Abstract
Introduction and hypothesis
The objective was to overview the literature on the existing pelvic floor procedure registries and databases and to identify patient demographic, clinical and/or patient-reported data items for inclusion in the Australasian Pelvic Floor Procedure Registry (APFPR) Minimum Data Set (MDS).
Methods
We conducted a literature search on the MEDLINE, Embase, CINAHL and PsycINFO databases in addition to Google Scholar and grey literature to identify studies in the period January 2008 to January 2020. All were English studies of registries and databases on female adults undergoing surgery for pelvic floor disorders including stress urinary incontinence (SUI) and pelvic organ prolapse (POP). Studies were assessed on demographic and clinical patient characteristics, procedure or treatment type, health-related quality of life, adverse events and safety outcomes, captured by pelvic floor procedure registries or databases that have been established to date.
Results
From 1662 studies, 29 publications describing 22 different pelvic floor registries and databases were included for analysis, 12 (55%) of which were multicentre. Six (27%) registries and databases involved solely SUI, eight (36%) were regarding POP, and the remaining eight (36%) focussed on both conditions. The majority of registries and databases captured similar details on patient characteristics, comorbidities and other clinical features, procedure or treatment type, health-related quality of life, adverse events, safety and efficacy.
Conclusion
The findings of this scoping review will assist in determining the MDS for the APFPR, an initiative of the Australian government, to improve health and quality of life outcomes of women who undergo pelvic floor reconstructive procedures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pelvic organ prolapse (POP) and stress urinary incontinence (SUI) are common pelvic floor disorders that can substantially affect a woman’s daily living and quality of life [1]. POP is defined as the “descent of one or more of the anterior vaginal walls, posterior vaginal wall, the uterus (cervix), or the apex of the vagina (vaginal vault or cuff scar after hysterectomy)” and correlated with symptoms that may include a vaginal bulge, pelvic pressure, a need to digitally replace the prolapse or bleeding/discharge/infection resulting from ulceration. SUI is defined as a “complaint of involuntary loss of urine on effort or physical exertion (e.g., sporting activities), or on sneezing or coughing” [2]. Treatment options for POP and/or SUI include lifestyle measures, pelvic floor muscle training, mechanical support devices and surgical procedures. These procedures include native tissue or prosthesis-based procedures that may be performed via a trans-vaginal or abdominal approach [3]. Prosthesis-based procedures involve the implantation of a fabricated substitute to augment or stabilize an anatomical structure and may consist of a synthetic “mesh” or biological “graft” material [4].
In Australia each year over 20,000 women undergo pelvic floor reconstructive surgical procedures to treat POP and SUI [5]. The pursuit of improved anatomical and functional outcomes has driven the development and uptake of techniques and products using prosthetic materials to augment deficient tissue and suspend the pelvic floor and pelvic organs [3, 5]. Until recently, approximately one quarter of all procedures involved the use of a prosthesis mesh product, with an estimated 150,000 mesh devices being implanted since 1998. However, the use of such devices has not always been associated with improved outcomes for women when compared with native tissue approaches or in relation to complications [1]. Unfortunately, outcome data for these procedures are not systematically or routinely collected in Australia, with no capacity to analyse their benefits and risks [1].
Outside Australia, clinical registries and databases have been established to routinely monitor the number, type and outcomes of pelvic floor procedures to support improved quality of care in the surgical management of pelvic floor disorders [6,7,8,9]. The data collected by these registries and databases have been used to inform a number of scientific studies of specific urogynaecological topics, broader epidemiological topics and the use to describe patient-reported outcome measures (PROMs) in this area (Fig. 1).
Clinical quality registries (CQRs) aim to systematically monitor the quality of health care, within specific clinical domains, by routinely collecting, analysing and reporting health-related information. Well-designed and managed CQRs provide clinical information which is richer and more reliable than information obtained from hospital administrative systems [10]. A key attribute of CQRs is that they provide feedback to stakeholders including clinicians, managers, funders, policy makers and researchers through benchmarking activities that identify significant variation in quality of care [11, 12].
In 2018, the Australian Senate Community Affairs Reference Committee investigated the number of women with transvaginal mesh implants and related matters and reported that for many Australian women there has been significant suffering associated with the complications and long-term effects of pelvic floor mesh [5, 13]. The Australian government supported the Senate Inquiry’s recommendation for the development of a CQR for pelvic floor-related procedures to support the monitoring of pelvic device outcomes, and in 2019, the Federal Health Minister announced the Australian government would invest $2.3 million over 3 years to establish the Australasian Pelvic Floor Procedure Registry (APFPR). The establishment of the APFPR will inform evidence-based care driving improvement in patient safety and provide an opportunity to collect real-world outcomes from patients undergoing SUI and POP procedures that utilize prostheses.
Establishing a CQR for collecting clinical and patient-reported outcomes and outcome measures on pelvic floor surgical procedures will address gaps in the collection, analysis and reporting of pelvic floor procedures and provide feedback to clinicians and patients regarding the status of pelvic floor interventions which have the potential to provide significant improvements in health-related quality of life (HRQoL) [5].
