Abstract
Introduction and hypothesis
Intraabdominal pressure acts on the pelvic floor through an aperture surrounded by bony and muscular structures of the pelvis. A small pilot study showed the area of the anterior portion of this plane is larger in pelvic organ prolapse. We hypothesize that there is a relationship between prolapse and anterior (APA) and posterior (PPA) pelvic cross-sectional area in a larger, more diverse population.
Study design
MRIs from 30 prolapse subjects and 66 controls were analyzed in this case-control study. The measurement plane was tilted to approximate the level of the levator ani attachments. Three evaluators made measurements. Patient demographic characteristics were compared using Wilcoxon rank-sum and Fisher’s exact tests. A multivariable logistic regression model identified factors independently associated with prolapse.
Results
Controls were 3.7 years younger and had lower parity, but groups were similar in terms of race, height, and BMI. Cases had a larger APA (p < 0.0001), interspinous diameter (ISD) (p = 0.001), anterior-posterior (AP) diameter (p = 0.01), and smaller total obturator internus muscle (OIM) area (p = 0.002). There was no difference in the size of the PPA(p = 0.12). Bivariate logistic regression showed age (p = 0.007), parity (p = 0.009), ISD (p = 0.002), AP diameter (p = 0.02), APA (p < 0.0001), and OIM size (p = 0.01) were significantly associated with prolapse; however, PPA was not (p = 0.12). After adjusting for age, parity, and major levator defect, prolapse was significantly associated with increased anterior pelvic area (p = 0.001).
Conclusions
We confirm that a larger APA and decreasing OIM area are associated with prolapse. The PPA was not significantly associated with prolapse.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
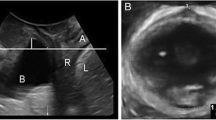
There are many factors involved in causing pelvic organ prolapse (POP), with age, parity, operative vaginal delivery, and levator ani injury being the most commonly cited [1,2,3]. Factors that affect the loads placed on the pelvic floor could also contribute. Abdominal pressure acts on the pelvic floor whose aperture is bordered by the pubic bone anteriorly, sacrum, sacrospinous ligaments and coccygeus muscles posteriorly, and obturator internus muscles laterally (Fig. 1a–c). Given that force equals pressure multiplied by the area to which it is applied, it may be inferred that changes in area with similar pressures would result in linear changes in force. Thus, it may stand to reason that women with larger pelvic floor areas would be subject to higher forces because of intra-abdominal pressure compared to women with smaller pelvic floor areas, potentially leading to development of pelvic organ prolapse.
a Intraabdominal pressure (IAP) translates to increased force on the pelvic floor (PF). b Plane at the level of the insertion of the levator ani muscles. c Study measures: white circle = inferior pubic point, yellow triangles = ischial spines, white dashed lines = obturator internus muscles (OIM), red solid line = anterior pelvic area, white dashed-dot line = interspinous diameter, blue solid line = posterior pelvic area, white solid line = anterior-posterior diameter
Individual variations in the bony pelvic shape or muscle bulk have been associated with pelvic organ prolapse [4,5,6,7]. These variations can affect the size of the pelvic floor aperture. This area is significant because it is spanned by the levator ani muscle attachments to the arcus tendinous fascia pelvis, and it is well known that levator ani injury or avulsion from this attachment is associated with pelvic organ prolapse [3].
A technique development pilot study performed by members of the Society of Gynecologic Surgeons Pelvic Anatomy Group-Imaging (SGS-PAG Imaging) showed that the anterior portion of this plane is 17% larger in women with pelvic organ prolapse [7]. In the pilot study, we were able to demonstrate a relationship between POP and the cross-sectional area of the pelvic floor at the level at which levator injury and paravaginal separation occur by performing the measurement in an oblique rather than an orthogonal plane. However, that study was limited by the small sample size, which prevented evaluation of additional relationships such as how the posterior cross-sectional area and obturator internus cross-sectional area measured in the oblique plane relate to POP. In addition, in this study the posterior area of this plane had not been previously assessed. Racial differences in the posterior bony pelvic dimensions have been reported in prior studies and so could be relevant [5]. The current study aims to (1) repeat our analysis of the anterior pelvic area in a larger sample to see if our findings can be replicated, (2) determine whether reduced obturator bulk is associated with a larger aperture, (3) determine if the posterior pelvic opening is different between women with and without prolapse, and (4) to perform multivariable analysis to look for associations among several factors.
