was done using the online electronic survey tool SimpleSurvey (SimpleSurvey, Montreal, Canada). Approval for this study was granted by the institutional Research Ethics Board (ID# 19-0263-E).
Participants
The survey was distributed electronically to email list members of two EDS patient advocacy groups (EDS Canada and The ILC Foundation) and was posted on EDS-focused social media accounts. In addition, recruitment posters were displayed in obstetric and gynecological outpatient clinics at a tertiary care centre in Toronto, Canada. The invitation targeted cisgender women with a diagnosis of EDS, with an aim of understanding their obstetric and gynecological history, as well as the impact of gynecological health on their quality of life. Participants provided consent and were instructed to complete the survey only once on the landing page of the online survey. All results were based on self-reported measures and participants who reported no official diagnosis from a physician, identified as male or other, or reported an age under 18 were excluded from the study.
Survey design
An online survey was created by the study investigators that used a combination of history-based questions and validated questionnaires to gather information on pelvic floor symptoms, sexual function, and pelvic pain, as well as fertility and obstetric history, gynecological history, menstrual symptoms, and menopause symptoms. Four experts in obstetrics and gynecology (with subspecialty expertise in Urogynecology, Reproductive Endocrinology and Infertility, Maternal Fetal Medicine, and Menopause) were consulted on the survey content and provided qualitative feedback. A pilot group of five participants with a known diagnosis of EDS was invited to complete the survey. The pilot participants’ answers were assessed for completion and comprehension and acted as an estimate of external validity of the survey. Expert and pilot participant feedback was incorporated into the final version of the survey, which is included as electronic supplementary material for this study. For this planned analysis of pelvic floor symptoms and impact on quality of life, history and validated questionnaire data addressing bladder symptoms, bowel symptoms, POP symptoms, and sexual function were extracted from the survey responses, as well as pelvic pain-related numerical rating score (NRS) information.
In addition to history-based questions, three validated questionnaires related to pelvic health were included in this study. The Pelvic Floor Disability Index (PFDI-20) and the Pelvic Floor Impact Questionnaire (PFIQ-7) are validated questionnaires used to measure lower urinary tract, lower gastrointestinal tract, and POP symptoms and their effect on quality of life over the last 3 months [15]. The PFDI-20 assesses the presence and severity of symptoms. It consists of 20 questions divided into three subscales: the Urinary Distress Inventory (UDI-6), the Colorectal-Anal Distress Inventory (CRADI-8), and the Pelvic Organ Prolapse Distress Inventory (POPDI-6). For each question in the PFDI-20, the participant is asked whether the symptom exists and, if it does, to quantify how bothersome it is from “not at all” to “quite a bit.” The PFIQ-7 assesses the impact of symptoms on quality of life. Similarly, it consists of three subscales: the Urinary Impact Questionnaire (UIQ-7), the Colorectal-Anal Impact Questionnaire (CRAIQ-7), and the Pelvic Organ Prolapse Impact Questionnaire (POPIQ-7). For each question in the PFIQ-7, the participant is asked to quantify how pelvic floor symptoms affect their quality of life, from “not at all” to “quite a bit.” For both the PFDI-20 and the PFIQ-7, each subscale is scored from 0 (least symptomatic or least distress) to 100 (most symptomatic or most distress) and summed, for a total score for each questionnaire ranging from 0 to 300. The six-item Female Sexual Function Index (FSFI-6) is a validated questionnaire that screens for female sexual dysfunction [16]. It addresses six domains of sexual function: desire, arousal, lubrication, orgasm, satisfaction, and pain, over the last 4 weeks. The FSFI-6 is scored from 2 (highest likelihood of sexual dysfunction) to 30 (lowest likelihood of sexual dysfunction).
Sample size
We assumed a 1 in 5,000 EDS prevalence in the general population, with an estimated 70% prevalence in women [3, 4]. To achieve a confidence level of 95% with an absolute precision of 5% for estimating the most conservative EDS prevalence in women (50%), a total of 384 completed surveys were required for these analyses.