As this is a scoping review intended to map and describe the extant literature, we did not undertake a detailed critical analysis of study quality and results. The aim of this scoping review was to identify and describe patient demographic and clinical information, outcomes and outcome measures collected and reported by pelvic floor procedure registries and databases that have been established to date to inform the development of the minimum dataset (MDS) for the APFPR. The development of a MDS is a significant registry activity that ensures that the data are clinically meaningful and will achieve quality improvement and research goals [14].
Materials and methods
Protocol and registration
This protocol follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews (PRISMA-ScR) format [15].
The protocol was registered in the Prospective Register of Ongoing Systematic Reviews (PROSPERO), registration number: CRD42020145496.
Information sources
This review searched the following electronic databases: MEDLINE, EMBASE, CINAHL and PsycINFO, as well as Google Scholar. Grey literature was also included. For each article selected for inclusion, abstracts and full articles were obtained. Reference lists of the included studies and systematic reviews were examined during the review. Articles were restricted to those written in English and published between January 1, 2008, and January 1, 2020. Articles were excluded if they were: written in a language other than English, published prior to 1 January, 2008, procedures were performed for males or were colorectal procedures. Unpublished manuscripts, dissertations, government reports, books and book chapters, randomized control trials, conference proceedings and meeting abstracts were also excluded.
Search strategy
The search strategies were developed by two authors (RR, JOD). We used Medical Subject Heading (MeSH) keywords and free text search terms. The database records and details of how the literature search was undertaken were maintained at each stage of the review process. Also, a manual search using Google was performed. The key search terms were ([“pelvic organ prolapse” OR “pelvic floor disorder” OR “stress incontinence”] AND [“device” OR “procedure” OR “graft” OR “sling” OR “mesh” OR “fascia” OR “suspension” OR “injection” OR “intervention”] AND [“registry” OR “database” OR “dataset” OR “audit”]). We adapted the search strategy to the remaining databases mentioned above. The terms were combined by means of Boolean operators.
Eligibility criteria
Quantitative studies (e.g. cohort, longitudinal, case studies, prospective and retrospective) describing the development, structure, outcomes or outcome measures collected and reported in the female pelvic floor surgical procedure registries and databases were included. Randomized controlled trials were excluded from this review, as they provided limited information on the development and structure of the pelvic floor surgical procedure registries and databases.
This review included female adults undergoing surgery for pelvic floor disorders including SUI and POP. Patients undergoing colorectal procedures were excluded.
The main phenomena of interest in this review were safety and effectiveness outcomes and outcome measures, including clinical and patient reported, related to surgical pelvic floor procedures using native tissue and mesh/graft prosthetic materials captured by registries or databases. The topics of focus for this review included demographic and clinical patient characteristics, procedure or treatment type, HRQoL, adverse events and safety outcomes.
Study selection
A three-phase screening process was applied. In phase one, one researcher conducted the initial search of the literature. During the second phase, two researchers independently screened the titles and abstracts of all articles identified in the search strategy to determine eligibility and classify studies as relevant, possibly relevant and irrelevant. During the last phase, the researchers independently reviewed the full texts to make a final determination of eligibility.
Data management and analysis
The search was carried out in the databases mentioned above and then loaded into EndNote™ X8 software, management software for references that allows the identified references to be organized into different electronic databases. All the results were inserted in a single EndNote folder, and the duplicated studies were identified and removed. After the duplicate removal, the research results were loaded onto Covidence™ (https://www.covidence.org/), a software that assists the article trials, database extraction and cooperation among multiple assessors.
Data were extracted using a standardized data extraction form in Microsoft Excel. Data were analysed on a study-by-study basis with the generation of reported outcomes and the techniques/tools used to assess each outcome.
Results
General description of the literature
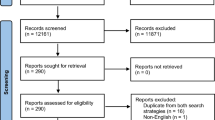
The search strategy yielded 1662 documents (Fig. 1). Their titles and abstracts were screened according to the inclusion criteria. Of those, 1397 references were reviewed and full copies of 183 articles were retrieved. The screening of full texts resulted in 29 papers.
Seven (23%) articles were published between 2008 and 2012, 11 (37%) articles between 2013 and 2016 and the remaining 11 (40%) between 2017 and 2019. Of the 29 studies, 18 (60%) were published in Europe and the UK, 10 (33%) in the USA and 1 (6%) in New Zealand.
Twenty-two different registries and databases were described in these studies, 12 (55%) of which were multicentre. Six (27%) registries and databases involved solely SUI, eight (36%) were regarding POP, and the remaining eight (36%) focussed on both conditions. The number of participants described in these studies ranged from 12 [16] to > 116,000 patients [17,18,19].
Data entry methods
Prospective online data entry was reported by most of the registries and databases with the exception of the Norwegian National Incontinence registry [20,21,22], where the data were collected retrospectively by scanning the data forms and transferring them to the registry. A similar method was also used to enter the retrospective data from electronic medical records into the POP database of women who underwent apical suspension procedures performed by seven female pelvic medicine and reconstructive surgery surgeons at two hospitals within a tertiary medical centre in Pittsburgh, PA (USA) [23] (Table 1).