Materials and methods
This was a case-control mechanistic study using methods similar to the pilot investigation [7]. MR images came from the Michigan Pelvic Floor Research Group MRI Repository. This collection contains images from female participants in multiple National Institute of Health-funded research studies on pelvic floor disorders. Women with and without pelvic floor disorders in these studies had been recruited to be demographically similar. The intent of the present study a priori was to perform a 2:1 match of controls to cases based on age and parity. Equal numbers of subjects with anterior and posterior predominant prolapse were included in the study. A total of 30 cases and controls from the original study were included in the current study; however, new measurements of these patients were made. Using a 7 cm2 (12%) difference in anterior pelvic area as determined in Sammarco et al. 2019, a sample size of 20, or 10 per group, is needed to obtain statistical power at the recommended 80% level with 95% two-tailed confidence interval. Because other measurements of interest in this plane are smaller than the anterior pelvic area, and due to the intention to diversify the study population in terms of parity and age, additional patients were included in the case and control groups.
Pelvic MRIs from women with and without prolapse were included in the current study. Prolapse was defined as the leading edge of the vagina at least 1 cm beyond the hymen on POP-Q examination. All patients had a uterus in situ and had not had any prior surgery for pelvic floor disorders. Women with pelvic floor disorders other than prolapse were excluded from the study. Demographic data were assessed as well as clinical data including the Pelvic Organ Prolapse Quantification scale. Levator ani defects were scored on a scale of 0–3 on each side of muscle attachment, with a total possible score of 6 for any given patient. A score of 0 on one side indicates no muscle detachment from the insertion, while a score of 3 represents complete detachment of the muscle from its insertion. Major levator ani defect was defined as a unilateral score of 3 or a combined score of 4 or more in an individual. The current study (HUM00144643) was approved by University of Michigan Institutional Review Board.
The imaging protocols have been previously published [3, 8,9,10,11]. To summarize, in older patients with and without prolapse, MRI images were obtained using either a 1.5-T or 3-T Philips Achieva scanner (Philips Medical Systems, Best, The Netherlands). For primiparous and mulitparous controls, proton density 2D fast spin images of the pelvic organs were obtained using a 1.5-T superconducting magnet (Signa; General Electric Medical Systems, Milwaukee, WI). For nulliparous patients, proton density weighted fast spin imaging was performed using a 3-T Ingenia MRI scanner (Philips Medical Systems, Best, The Netherlands).
Using 3D Slicer software (v 4.5.0, www.slicer.org, Brigham and Women’s Hospital, Boston, MA), the measurement plane was tilted to include the ischial spines and the inferior pubic point, approximating the level of the levator ani attachments (Fig. 1b,c). The borders of the measurements can be seen in Fig. 1c and include: the interspinous diameter, the anterior-posterior (AP, distance between inferior pubic point and sacrum or coccyx) diameter, the cross-sectional area of the obturator internus muscles, the anterior pelvic area (bordered by the pubic bone anteriorly, the medial edge of the obturator internus muscles laterally, and the interspinous diameter posteriorly), and the posterior pelvic area (bordered by the interspinous diameter anteriorly, the sacrum or coccyx posteriorly, and the medial edge of the coccygeus muscle laterally).