Statistical analysis
Statistical analyses were performed using R (version 4.0.5, R Core Team (2021)). Survey software forcing functions ensured that there were no missing data in the surveys submitted. Descriptive statistics were used to summarize participant characteristics. Groups based on age and EDS type were compared using the nonparametric Kruskal–Wallis test for continuous variables, and the Chi-squared test for categorical variables, with statistical significance based on an alpha of 0.05. When considering pelvic pain, as there were 14 items within this analysis, we used Bonferroni correction to adjust for multiple comparisons.
Results
A total of 2,084 respondents agreed to participate in the survey, of whom 1,526 submitted complete responses (73% completion rate). Of these, 1,303 responses met the eligibility criteria and were included in the analysis. Baseline characteristics of the sample group are summarized in Table 1. The most common EDS types were hypermobile EDS (hEDS, 84%), classical EDS (cEDS, 8%), vascular EDS (vEDS, 2%), and classical-like EDS (clEDS, 2%). Most participants reported white ethnicity (95%). The median number of total pregnancies was 1 (interquartile range [IQR) 0–3]. Previous hysterectomy was reported by 14% of participants.
Lower urinary tract symptoms
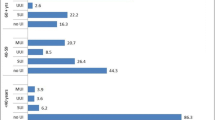
Daily UI was reported by 22% of all participants. Lifetime prevalence of stress urinary incontinence (SUI) increased with age, from 55% for participants aged 18–39 and up to 76% for participants aged 50 or higher. Lifetime prevalence of urgency urinary incontinence (UUI) ranged from 52% (age 18–39) to 66% (age 50 or higher). Nocturia (two or more episodes per night) was reported by 18% of all participants, with no significant differences between age groups. There were no differences in lower urinary tract symptom prevalence between EDS types. The median UDI-6 score was 29 (IQR 17–50) and the median UIQ-7 score was 14 (IQR 0–33). There were no differences in the UDI-6 or UIQ-7 scores between age groups or between EDS types. Lower urinary tract symptoms are summarized in Table 2.
Lower gastrointestinal tract symptoms
Lifetime FI was reported by 24% of all participants, ranging from 20% in participants aged 18–39 and up to 30% in participants over age 40. Lifetime rectal prolapse (RP) was reported by 18% of all participants. RP prevalence was significantly different between age groups, from 15% (age 18–39) to 33% (age 50 or higher). There were no differences in the prevalence of FI or RP between EDS types. The median CRADI-8 score was 28 (IQR 16–44) and the median CRAIQ-7 score was 14 (IQR 0–38). There were no differences in the CRADI-8 or CRAIQ-7 scores between EDS types or between age groups. Lower gastrointestinal tract symptoms are summarized in Table 3.
Pelvic organ prolapse symptoms
Lifetime POP was reported by 21% of all participants. Prevalence increased with age, ranging from 15% in participants aged 18–39, 28% for participants aged 40–49, and up to 37% for participants aged over 50. There were no differences in prevalence of POP between EDS types. The median POPDI-6 score was 25 (IQR 7–42) and did not differ between age groups or between EDS types. The median POPIQ-7 score was 14 (IQR 0–38). Participants aged 18–39 had significantly higher impact scores (POPIQ-7 19 [IQR 0–43]) than those aged 40–49 and 50 or more (p<0.001). POP symptoms are summarized in Table 4.
Pelvic pain
Symptoms of pelvic pain were reported by 71% participants, of whom 45% reported having had these symptoms for more than 10 years. Using a numerical rating scale (0–10), the highest pelvic pain scores were reported for muscle/joint pain (median 7; IQR 6–8), backache (median 7; IQR 5–8), dysmenorrhea (median 7; IQR 4–8), premenstrual pain (median 5; IQR 2–7), and dyspareunia (median 4; IQR 2–7). Comparing age groups, differences in NRS scores were found in cyclic pain types (premenstrual, ovulation, dysmenorrhea, postmenstrual) consistent with lessening impact of these symptoms with age and/or recency effect. NRS scores for pain with sitting were higher in respondents over age 50 (p=0.002). Comparing EDS types, NRS scores were only significantly different for pain with a full bladder (clEDS highest, median 6; IQR 3–8.5). Of 980 participants who reported being peri- or pre-menopausal, 55% described missing one or more day of work or social activities during an average menstrual period because of pain. Details of pelvic pain symptoms are summarized in Table 5.