Data reporting and feedback
The British Society of Urogynaecology BSUG [17,18,19] provides the ability to generate automated reports on a regular basis. Annual reports published on a 3-year rolling cycle are available from the British Association of Urological Surgeons (BAUS) audit tool [24]. Annual reports are also produced by the Pelvic Floor Disorders Registry (PFDR), launched by the American Urogynecologic Society [7, 25] and the DugaBase in Denmark [26]. In addition, data in this registry are reported on a quarterly basis for the centres to review their own performance. Similarly, twice a year the participating centres receive a report where their own data are compared with the national average in the Norwegian National Incontinence Registry [20,21,22]. In a German pilot registry of urogynaecological implants, users are able to extract their data from the web interface directly [27].
Outcomes and outcome measures collected by the registries and databases
Table 2 summarizes outcomes and outcome measures captured by SUI and POP registries and databases described in this scoping literature review.
Patient demographic details and anthropometric variables
Most of the registries and databases described in this review captured anonymised patient details, except the BSUG [17,18,19] and Swedish Gynaecological Surgery (GYNop) [28] registries that collected identifiable details such as patient’s name and surname. In addition, contact details were captured in the PFDR [7, 25]. Race was mainly collected in the US registries and databases, for example in the PFDR [7, 25], the Faculty Practice Solutions Center (FPSC) database [29], the Pittsburgh Database from two hospitals in the USA [23] and the Michigan Observational prolapse database in the USA [30]; ethnicity was also captured in the New Zealand Urogynaecology database [31].
Other information commonly captured by most of the data sources included the patient’s age or date of birth, body mass index, smoking habits, alcohol consumption, parity and menopausal status.
Diagnostic and clinical data variables
Most commonly captured clinical data included prior surgery details, patient comorbidities, pelvic floor disorder symptoms and urodynamic testing data (i.e. urodynamic stress incontinence, detrusor overactivity, voiding dysfunction). Prolapse was assessed using the International Continence Society (ICS) Pelvic Organ Prolapse Quantification (POP-Q) system. In addition to the previous surgery details, clinical characteristics captured in the Austrian Transvaginal Mesh Registry included anterior and posterior vaginal wall prolapse and bladder, bowel and sexual symptoms [6]. The Prolift™ registry (France) [32] captured medical, surgical and obstetric history, functional urination and rectal problems, and the impact of the prolapse on daily life (including sexual activity). Moskowitz et al. [33] used a prospective institutional database recording retropubic and transobturator SUI procedures that included prior surgery, preoperative urodynamic evaluation (UDS) performed according to International Continence Society guidelines, Valsalva leak point pressure (VLPP) and detrusor overactivity (DO) and longitudinally reported the completely dry rate at 3, 5 and 10 years.
Treatment, procedure and device information
Original and repeat procedure details were collected by BSUG. These included mid-urethral sling (MUS) procedures such as transobturator tape (TOT) and the transobturator tape inside-out (TVT-O), with the unique device identifier and mesh material captured [17,18,19]. Similar procedures and treatment details were also recorded by BAUS [24], the Tension Free Vaginal Tape (TVT) Worldwide Observational Registry [34], the DugaBase in Denmark, by the Norwegian National Incontinence Registry [26] and the Institutional database in the US (VA, WA, NJ) in Texas [35].
The Austrian registry recorded multiple procedures and their details including mesh type and manufacturer, hysterectomy, vaginal repairs, posterior colporrhaphy, sacrospinous fixation, sacrocolpopexy, abdominal and/orlaparoscopic colposuspension, and tape for urinary incontinence and other procedures [6]. Similar information was collected in the Belgian EPILAPSUS registry [36] and GynOp [28] and by single-centre databases such as the Faculty Practice Solutions Center (FPSC) database of women with SUI procedures in Sacramento, California [29], and other similar databases in the USA [30, 37]. More details on mesh type, mechanical and chemical characteristics were captured by the German European Registry of urogynaecological implants (EURUGI) [38], an Italian single-centre database described by Costantini et al. [16] and the New Zealand Urogynaecology database [31, 39].
The procedure data elements were clearly defined and described by the PFDR [7, 25]. The PFDR was developed specifically to allow the capture of safety and effectiveness data within the context of a pragmatic study design to capture diverse, “real-world” POP treatment settings [25].
Safety outcomes and complications
Safety outcomes and complication details described in this review comprised intraoperative, perioperative, postoperative and long-term complications. Reoperation details were also captured. Surgical complications were graded according to the ICS and International Urogynecological Association (IUGA) [2] and Clavien-Dindo Grade classification systems [40].
Perioperative complications (i.e. ureteric injury, bladder injury, bowel injury, vaginal buttonhole tear, urethral injury, blood loss > 500 ml, device injury) were captured by most of the registries and databases in this review [6, 7, 17,18,19,20, 24, 26, 32, 34, 36, 41, 42]. Long-term problems and complications included de novo operative bladder symptoms, prolapse, issues with bladder or bowel function, sexual function problems, chronic pain and psychological issues, change in incontinence, dyspareunia, sling erosion and others.
The GynOp registry [28] captured severe medical and surgical complications that included organ lesions, excessive bleeding, deep venous thrombosis or severe infection. A postoperative infection was recorded if patients received treatment with antibiotics because of surgical site or urinary tract infection. Similarly, in a Pennsylvanian database in the USA, a major complication was considered present when any of the following occurred: visceral injury (bladder, bowel or ureter), blood transfusion, conversion to laparotomy, infection, readmission, return to the operating room, small bowel obstruction/ileus or mesh complications [23].