Three raters from the SGS-PAG Imaging made independent measurements, and interrater reliability was assessed by calculating the intra-class correlation coefficient (ICC). Each rater was trained in the use of the software and measurements over a series of screen-sharing conference calls and in-person meetings. All variables were summarized using descriptive statistics such as means, medians and proportions as necessary. The normality of all continuous variables was assessed after observation of skewness and kurtosis values and the Shapiro-Wilk test. Results from normally distributed variables were presented with mean and standard deviation and non-normally distributed variables with median and interquartile range. Bivariate analysis was performed to compare demographic characteristics and pelvic area measurements between women with and without prolapse. Comparisons of categorical variables were performed using chi-square tests or Fisher’s exact test, where appropriate. Continuous variables were compared using Student’s t-test or Wilcoxon rank test, where appropriate. Variables independently associated with prolapse from bivariate analysis and other clinically relevant variables were considered candidates for multivariable logistic regression modeling. All data management and analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC), and p < 0.05 was used to indicate statistical significance.
Results
Sixty-six women without and 30 women with prolapse were included in the study. Controls were an average of 3.7 years younger than cases (median [interquartile range] controls: 54.1 [32.1–59.7] vs. cases: 57.8 [52.4–63.6], p = 0.02) and had lower parity (controls: 1 [1–2] vs. cases: 2 [1–3], p = 0.01). Groups were similar in terms of race, height, and BMI (Table 1). As intended with our study design, prolapse patients had a median prolapse size of 2 [1–3] cm beyond the hymen, while all controls had a maximum prolapse that was 2 [−2.5 – −1] cm above the hymen (p < 0.0001). Genital hiatus on physical examination was 67% larger in women with prolapse compared to controls, and there were three times more major levator ani defects in the prolapse group (Table 1). The left and right obturator internus muscles had similar cross-sectional areas comparing either the prolapse or control group, so left and right muscles were added for the remainder of the analysis (controls: 7.8 ± 1.4 cm2 left vs.7.7 ± 1.6 cm2 right, p = 0.65; cases: 6.9 ± 1.5 cm2 left vs. 6.9 ± 1.7 cm2 right, p = 0.97). The intraclass correlation coefficient was 0.92, indicating excellent interrater reliability.
Patients with prolapse had a 4.7% larger interspinous diameter (p = 0.001), 3.4% larger AP diameter (p = 0.01), 12.5% larger anterior pelvic area (p < 0.0001), and 13.9% smaller obturator internus area (p = 0.002) compared to controls (Table 1). The difference in the posterior pelvic area measurements between women with and without prolapse did not reach statistical significance (p = 0.12, Table 1). The total pelvic area (sum of anterior and posterior pelvic areas) was 10.5% larger in prolapse patients than in controls (95.1 ± 6.9 cm2 vs. 86.1 ± 8.4 cm2, p < 0.0001), largely because of the 12.5% larger anterior pelvic area.
Bivariate logistic regression showed that anterior pelvic area, total pelvic area, and obturator internus size were significantly associated with prolapse as well as age, parity, genital hiatus, major levator defect, interspinous diameter, and anterior-posterior diameter (Table 2). Posterior pelvic area was not significantly associated with prolapse [OR 1.06, (95% CI: 0.99, 1.14), p = 0.12]. Variation in genital hiatus and levator defect score accounted for the most amount of variation in prolapse (r2 = 0.39 and 0.29, respectively, Table 2).
Because interspinous diameter, AP diameter, and obturator internus are all borders and dimensions of the anterior pelvic area and therefore colinear, only anterior pelvic area was included in the final logistic regression model. While total pelvic area is also significantly associated with prolapse, this is largely because of the changes in the anterior pelvic area, as the posterior pelvic area changes were not significantly associated with prolapse in bivariate regression. After adjusting for age, parity, and major levator defect, prolapse was significantly associated with increased anterior pelvic area [OR 1.27 (95% CI: 1.10, 1.46), p = 0.001, Table 3].