Sexual function
The majority of respondents (59%) reported having been sexually active in the last 4 weeks. Of those who had been sexually active, 36% reported symptoms of dyspareunia more than half the time. Prevalence of dyspareunia ranged from 40% for participants aged 18–39, to 29% for participants over the age of 50. There were no differences in prevalence of dyspareunia between EDS types. The median FSFI-6 total score for all respondents was 20 (IQR 15–24). FSFI-6 total scores were lower in respondents over 40 than in those aged 18–39, indicating greater sexual dysfunction in the older age group. There were no differences in FSFI-6 total scores between EDS types. Using a FSFI-6 total score cut-off of ≤19 [16], 49% of all participants screened positive for sexual dysfunction. Details of sexual function are summarized in Table 6.
Treatments for pelvic floor disorders
The number of respondents that reported attempting various treatment options for each of the pelvic floor disorders is summarized in Table 7. Patients were specifically asked if they had tried any of these treatments and if so, did these treatments improve or not improve their symptoms. For SUI, of those who had surgery, 74.6% stated the surgery had improved their symptoms, whereas a large percentage of patients who had tried diet/lifestyle modifications (75.2%) and supportive appliances (i.e., tampon or pessary; 83.3%) found no improvement. For UUI, more patients who had tried medication found that they had improved symptoms (59.1%), whereas most who tried diet/lifestyle modifications saw no improvement (73.1%). For FI, more patients who had previously had surgery reported improvements (79.5%) than those who had tried diet/lifestyle modifications without improvement (65.9%). For both RP and vaginal POP, more patients who had undergone surgery reported improvements in symptoms (80.0% and 80.5% respectively) and more patients who had tried supportive appliances reported no improvement (76.9% and 75.5% respectively).
Discussion
This survey study describes pelvic floor symptoms in a large international sample of cisgender women with a diagnosis of EDS. A high prevalence of urinary tract symptoms, gastrointestinal symptoms, pelvic organ prolapse, and pelvic pain were reported. Sexual dysfunction screening was positive in almost half of respondents. In addition, this study quantified the severity and impact of pelvic floor symptoms on the quality of life in this population, using the PFDI-20 and PFIQ-7 validated questionnaires.
Respondents in this study reported a high prevalence of lower urinary tract symptoms, including daily UI in 22% of participants and nocturia in 18% of participants. These findings are consistent with those of previous case series of women with EDS, which described daily UI ranging from 13% to 20% [6, 7]. Lifetime prevalence of SUI and UUI in the present study were high, at 60% and 54% respectively. In large survey studies of the general population, estimates of SUI prevalence in women range from 9% to 46%, with estimates of UUI in women ranging from 4% to 31% [17,18,19]. In the present study, women of all age groups reported both SUI and UUI higher than the general population estimates. Overall, UI in this study increased with age, in accordance with age-related increases also seen in the general population. Specifically, the present study demonstrated that SUI and UUI were 55% and 52% for participants aged 18–39, 65% and 54% for those 40–49, and up to 76% and 66% for participants over 50 respectively. These prevalence rates far exceed recent estimated prevalence of SUI and UUI in the general US population, which was 33.9% and 17.6% for women aged 20–39, 51.3% and 27.9% for women aged 40–59, and 53.1% and 49.5% for those over 60 respectively [17], and are considerably higher than that reported by the international Epic Study, whereby SUI and UUI were reported in only 3.7% and 1% in those under 39, 7.9% and 1.1% in those 40–59, and 8% and 2.5% for those over 60 respectively [18]. Other general population studies support a peak in SUI prevalence between 40 and 50 years of age, with declining prevalence at older ages [20]. It is theorized that this pattern may be related to increased UUI and mixed UI in older individuals, decreased physical activity, or to increased SUI treatment in the fourth and fifth decades of life [20]. In contrast, 76% of respondents over age 50 in the present study reported SUI, which was notably higher than in younger study participants. The high burden of SUI in women with EDS may be related to collagen abnormalities resulting in increased urethral hypermobility. It is also possible that the risk of poor tissue healing in EDS patients, as well as a theoretical risk of higher pain after a mesh implant such as a midurethral sling, has resulted in these patients not being offered incontinence procedures as readily as the general population. As such, this would result in persistently increased reports of SUI in women with EDS at older ages.