Patient-reported outcome measures
Most commonly captured condition-specific patient-reported outcome measures (PROMs) included the International Consultation on Incontinence Questionnaire Urinary Incontinence module (ICIQ-UI) [7, 13, 17,18,19, 24,25,26], the Patient Global Impression of Improvement (PGI-I) [7, 17,18,19, 25, 27, 33] and the Urinary Distress Inventory (UDI) [16, 28, 33, 35, 43, 44]. The other condition-specific and validated measures included the Pelvic Organ Prolapse/Urinary Incontinence Questionnaire (PISQ-12) [7, 25, 30], the Pelvic Floor Distress Inventory (PFDI) [7, 25, 30], the Pelvic Floor Impact Questionnaire (PFIQ-7) [7, 25], the Incontinence Quality of Life Instrument (I-QOL) [34] and the Incontinence Impact Questionnaire (IIQ) [35, 43, 44].
Pain symptoms were assessed using the Brief Pain Inventory (BPI) after the surgery and at 12-month follow-up in the TVT Worldwide Observational Registry [34]. To assess pain, other registries and databases used a visual analogue pain scale (VAS) [36, 42, 44,45,46] or a binary question of persistent postoperative pain at baseline, 6 and 12 months postoperatively [7, 25, 47].
Many registries and databases captured patient and physician satisfaction with the surgery. In addition, some registries conducted qualitative interviews with patients to identify impaired function and poor tolerance of the prosthesis, new onset or lack of improvement in urinary or digestive problems, level of their satisfaction and impact on sex life [48, 49].
Discussion
This scoping review examined existing international registries and databases to inform the development of the MDS for the APFPR modules which will comprise SUI mesh and mesh revision procedures initially, followed by POP mesh and related revisions. The results of this scoping review will be used by the registry to provide a framework to determine what data items are important to measure safety and effectiveness with respect to all SUI and POP procedures including device implantation, revision and removal.
The APFPR will address systemic deficits in the collection, analysis and reporting of pelvic floor devices to establish early warning systems and provide feedback to clinicians, hospitals, regulatory bodies and ultimately the public regarding the status of pelvic floor interventions. The ability to monitor and measure care and safety outcomes requires the collection of high-quality epidemiological data collected on all patients undergoing SUI and POP pelvic floor procedures. An important secondary purpose of the APFPR will also be to provide a platform for further research.
Twenty-two registries and databases from 29 publications were identified. Most publications did not describe data elements captured by the registries or databases explicitly; however, these publications summarized findings arising from the studies captured by the relevant registries and databases.
The domains of interest for the APFPR MDS included patient demographics, clinical history, comorbidities and diagnostic data that may serve as risk adjustment factors; procedure or treatment type; HRQoL; adverse events and safety; and efficacy measures and outcomes. Most commonly captured demographic data elements described in this scoping review included patient identification and age. Only two, the BSUG and BAUS registries, captured identifiable patient details. Identifiable personal information is required for a registry to provide information to patients if the registry is responsible for patient recruitment and send patients follow-up questionnaires and PROMs. As well as for use in registry operations as described above, the APFPR will collect patient identifiers but will not release identified information other than to the treating surgeon to state/national death registries for linkage for quality assurance purposes.
Anthropometric data such as weight, height and body mass index (BMI) were captured by all registries and databases in this review. In addition, some registries also collected lifestyle factors such as smoking and alcohol consumption. Factors such as age, BMI and smoking status are risk factors that may, if not adjusted for at the time of registry data analysis, affect interpretation of the outcomes [50]. Evidence suggests that a raised BMI adversely affects success of SUI surgery [51] and that in those with a BMI > 35 kg/m² the cure rate is decreased after TVT insertion.
Additional details collected by the registries included ethnicity, education and employment, insurance and social security information. These factors may be particularly relevant as risk-adjustment factors, i.e. factors (beyond the control of the surgeon) in the USA or other countries that do not have universal healthcare systems. While this may not be as relevant in Australia, which provides universal health coverage, health insurance status may be important given the APFPR is collecting whether the surgery is undertaken in a public or private hospital, where it is known that approximately 80% of pelvic floor procedures occur in private settings [52].
Most commonly captured diagnostic/clinical variables included patient diagnosis, comorbidities, prior surgery details, urodynamic data, pelvic floor status, menopausal status, baseline sexual function and dyspareunia [6, 7, 17, 18, 24, 35, 36]. These data variables are mainly collected for risk adjustment.
Procedure types and treatments were captured in detail by all the registries and databases. Procedure and device information, prosthetic material and mesh type, and associated surgeries were consistently recorded. The type and details of the procedures and devices varied among the registries, depending on the procedure performed (SUI or POP). Most of the registries used unique device identifier and mesh details [6, 17, 26, 31]. These details were captured for primary and any repeat procedures. The amount of information collected varied and needs to considered carefully for inclusion in the context of the Australian health system, particularly with respect to the methods for identifying devices.