Discussion
We confirm that the anterior pelvic area is 12% larger in women with prolapse than in those without, a fact that would be consistent with a similarly larger load that it is subjected to for any given abdominal pressure. Many of the other study measures, which include the borders of the anterior pelvic area (i.e., interspinous diameter, anterior-posterior diameter, and obturator internus size), were significantly associated with prolapse in our bivariate analysis. However, the r2 values for these were much lower than that of the anterior pelvic area. The posterior measurements of this plane were not significantly associated with prolapse. This indicates that the anterior pelvic area is likely the more important measurement to consider when evaluating the effect of pressure on the pelvic floor.
The anterior pelvic area, a composite of both bony and muscular borders of the cross-sectional area at the level of the levator ani muscle insertion, was 12% larger in prolapse patients compared to controls in this study. This is consistent with a prior study that showed that this same area was 14.4% larger in patients with prolapse compared to similarly aged primiparous controls [7]. The current study builds upon the prior literature by confirming this finding in a larger sample size of varying parity compared to the prior study. This area explains 26% of the variation in prolapse and was the strongest of our study measures. The total pelvic area, which consisted of the sum of the anterior and posterior pelvic areas, is 10.5% larger in prolapse, mainly because of the variation in anterior pelvic area, as the posterior area was not significantly different between women with and without prolapse.
The cross-sectional area of the obturator internus muscles was 14% smaller in prolapse, accounting for 8% of the variation in prolapse. Prior studies looking at this measure showed that it was 11–15% smaller in prolapse, but were unable to show significance presumably because of inadequate power [7]. The present study confirms that these muscles are in fact smaller in patients with prolapse. The cause for this may be multifactorial with possible causes being age, sarcopenia, or a combination of both [12]. There is about a 25–40% decrease in muscle volume between the 3rd and 7th decade of life [12,13,14]. This means that the age difference of 3 years between our groups would only account for 1.5–3% difference in muscles size and that this finding is still important.
Many of the study measures, which included assessments of bony and muscular landmarks of the pelvis, were significantly different in women with prolapse; however, these measures are all related or form the borders of the anterior pelvic area and are therefore collinear. These measures also played a smaller role compared to the anterior pelvic area in accounting for the variation in prolapse on bivariate regression. The AP diameter was 3.3% larger in patients with prolapse, and while this small difference was significant, it accounted for only 6% of the variation in prolapse. The interspinous diameter was 5% larger in prolapse, which is also consistent with prior findings, [4, 6, 7, 15] and accounted for 11% of the variation in prolapse. Not all published data on the interspinous diameter are congruent, however, and some studies report no difference in this measure in women with and without prolapse [16].
Clinical Implications
This mechanistic study adds to our understanding of the clinical significance of the anterior pelvic area. The APA is measured at the level of the attachment of the levator ani muscles to the arcus tendineous fascia pelvis, making it an important area, as this is where levator injury occurs. The levator defect score and genital hiatus accounted for the most variation in prolapse in this cohort (29% and 39%, respectively), relationships which have been well established in prior literature [3, 17, 18]. Tracey et al. evaluated maternal birth canal capacity with biomechanical modeling and found that women with a smaller capacity are more likely to have a predicted levator ani injury than those with a larger capacity [19]. Conversely, levator ani defects have been associated with some smaller pelvic dimensions such as a shorter sacrococcygeal-inferior pubic point (SCIPP) line [20], and in this study we find that a smaller anterior pelvic area is associated with not having prolapse. These opposing but logical observations belie the complex interactions that can influence prolapse. A small pelvis predisposes to support structure injury, but a large pelvic aperture results in increased load. Therefore, it is possible that there is set of ideal pelvic dimensions that are protective against developing prolapse, which include a balance between having a pelvis large enough to deliver a baby without levator injury and having pelvic dimensions such that they limit the amount of force over time transferred to the pelvic floor. Further studies are needed to evaluate these relationships.