In this study, women with EDS also reported a high lifetime prevalence of FI (24%) and RP (18%). The present study demonstrated that FI was reported by 20% of participants aged 18–39, 31% of those 40–49, and 30% of those over 50. These results are far higher than those seen in a Norwegian general population study of women who reported symptoms of FI in 1.7% of those aged 30–39, 1.5% of those 40–49, 2.2% of those 50–59, and 3.8% of those 60–69 [21]. Another general population study of women living in Minnesota also described fewer symptoms of FI, as reported by 1.8% of those aged 18–39, 4.4% of those 40–59, and 7.5% of those over 60 [22]. Although epidemiological studies of RP are rare, general population prevalence is estimated at less than 0.5% [23]. Previous studies of GI symptoms in EDS patients have not disaggregated data in women compared with men [8, 11,12,13]. A broad range of bowel symptoms have been reported in EDS patients, with some reporting lower FI at 2% to 6% and RP at 2% to 3% [11, 13]. In more recent studies, bowel symptoms in EDS patients were reported, with a prevalence similar to our findings, with FI ranging from 13% to 19% [8, 12], and RP at 16% [8]. These differences may be related to the reclassification of EDS diagnoses in 2017, which makes direct comparison of study populations challenging and emphasizes the need for replication studies looking at sex-disaggregated symptom prevalence in patients with a confirmed EDS diagnosis.
Overall prevalence of lifetime POP in this study population was 21% and symptoms increased with age, from 15% in participants aged 18–39, 28% in those 40–49, and up to 37% in participants over the age of 50. Across age groups, these are higher than the 3–6% prevalence of POP symptoms that has been described in population-based surveys of women [24]. This is also notably higher than the prevalence of POP found in the recent general population study of Minnesota women, whereby 4.0% aged 18–39, 5.7% aged 40–59, and 7.9% over 60 reported symptoms of POP [22]. In previous studies of POP in women with EDS, hEDS has been the most frequently studied subtype, with reported POP ranging widely between 13% and 75% [5, 6, 8, 14], similar to those found in the present study. Interestingly, in a recent cross-sectional study of 45 women with molecularly confirmed cEDS, only one participant had pelvic organ prolapse [25]. Conversely, genetic studies of POP have identified COL3A1 (the major gene locus involved in vEDS) as a candidate gene that may predispose white women to POP [26]. In future studies of pelvic floor symptoms, targeted recruitment of participants according to EDS type will be important to help clarify symptom prevalence within each type.
Sexual function in patients with EDS has not previously been a focus of quantitative research. Qualitatively, EDS patients describe pain, fatigue, and POP symptoms as impacting sexual function [27]. In the present study, sexual dysfunction screening using the FSFI-6 validated questionnaire was positive in almost half of respondents. Dyspareunia was described more than half the time by 36% of respondents, consistent with previously published dyspareunia in 30–77% in female patients with EDS [2, 5, 7, 9, 10, 14].
Chronic pain is a known symptom of EDS, and this is the first study to date that has demonstrated the prominent role of pelvic pain in the EDS experience. Types of pelvic pain characterized in this study included dysmenorrhea, dyspareunia, and lower urinary tract pain. The median NRS rating described for dysmenorrhea was 7 [IQR 4–8], similar to muscle/joint pain and backache NRS ratings. Fifty-five percent of pre-menopausal participants reported at least one day of missed work or social activities in an average menstrual period due to pain. Compared with women with primary dysmenorrhea, of whom 17% reported missing school or work [28], this suggests a greater functional impact of dysmenorrhea in women with EDS.