For up to one in ten women who underwent pelvic floor procedures with mesh, complications included chronic abdominal, buttock and leg pain, bleeding and discharge, and difficulties with sex, which may impact the ability to work and maintain social activities and relationships [53]. Complications reported in the review were both perioperative and long term. Perioperative complications included ureteric injury, bladder injury, bowel injury, vaginal buttonhole tear, urethral injury, blood loss > 500 ml and device injury. Long-term problems included de novo operative bladder symptoms or prolapse, bladder, bowel, affected sexual activity, mesh exposure, chronic pain, changes in incontinence, catheter requirement, dyspareunia and sling erosion. These were captured by the majority of the registries. In addition, several studies [30, 33, 54, 55] described physical and psychological issues reported by patients. Adverse event reporting systems such as the Therapeutic Goods Administration (TGA)'s notification system rely on public reporting and are known to capture only a proportion of likely overall pelvic mesh complications.
One of the main drivers for the establishment of the APFPR is the monitoring of safety outcomes and complications related to mesh-related pelvic floor prostheses, particularly new symptoms that may present shortly or long after the procedure. However, most registries and databases also looked more broadly and included non-mesh-related complications, i.e. related to the procedure but not the prosthesis used, that may occur intra- and postoperatively and included reoperation. There appears to be broad consensus around the non-mesh complications of intraoperative visceral injury and haemorrhage to be collected. The development of new functional bladder and pelvic floor symptoms, sexual dysfunction and pelvic pain, that may or may not be mesh-related, along with mesh-related complications such as exposure, was also captured by most. However, it is important to distinguish mesh from non-mesh complications, i.e. those that may also occur with a non-mesh SUI procedure, e.g. pubovaginal sling. Use of a standardized categorisation system such as the ICS/IUGA mesh complication classification system [2], the only one in common use, is important for distinguishing those mesh-related complications, while the Clavien-Dindo system [40] provides a method of categorizing the severity of surgical complications based on the treatment required. This review makes it apparent that any pelvic floor registry MDS needs to have the capacity to record and categorize mesh and non-mesh complications at different time points in a standardized manner. The collection of risk factors enables the ability to adjust for variables that may increase risk of complications in patient or clinician cohorts, facilitating the production of meaningful reports that may be provided to clinicians and health system stakeholders to identify opportunities for improved quality of care or investigation of certain prostheses.
The collection of PROMs is a critical activity of the APFPR that provides additional information to support safety monitoring of mesh-related adverse events. This is particularly important as the PROMs will provide baseline information about a participant’s condition prior to surgery as well as monitor them beyond the usual post-surgical follow-up time period. Most pelvic floor registries included patient-reported outcomes collected by commonly used condition-specific validated PROMs in the domains of urinary incontinence, urinary and pelvic floor dysfunction impact, sexual function and HRQoL at baseline, and 6 and 12 months postoperatively [6, 18, 19, 24, 26, 38, 39]. Most commonly captured data included SUI and POP- specific information; however, generic HRQoL information, evaluation of pain and satisfaction with success of the procedure were also included. PROMs data were captured electronically in most of the registries and databases. Despite the risks associated with the use of mesh, relatively few used validated pain questionnaires. Given the goal of treatment for most pelvic floor procedures to improve HRQoL, a key outcome of the APFPR will be to report patient-reported outcomes, in terms of both the efficacy and adverse effects of mesh-related procedures. From this review it appears there is no single PROM that incorporates the range of mesh-related complication symptoms experienced by patients. This may indicate the need for development of such a PROM, particularly when reporting the outcomes of procedures to treat mesh complications.
Strengths and limitations
This study has systematically and comprehensively reviewed the data collected and outcomes reported by international pelvic floor registries providing an initial inventory of data items from which to develop the APFPR MDS.
To appreciate the findings in this review, the following limitations should be considered. First, studies were excluded when they did not have full text available. This may have led to exclusion of a selection of relevant registries and databases. Second, not all information about the outcomes and outcome measures captured by pelvic floor procedure registries and databases is published in the academic literature and hence we may have missed some relevant information. Therefore, our goal was not to perform a comprehensive search, but rather to overview the literature on the existing pelvic floor procedure registries and databases and to identify most commonly captured outcomes and outcome measures to be considered for inclusion in the APFPR.
Conclusions and next steps
This review has identified the domains and data items used by existing pelvic floor registries internationally as the first step in developing an evidence-based MDS facilitating the analysis and reporting of outcomes of mesh-related pelvic floor procedures as part of the APFPR. This inventory will be refined to ensure the final MDS contains only items that are important and feasible to collect, through further consultation with patients and clinicians, to maximize participation in the registry. The review has also identified potential gaps where additional instruments may need to be developed to measure adverse outcomes of mesh procedures. As the APFPR evolves, additional items may be required to report clinical quality indicators.
The APFPR, as a clinician-led national registry, is relatively unique in that it has been established with government funding and aims to work closely with the national device regulator (TGA) to support early detection and intervention where device issues are detected.
Australia does not yet have a unique device identifier (UDI), which has made the task of collecting large-scale medical implanted device outcome information difficult. However, recent funding announced by the Commonwealth government [5] has been established for UDI development, which is very timely for the APFPR. The APFPR has also determined that it will utilize a broader definition of ‘prosthesis’ for the inclusion of devices within the registry, which will include bulking devices as well as mesh products. This is broader than a number of other device databases currently in existence.