As with any new measurement, it will take time to learn how differences in the APA factor into the development, clinical management, and counseling regarding prolapse. It is unknown as of yet whether any of these measurements are modifiable in women with and without prolapse. This research adds specific data regarding one element of the pelvic organ support system that can be added to the growing number of observations about specific factors involved in causing prolapse. Now that a system for quantifying this feature is established and its relation to prolapse demonstrated, its interaction with other factors such as muscle atrophy, injury, pelvic bone and muscular structure, and force measurements on the pelvic floor can be carried out. In this study, there were no associations between prolapse and BMI. BMI contributes to increased intraabdominal pressure, which could then increase pressure and thereby force on the pelvic floor. Further studies are indicated to further explore whether any relationship exists between BMI, as an indicator of force, and anterior pelvic area.
Previous studies have shown in a smaller group of primiparous women that the novel measurement of the anterior pelvic area was a potentially important measure regarding pelvic organ prolapse but were under-powered to evaluate some of the other measurements in this plane. One strength of this study is that it includes a larger number of women from a diverse background of parity and age for both controls and cases. In this cohort we were able to confirm previous findings that anterior pelvic area is associated with prolapse and affirm that prior non-significant trends regarding the size of the obturator internus muscles are significant and important. This area measurement factors in the relationship of muscle size, which is known to change with age, in addition to the static bony dimensions of the pelvis [12]. By tilting the plane to include the bilateral ischial spines and inferior pubic point in each individual subject, we were able to overcome the effect of how subjects may be laying, and their individual pelvic tilt, in the MRI machine. The interrater reliability in this study was high, indicating that measurements in this novel plane are reproducible.
The study is limited by a lack of racial diversity. Our population was mostly Caucasian, limiting the generalizability of the findings, as prior studies have reported racial differences in similar pelvic floor measures [5, 21]. This is not a population-based sample, and despite aims to match based on age and parity, both were slightly lower in the control group. Age is known to affect pelvic floor support and obturator internus muscle size [10, 12]. There were also, as expected, fewer major levator ani defects in the control group compared to the cases. Therefore, we controlled for age, parity, and presence of major levator defects in our final model. The study was not powered to assess the relationship between anterior and posterior predominant prolapse with the study measures, and this is an area for future research. Many of the variables that were significantly associated with prolapse in bivariate regression were collinear with each other because of some measurements forming the borders of the pelvic cross-sectional area, thus limiting what could be included in the final logistic regression model. Because of this collinearity, however, these measures are reflected in the pelvic area measurements, and bivariate regression showed that the anterior pelvic area accounted for the largest percentage of variability in prolapse of the study measures.
The relationship among force, stress, and area is linear and most often relates to orthogonal application of stress to an area in question; while the direction of IAP on the APA approximates orthogonal application stress, the pelvic floor mechanism also experiences stress applied at oblique angles due to contraction of the supporting musculature [10]. Thus, another limitation of this study is the inability to control for these additional stressors as well as for tissue integrity, both of which likely contribute to the development of POP. Defining patients with prolapse as those with the lead point 1 cm or more beyond the hymen represents another potential limitation as the control group would likely include patients with prolapse, albeit non-visible or asymptomatic, thereby leading to a potential underestimation of the effect of the APA on development of prolapse. Future studies should include the leading point as an ordinal rather than a categorical variable.
We confirm that the anterior pelvic area is related to prolapse extending 1 cm or more beyond the hymen, after adjusting for levator ani defect and age. Reduced bulk of the obturator internus muscle contributes to this increase in area. Other factors that affect the size of this area include the muscular and boney dimensions that affect the borders, some of which change with age (muscle), while others remain relatively constant (bone). Understanding the relationship between pelvic structures and spaces and their relationship to the force on the pelvic floor is critical. A more complete and evidence-based understanding of pelvic floor biomechanics is needed to develop interventions for prevention and treatment.
References
Nygaard I, et al. Prevalence of symptomatic pelvic floor disorders in US women. Jama. 2008;300(11):1311–6.
Sze EH, Sherard GB 3rd, Dolezal JM. Pregnancy, labor, delivery, and pelvic organ prolapse. Obstet Gynecol. 2002;100(5 Pt 1):981–6.