The PFDI-20 and its subscales are commonly used validated measures that quantify how bothersome a respondent finds their bladder, bowel, and/or prolapse symptoms. The PFIQ-7 and its subscales quantify their impact on quality of life [15]. Normative PFDI-20 and PFIQ-7 data have been described for women presenting for routine gynecological care, with median scores of 0 for the POPDI-6, CRADI-8, UIQ-7, CRAIQ-7, and POPIQ-7 subscales, and a UDI-6 median score of 8 [29]. In our study, women with EDS (all subtypes and age groups) had higher PFDI-20 scores and PFIQ-7 scores than normative populations, across all questionnaire subscales. Degree of bother was highest for bladder symptoms (UDI-6 score: 29), followed by bowel (CRADI-8 score: 28) and prolapse symptoms (POPDI-6 score: 25). Impact of bladder, bowel, and prolapse symptoms in this group of women was comparable, with median scores of 14 on each subscale. Women under age 40 experienced a greater impact of prolapse symptoms on quality of life than older participants, with a POPIQ-7 of 19.
This study also reported on the number of EDS patients who tried various treatment options and whether these treatments had improved their pelvic floor symptoms. For each condition, the majority of patients had tried pelvic floor muscle exercises on their own. For SUI, FI, RP, and POP most patients who had reported undergoing surgery had seen improvements in their symptoms. Those with UUI who had tried medication also reported greater improvements in their symptoms. Diet and lifestyle modifications appeared to have the lowest impact on improving symptoms for all conditions, and the use of supportive appliances, such as tampons or pessaries, also showed the least improvement in symptoms for those patients with SUI, RP, and POP. Although this is to our knowledge the first report to include pelvic floor disorder treatment options tried by women with EDS, it is notably limited by the self-reported nature of these results. The duration and frequency of these treatment options, the type or dose of medications tried, and the type of surgical procedures performed were not recorded.
A strength of this study is the size of the sample and the global reach of the participants. The increasing prevalence of UI, FI, and POP with advancing age, as expected in the general population, contribute to the external validity of this sample. The potential for self-selection bias exists in the online recruitment process. However, this effect was likely mitigated by the fact that recruitment did not target pelvic floor symptoms; rather, this was a sub-analysis extracted from a more extensive survey that broadly evaluated obstetric and gynecological experiences of cisgender women with EDS.
As this was a survey study, it is limited by the data being collected anonymously by self-report, without medical record confirmation. Comparisons of pelvic floor symptoms and impact were made with previous studies rather than a control group. The online nature of the study and of the recruitment process may have led to the exclusion or attrition of potential study participants in older age groups, who may have less experience participating in online research. The sample population is predominantly white and recruitment was aimed at cisgender women; thus, generalizability to other populations is lower.
Conclusion
This large international survey study demonstrated a high prevalence of pelvic floor symptoms, including UI, FI, and POP, in cisgender women with EDS. Pelvic pain, especially dysmenorrhea and dyspareunia, were also common and sexual dysfunction was likely. Women with EDS are bothered by bladder, bowel, and prolapse symptoms, as demonstrated by PFDI-20 and PFIQ-7 scores. Prolapse symptoms in younger women with EDS have the greatest impact on quality of life. The results of this study provide a foundation for future investigations into pelvic floor disorders in this population. Further research, with a focus on recruiting participants with less common EDS types and more diverse racial backgrounds, will provide valuable data to improve clinicians’ understanding of patients’ experiences and allow for more individualized counseling and management.
References
Malfait F, Francomano C, Byers P, et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017;175(1):8–26. https://doi.org/10.1002/ajmg.c.31552.
Hugon-Rodin J, Lebegue G, Becourt S, Hamonet C, Gompel A. Gynecologic symptoms and the influence on reproductive life in 386 women with hypermobility type Ehlers-Danlos syndrome: a cohort study. Orphanet J Rare Dis. 2016;11:124–9. https://doi.org/10.1186/s13023-016-0511-2.
Castori M, Camerota F, Celletti C, Grammatico P, Padua L. Ehlers-Danlos syndrome hypermobility type and the excess of affected females: possible mechanisms and perspectives. Am J Med Genet A. 2010;152(9):2406–8. https://doi.org/10.1002/ajmg.a.33585.