The APFPR is currently finalizing its MDS and PROMs for its SUI module and plans to pilot the dataset in over 20 hospitals across Australia in early 2021. New Zealand has also expressed interest in participating in the APFPR. While the registry will not assist women, who have already been affected by complications from pelvic mesh, it is hoped that, in conjunction with similar activities internationally, this will enable pelvic floor devices and procedures that have higher than expected adverse outcomes to be detected and acted on far earlier than has occurred in the past.
References
Australian Government response to the Senate Community Affairs References Committee report: The number of women in Australia who have had transvaginal mesh implants and related matters. 28 March 2018. © Commonwealth of Australia 2018. ISBN 978-1-76010-701-7 https://www.aph.gov.au/Parliamentary_Business/Committees/Senate/Community_Affairs/MeshImplants/Report.
Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4–20. https://doi.org/10.1002/nau.20798.
Weber AM, Walters MD, Piedmonte MR, Ballard LA. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185(6):1299–304; discussion 1304-1296. https://doi.org/10.1067/mob.2001.119081.
Haylen BT, Freeman RM, Swift SE, Cosson M, Davila GW, Deprest J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint terminology and classification of the complications related directly to the insertion of prostheses (meshes, implants, tapes) and grafts in female pelvic floor surgery. Neurourol Urodyn. 2011;30(1):2–12. https://doi.org/10.1002/nau.21036.
Daly J, Ahern S, Herkes R, O'Connell H. The Australasian pelvic floor procedure registry: not before time. Aust N Z J Obstet Gynaecol. 2019;59:473–6.
Bjelic-Radisic V, Aigmueller T, Preyer O, Ralph G, Geiss I, Muller G, et al. Vaginal prolapse surgery with transvaginal mesh: results of the Austrian registry. Int Urogynecol J. 2014;25(8):1047–52. https://doi.org/10.1007/s00192-014-2333-x.
Bradley CS, Visco AG, Weber LeBrun EE, Barber MD. The pelvic floor disorders registry: purpose and development. Female Pelvic Med Reconstr Surg. 2016;22(2):77–82. https://doi.org/10.1097/SPV.0000000000000254.
Hansen MF, Lose G, Kesmodel US, Gradel KO. Repeat surgery after failed midurethral slings: a nationwide cohort study, 1998-2007. Intl Urogynecol J. 2016;27(7):1013–9. https://doi.org/10.1007/s00192-015-2925-0.
Weber LeBrun E, Adam RA, Barber MD, Boyles SH, Iglesia CB, Lukacz ES, et al. Pelvic floor disorders registry: study design and outcome measures. Female Pelvic Med Reconstr Surg. 2016;22(2):70–6. https://doi.org/10.1097/SPV.0000000000000237.
Wilcox N, McNeil JJ. Clinical quality registries have the potential to drive improvements in the appropriateness of care. Med J Aust. 2016;205(10):S27–s29.
Evans SM, Bohensky M, Cameron PA, McNeil J. A survey of Australian clinical registries: can quality of care be measured? Intern Med J. 2011;41(1a):42–8. https://doi.org/10.1111/j.1445-5994.2009.02068.x.
Md Emdadul Hoque D, Ruseckaite R, Lorgelly P, McNeil JJ, Evans SM. Cross-sectional study of characteristics of clinical registries in Australia: a resource for clinicians and policy makers. Int J Qual Health Care. 2018;30(3):192–9. https://doi.org/10.1093/intqhc/mzx196.
Australian Parliament, Senate, Community Affairs References Committee, Siewert R. Number of women in Australia who have had transvaginal mesh implants and related matters, 2018. https://www.aph.gov.au/Parliamentary_Business/Committees/Senate/Community_Affairs/MeshImplants.
Håkonsen SJ, Pedersen PU, Bygholm A, Peters MD, Bjerrum M. Speaking the same language: development of a nutrition minimum data set for healthcare professionals in primary healthcare. Health Informatics J. 2020;26(1):248–63. https://doi.org/10.1177/1460458218824707.
Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73. https://doi.org/10.7326/m18-0850.
Costantini E, Zucchi A, Lazzeri M, Del Zingaro M, Vianello A, Porena M. Managing mesh erosion after abdominal pelvic organ prolapse repair: ten years' experience in a single center. Urol Int. 2011;86(4):419–23. https://doi.org/10.1159/000324243.
Moran P, Foon R, Assassa P. The BSUG national database: concept, design, implementation and beyond. Obstet Gynaecol. 2013;15:120–7.
Patil A, Moran P, Duckett J, British Society of Urogynaecology Audit C. How do urogynaecologists treat failed suburethral slings? Experience from the British Society of Urogynaecology database and literature review. J Obstet Gynaecol. 2011;31(6):514–7. https://doi.org/10.3109/01443615.2011.581319.
Trochez RD, Lane S, Duckett J. The use of synthetic mesh for vaginal prolapse in the UK: a review of cases submitted to the British Society of Urogynaecology database. Intl Urogynecol J. 2018;29(6):899–904. https://doi.org/10.1007/s00192-018-3595-5.
Dyrkorn OA, Kulseng-Hanssen S, Sandvik L. TVT compared with TVT-O and TOT: results from the Norwegian National Incontinence Registry. Int Urogynecol J. 2010;21(11):1321–6. https://doi.org/10.1007/s00192-010-1195-0.
Moe K, Schiotz HA, Kulseng-Hanssen S. Outcome of TVT operations in women with low maximum urethral closure pressure. Neurourol Urodynam. 2017;36(5):1320–4. https://doi.org/10.1002/nau.23044.