DeLancey JO, et al. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109(2 Pt 1):295–302.
Sze EH, et al. Computed tomography comparison of bony pelvis dimensions between women with and without genital prolapse. Obstet Gynecol. 1999;93(2):229–32.
Baragi RV, et al. Differences in pelvic floor area between African American and European American women. Am J Obstet Gynecol. 2002;187(1):111–5.
Handa VL, et al. Architectural differences in the bony pelvis of women with and without pelvic floor disorders. Obstet Gynecol. 2003;102(6):1283–90.
Sammarco AG, et al. A novel measurement of pelvic floor cross-sectional area in older and younger women with and without prolapse. Am J Obstet Gynecol. 2019;221(5):521.e1–7.
Chen L, et al. Structural failure sites in anterior Vaginal Wall prolapse: identification of a collinear triad. Obstet Gynecol. 2016;128(4):853–62.
DeLancey JO, et al. The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet Gynecol. 2003;101(1):46–53.
Swenson CW, et al. Aging effects on pelvic floor support: a pilot study comparing young versus older nulliparous women. Int Urogynecol J. 2019.
DeLancey JO, et al. Stress urinary incontinence: relative importance of urethral support and urethral closure pressure. J Urol. 2008;179(6):2286–90 discussion 2290.
Morris VC, et al. A comparison of the effect of age on levator ani and obturator internus muscle cross-sectional areas and volumes in nulliparous women. Neurourol Urodyn. 2012;31(4):481–6.
Brooks SV, Faulkner JA. Skeletal muscle weakness in old age: underlying mechanisms. Med Sci Sports Exerc. 1994;26(4):432–9.
Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50 Spec No:11–6.
Brown KM, et al. Three-dimensional shape differences in the bony pelvis of women with pelvic floor disorders. Int Urogynecol J. 2013;24(3):431–9.
Stein TA, et al. Comparison of bony dimensions at the level of the pelvic floor in women with and without pelvic organ prolapse. Am J Obstet Gynecol. 2009;200(3):241.e1–5.
Andrew BP, et al. Enlargement of the levator hiatus in female pelvic organ prolapse: cause or effect? Aust N Z J Obstet Gynaecol. 2013;53(1):74–8.
Delancey JO, Hurd WW. Size of the urogenital hiatus in the levator ani muscles in normal women and women with pelvic organ prolapse. Obstet Gynecol. 1998;91(3):364–8.
Tracy PV, DeLancey JO, Ashton-Miller JA. A geometric capacity-demand analysis of maternal Levator muscle stretch required for vaginal delivery. J Biomech Eng. 2016;138(2):021001.
Berger MB, Doumouchtsis SK, Delancey JO. Are bony pelvis dimensions associated with levator ani defects? A case-control study. Int Urogynecol J. 2013;24(8):1377–83.
Handa VL, et al. Racial differences in pelvic anatomy by magnetic resonance imaging. Obstet Gynecol. 2008;111(4):914–20.
Funding
Supported by National Institutes of Health (NIH) ORWH SCOR grant P50 HD044406, the Eunice Kennedy Shriver National Institute of Child Health and Human Development R01 HD038665 and National Institute of Diabetes, Digestive, and Kidney Diseases R01 DK51405 and R21HD079908. Investigator support for C.W.S. was provided by the National Institute of Child Health and Human Development WRHR Career Development Award K12 HD065257. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper was presented as a Poster at the 46th Annual Scientific meeting of the Society of Gynecologic Surgeons in Jacksonville, FL, July, 2020.
Rights and permissions
About this article
Cite this article
Sammarco, A.G., Sheyn, D., Hong, C.X. et al. Pelvic cross-sectional area at the level of the levator ani and prolapse. Int Urogynecol J 32, 1007–1013 (2021). https://doi.org/10.1007/s00192-020-04546-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-020-04546-4