Kulas Søborg M-L, Leganger J, Quitzau Mortensen L, Rosenberg J, Burcharth J. Establishment and baseline characteristics of a nationwide Danish cohort of patients with Ehlers-Danlos syndrome. Rheumatology (Oxford). 2017;56:763–7. https://doi.org/10.1093/rheumatology/kew478.
Castori M, Camerota F, Celletti C, et al. Natural history and manifestations of the hypermobility type Ehlers-Danlos Syndrome: a pilot study on 21 patients. Am J Med Genet. 2010;152A(3):556–64. https://doi.org/10.1002/ajmg.a.33231.
Carley ME, Schaffer J. Urinary incontinence and pelvic organ prolapse in women with Marfan or Ehlers Danlos syndrome. Am J Obstet Gynecol. 2000;182(5):1021–3. https://doi.org/10.1067/mob.2000.105410.
McIntosh LJ, Mallett VT, Frahm JD, Richardson DA, Evans MI. Gynecologic disorders in women with Ehlers-Danlos syndrome. J Soc Gynecol Investig. 1995;2(3):559–64. https://doi.org/10.1016/1071-5576(94)00050-b.
Nee J, Kilaru S, Kelley J, et al. Prevalence of functional GI diseases and pelvic floor symptoms in Marfan Syndrome and Ehlers-Danlos Syndrome. J Clin Gastroenterol. 2019;53(9):653–9. https://doi.org/10.1097/MCG.0000000000001173.
Hurst BS, Lange SS, Kullstam SM, et al. Obstetric and gynecologic challenges in women with Ehlers-Danlos syndrome. Obstet Gynecol. 2014;123(3):506–13. https://doi.org/10.1097/AOG.0000000000000123.
Glayzer JE, McFarlin BL, Castori M, et al. High rate of dyspareunia and probable vulvodynia in Ehlers-Danlos syndromes and hypermobility spectrum disorders: an online survey. Am J Med Genet C Semin Med Genet. 2021;187(4):599–608. https://doi.org/10.1002/ajmg.c.31939.
Nelson AD, Mouchli MA, Valentin N, et al. Ehlers Danlos Syndrome and gastrointestinal manifestations: a 20-year experience at Mayo Clinic. Neurogastroenterol Motil. 2015;27:1657–66. https://doi.org/10.1111/nmo.12665.
Abu-Farhaneh E, Tse Y, Parker CH, Liu LW. Gastrointestinal symptoms and disorders of gut brain interaction are common in patients with Ehlers-Danlos Syndrome (EDS) in tertiary referral center. J Can Assoc Gastroenterol. 2020;3(S1):143–4. https://doi.org/10.1093/jcag/gwz047.122.
Alomari M, Hitawala A, Chadalavada P, et al. Prevalence and predictors of gastrointestinal dysmotility in patients with hypermobile Ehlers-Danlos Syndrome: a tertiary care center experience. Cureus. 2020;12(4):e7881. https://doi.org/10.7759/cureus/7881.
Castori M, Morlino S, Dordoni C, et al. Gynecologic and obstetric implications of the joint hypermobility syndrome (a.k.a. Ehlers-Danlos syndrome hypermobility type) in 82 Italian patients. Am J Med Genet A. 2012;158A(9):2176–82. https://doi.org/10.1002/ajmg.a.35506.
Barber MD, Walters MD, Bump RC. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol. 2005;193:103–13. https://doi.org/10.1016/j.ajog.2004.12.025.
Isidori AM, Pozza C, Esposito K, et al. Development and validation of a 6-item version of the Female Sexual Function Index (FSFI) as a diagnostic tool for female sexual dysfunction. J Sex Med. 2010;7:1139–46. https://doi.org/10.1111/j.1743-6109.2009.01635.x.
Abufaraj M, Xu T, Cao C, et al. Prevalence and trends in urinary incontinence among women in the United States, 2005–2018. AJOG. 2021;225(2):166.e1–166.e12. https://doi.org/10.1016/j.ajog.2021.3.016.
Irwin DE, Milson I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006;50(6):1306–15. https://doi.org/10.1016/j.eururo.2006.09.019.
Coyne KS, Sexton CC, Thompson CL, et al. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and Sweden: results from the Epidemiology of LUTS (EpiLUTS) study. BJU Int. 2009;104(3):352–60. https://doi.org/10.1111/j.1464-410X.2009.08427.x.