Moksnes LR, Svenningsen R, Schiotz HA, Moe K, Staff AC, Kulseng-Hanssen S. Sling mobilization in the management of urinary retention after mid-urethral sling surgery. Neurourol Urodynam. 2017;36(4):1091–6. https://doi.org/10.1002/nau.23046.
Carter-Brooks CM, Lowder JL, Du AL, Lavelle ES, Giugale LE, Shepherd JP. Restoring genital Hiatus to normative values after apical suspension alone versus with level 3 support procedures. Female Pelvic Med Reconstr Surg. 2019;25(3):226–30. https://doi.org/10.1097/SPV.0000000000000528.
Cashman S, Biers S, Greenwell T, Harding C, Morley R, Cooper D, et al. Results of the British Association of Urological Surgeons female stress urinary incontinence procedures outcomes audit 2014-2017. BJU Intl. 2019;123(1):149–59. https://doi.org/10.1111/bju.14541.
Le Brun EW, Adam RA, Barber MD, Boyles SH, Iglesia CB, Lukacz ES, et al. Pelvic floor disorders registry: study design and outcome measures. Female Pelvic Med Reconstr Surg. 2016;22(2):70–6. https://doi.org/10.1097/SPV.0000000000000237.
Guldberg R, Brostrom S, Hansen JK, Kaerlev L, Gradel KO, Norgard BM, et al. The Danish urogynaecological database: establishment, completeness and validity. Intl Urogynecol J Pelvic Floor Dysfunct. 2013;24(6):983–90. https://doi.org/10.1007/s00192-012-1968-8.
Barski D, Gerullis H, Ecke T, Kranz J, Schneidewind L, Leistner N, et al. Registry of implants for the reconstruction of pelvic floor in males and females: a feasibility case series. Intl J Surg. 2017;42:27–33. https://doi.org/10.1016/j.ijsu.2017.04.028.
Bohlin KS, Ankardal M, Nussler E, Lindkvist H, Milsom I. Factors influencing the outcome of surgery for pelvic organ prolapse. Intl Urogynecol J. 2018;29(1):81–9. https://doi.org/10.1007/s00192-017-3446-9.
Cantrell AB, Rothschild J, Durbin-Johnson B, Gonzalez R, Kurzrock EA. Surgical trends in the correction of female stress urinary incontinence in academic centers within the United States. Neurourol Urodynam. 2017;36(2):394–8. https://doi.org/10.1002/nau.22940.
Gupta P, Gaines N, Bartley J, Ehlert M, Gilleran J, Fischer M, Killinger KA, Sirls LT (2016) Stress urinary incontinence after robotic assisted prolapse repair. Neurourol Urodynam 1):S102-S103. https://doi.org/10.1002/nau.22967
Karmakar D, Hayward L, Smalldridge J, Lin S. Vaginal mesh for prolapse: a long-term prospective study of 218 mesh kits from a single Centre. Intl Urogynecol J Pelvic Floor Dysfunct. 2015;26(8):1161–70. https://doi.org/10.1007/s00192-015-2658-0.
Simon M, Debodinance P (2011) Vaginal prolapse repair using the Prolifttm kit: A registry of 100 successive cases. Intl Urogynecol J Pelvic Floor Dysfunct 3):S1894. https://doi.org/10.1007/s00192-011-1521-1
Moskowitz D, Gioia KT, Wolff EM, Massman JD, Lucioni A, Kobashi KC, et al. Analysis of the completely dry rate over time after mid-urethral sling in a real-world clinical setting. Urology. 2019;126:65–9. https://doi.org/10.1016/j.urology.2018.12.036.
Tincello D, Lucente V, Khandwala S, Botha T, Grier D, Urquhart C, et al. One year results from a world-wide registry of TVT-SECURTM in women with stress urinary incontinence (SUI). Neurourol Urodynam. 2010;29(6):1033–4. https://doi.org/10.1002/nau.20973.
Lavelle RS, Christie AL, Alhalabi F, Zimmern PE. Risk of prolapse recurrence after native tissue anterior vaginal suspension procedure with intermediate to long-term Followup. J Urol. 2016;195(4):1014–20. https://doi.org/10.1016/j.juro.2015.10.138.
de Landsheere L, Smajda S, Oberweis D, Keuller H, Dehon S, Smets M, et al. Management of pelvic organ prolapse in French-speaking Belgium: the EPILAPSUS study. Gynecol Surg. 2016;13(3):165–72. https://doi.org/10.1007/s10397-016-0948-2.
Carter EJ, Pallin DJ, Mandel L, Sinnette C, Schuur JD. Emergency department catheter-associated urinary tract infection prevention: multisite qualitative study of perceived risks and implemented strategies. Infect Control Hosp Epidemiol. 2016;37(2):156–62. https://doi.org/10.1017/ice.2015.267.
Barski D, Arndt C, Gerullis H, Yang J, Boros M, Otto T, et al. Transvaginal PVDF-mesh for cystocele repair: a cohort study. Intl J Surg. 2017;39:249–54. https://doi.org/10.1016/j.ijsu.2017.02.006.
Karmakar D, Mostafa A, Abdel-Fattah M (2015) A new validated score for detecting patient-reported success on postoperative ICIQ-SF: A novel two-stage analysis from two large RCT cohorts. International Urogynecology Journal and Pelvic Floor Dysfunction 1):S81-S82. https://doi.org/10.1007/s00192-015-2713-x
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. https://doi.org/10.1097/01.sla.0000133083.54934.ae.
Barski D, Gerullis H, Georgas E, Bar A, Lammers B, Ramon A, et al. Coating of mesh grafts for prolapse and urinary incontinence repair with autologous plasma: exploration stage of a surgical innovation. BioMed Res Intl. 2014;2014:296498. https://doi.org/10.1155/2014/296498.
Collinet P, Ciofu C, Costa P, Cosson M, Deval B, Grise P, et al. The safety of the inside-out transobturator approach for transvaginal tape (TVT-O) treatment in stress urinary incontinence: French registry data on 984 women. Intl Urogynecol J. 2008;19(5):711–5. https://doi.org/10.1007/s00192-007-0514-6.
Rawlings T, Lavelle RS, Coskun B, Alhalabi F, Zimmern PE. Prolapse recurrence after transvaginal mesh removal. J Urol. 2015;194(5):1342–7. https://doi.org/10.1016/j.juro.2015.06.080.
Singla N, Aggarwal H, Foster J, Alhalabi F, Lemack GE, Zimmern PE. Management of urinary incontinence following suburethral sling removal. J Urol. 2017;198(3):644–9. https://doi.org/10.1016/j.juro.2017.02.3341.
Lavelle RS, Christie AL, Alhalabi F, Zimmern PE. Risk of prolapse recurrence after native tissue anterior vaginal suspension procedure with intermediate to long-term Followup. J Urol. 2016;195(4 Pt 1):1014–20. https://doi.org/10.1016/j.juro.2015.10.138.
Rawlings T, Christie A, Zimmern PE (2015) Cost analysis of the anterior vaginal wall suspension procedure in the repair of stress urinary incontinence with early grade anterior compartment prolapse. Neurourology and Urodynamics 1):S73. https://doi.org/10.1002/nau.22738
Madbouly KM, Youssef M. Laparoscopic ventral Rectopexy versus laparoscopic Wells Rectopexy for complete rectal prolapse: long-term results. J Laparoendosc Adv Surg Tech. 2018;28(1):1–6. https://doi.org/10.1089/lap.2017.0012.
Oversand SH, Staff AC, Spydslaug AE, Svenningsen R, Borstad E. Long-term follow-up after pelvic organ prolapse operations: results and need for reoperation. Neurourol Urodynam. 2012;31(6):853–5. https://doi.org/10.1002/nau.22287.
Simon M, Debodinance P. Vaginal prolapse repair using the Prolift™; kit: a registry of 100 successive cases. Eur J Obstet Gynecol Reproduct Biol. 2011;158(1):104–9. https://doi.org/10.1016/j.ejogrb.2011.04.027.
Withagen MI, Vierhout ME, Hendriks JC, Kluivers KB, Milani AL. Risk factors for exposure, pain, and dyspareunia after tension-free vaginal mesh procedure. Obstet Gynecol. 2011;118(3):629–36. https://doi.org/10.1097/AOG.0b013e31822ada95.
McKenna JB, Parkin K, Cheng Y, Moore KH. Objective efficacy of the tension-free vaginal tape in obese/morbidly obese women versus non-obese women, at median five year follow up. Aust N Z J Obstet Gynaecol. 2016;56(6):628–32. https://doi.org/10.1111/ajo.12516.
ABS (Australian Bureau of Statistics) 2017. Private hospitals A, 2015–16. ABS cat. no. 4390.0. Canberra: ABS. https://www.abs.gov.au/statistics/health/health-services/private-hospitals-australia/latest-release.
Kowalik CR, Lakeman MME, de Kraker AT, Roovers J. Effects of mesh-related complications in vaginal surgery on quality of life. Int Urogynecol J. 2019;30(7):1083–9. https://doi.org/10.1007/s00192-018-3680-9.
Hill AM, Pauls RN, Kleeman SD, Shatkin-Margolis A, Crisp CC (2018) National practice patterns regarding apical support procedures at time of hysterectomy for pelvic organ prolapse: A nsqipanalysis. Female Pelvic Medicine and Reconstructive Surgery 24 (5 Supplement 1):S102. https://doi.org/10.1097/SPV.0000000000000625
Hill B, Fletcher S, Blume J, Adam R, Ward R. Volume at first leak is associated with sling failure among women with stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2019;25(4):294–7. https://doi.org/10.1097/SPV.0000000000000549.
Funding
This work is supported by the Australian Department of Health.
Author information
Authors and Affiliations
Contributions
R Ruseckaite: Protocol development, Data collection/extraction, Manuscript writing.
JO Daly: Protocol development, Manuscript editing.
J Dean: Manuscript editing.
S Ahern: Manuscript editing.
Corresponding author
Ethics declarations
Financial disclaimer/conflict of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ruseckaite, R., Daly, J.O., Dean, J. et al. Outcomes collected in female pelvic floor surgical procedure registries and databases: a scoping review. Int Urogynecol J 32, 3113–3130 (2021). https://doi.org/10.1007/s00192-021-04839-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-021-04839-2