Reynolds WS, Dmochowski RR, Penson DF. Epidemiology of stress urinary incontinence in women. Curr Urol Rep. 2011;12(5):370–6. https://doi.org/10.1007/s11934-011-0206-0.
Rømmen K, Schei B, Rydning A, Sultan HA, Mørkved S. Prevalence of anal incontinence among Norwegian women: a cross-sectional study. BMJ Open. 2012;2(4):e001257. https://doi.org/10.1136/bmjopen-2012-001257.
Gabra MG, Tessier KM, Fok CS, Nakib N, Oestreich MC. Fischer J. Pelvic organ prolapse and anal incontinence in women: screening with a validated epidemiology survey. Arch Gynecol Obstet. 2022; https://doi.org/10.1007/s00404-022-06510-7.
Bordeianou L, Hicks CW, Kaiser AM, Alavi K, Sudan R, Wise PE. Rectal prolapse: an overview of clinical features, diagnosis, and patient-specific management strategies. J Gastrointest Surg. 2014;18:1059–69. https://doi.org/10.1007/s11605-013-2427-7.
Barber MD, Maher C. Epidemiology and outcome assessment of pelvic organ prolapse. Int Urogynecol J. 2013;24:1783–90. https://doi.org/10.1007/s00192-013-2169-9.
Ritelli M, Venturini M, Cinquina V, Chiarelli N, Colombi M. Multisystemic manifestations in a cohort of 75 classical Ehlers-Danlos syndrome patients: natural history and nosological perspectives. Orphanet J Rare Dis. 2020;15:197. https://doi.org/10.1186/s13023-020-01470-0.
Niu K, Chen Y, Lu Y. COL3A1 rs1800255 polymorphism is associated with pelvic organ prolapse susceptibility in Caucasian individuals: evidence from a meta-analysis. PloS One. 2021;16(4):e0250943. https://doi.org/10.1371/journal.pone.0250943.
Bennett SE, Walsh N, Moss T, Palmer S. Understanding the psychosocial impact of joint hypermobility syndrome and Ehlers-Danlos syndrome hypermobility type: a qualitative interview study. Disabil Rehabil. 2021;43(6):795–804. https://doi.org/10.1080/09638288.2019.1641848.
Kho KA, Shields JK. Diagnosis and management of primary dysmenorrhea. JAMA. 2020;323(3):268–9. https://doi.org/10.1001/jama.2019.16921.
Lowder JL, Ghetti C, Oliphant SS, Moalli PA, Zyczynski HM. Normative data for commonly used validated pelvic floor disorder questionnaires for women. Female Pelvic Med Reconstr Surg. 2010;16(5):296–8. https://doi.org/10.1097/SPV.0b013e3181e4f148.
Acknowledgements
We are grateful for the support of the following patient societies: EDS Canada, The ILC Charitable Foundation, The Ehlers Danlos Society, The Hypermobility Syndromes Association, and other patient advocates active on social media.
Author information
Authors and Affiliations
Contributions
O. Kciuk: project development, data collection, data analysis, manuscript writing; Q. Li: data analysis; E. Huszti: data analysis; C.D. McDermott: project development, data collection, manuscript editing.
Corresponding author
Ethics declarations
Conflicts of interest
Olga Kciuk has no financial disclaimer or potential conflicts of interest relating to this project to disclose. Qixuan Li and Ella Huszti are part of the Biostatistics Research Unit, which is paid for by the Department of Obstetrics and Gynecology at Mount Sinai Hospital for statistical support related to research. Colleen McDermott is a medical advisor for Szio Inc. and COSM Inc, and a speaker for Pfizer. These companies were not involved in any facet of this study, including project development, data collection, and manuscript writing.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 553 kb)
Rights and permissions
About this article
Cite this article
Kciuk, O., Li, Q., Huszti, E. et al. Pelvic floor symptoms in cisgender women with Ehlers–Danlos syndrome: an international survey study. Int Urogynecol J 34, 473–483 (2023). https://doi.org/10.1007/s00192-022-05273-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-022-05273-8