Abstract
Introduction and hypothesis
The International Consultation on Incontinence Questionnaire Overactive Bladder Symptoms Quality of Life (ICIQ-OABqol) Module evaluates the quality of life of individuals with overactive bladder (OAB) symptoms, and its use in scientific studies and clinical practice is recommended by the International Continence Society. The aim was to conduct the cross-cultural adaptation and validation of the Brazilian Portuguese version of the ICIQ-OABqol (ICIQ-OABqol_portuguese) in individuals with OAB symptoms.
Methods
An observational cross-sectional study was performed at the Clinical Physiotherapy of PUC MINAS in Belo Horizonte, MG, Brazil, between March 2017 and October 2018. The translation was previously carried out by the Mapi Research Institute. After receiving the translated questionnaire, the cross-cultural adaptation process was conducted as follows: (1) review by an expert committee (13 experts); (2) pre-test (n = 30); (3) cross-cultural adaptation; (4) validation of the ICIQ-OABqol_portuguese. We analyzed the intraexaminer reliability validation (n = 118) and internal consistency measurement (Cronbach’s α coefficient), test-retest reliability (ICC) and correlation between the ICIQ-OAB and ICIQ-OABqol_portuguese questionnaires through Pearson’s correlation coefficient and Bland-Altman scatter plot and concordance. Confirmatory factor analysis was used to confirm the domains of the instrument.
Results
The ICIQ-OABqol was cross-culturally adapted to Brazilian Portuguese and presented satisfactory internal consistency (α-Cronbach coefficient 0.88), adequate construct validity, strong reliability considering the test-retest with an interval of 19.68 (±6.98) days and moderate correlation with the ICIQ-OAB.
Conclusions
The Brazilian Portuguese version of the ICIQ-OABqol shows satisfactory psychometric properties and can be used to evaluate the quality of life of individuals of both sexes with OAB symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The International Continence Society (ICS) defines overactive bladder (OAB) as a clinical syndrome characterized by urinary urgency often associated with increased frequency and nocturia, whether or not accompanied by urinary incontinence, in the absence of any urinary tract infection or other obvious disease [1].
According to the latest consensus from ICS, OAB symptoms are generally present in 16.5% of the population (3–41% in women and 2–35% in men) [2]. For the Brazilian population, Teloken et al. (2006) reported a symptom prevalence of 23.2% in women and 18.9% in men [3], while Soler et al. (2018) reported 24% in women and 25% in men [4]. The higher incidence among women may be due to obstetric history, which involves the route and number of deliveries and the concomitant presence of other pelvic floor disorders such as pelvic organ prolapse associated with age, hormonal conditions and other comorbidities, e.g., obesity [5, 6].
OAB symptoms tend to be costly, having a negative effect on the quality of life of individuals and affecting interpersonal relationships and self-esteem, which leads to social isolation [7]. These symptoms can trigger anxiety and depression, reduce work productivity and have a significant impact on the mental and physical health of individuals [8].
The ICS recommends that quality of life assessments be included in both population surveys and clinical practice at different levels of health care to provide a better understanding of this clinical condition and its impact on the daily routine of individuals with urinary symptoms [9]. To achieve this, specific questionnaires validated to measure quality of life are useful, practical, reliable and inexpensive [9, 10].
To assess the impact of OAB symptoms on quality of life, Coyne et al. (2002) developed the instrument entitled International Consultation on Incontinence Questionnaire Overactive Bladder Symptoms Quality of Life (ICIQ-OABqol) Module, a self-administered questionnaire divided into four domains: sleep, social interaction, concern and coping with symptoms. The questionnaire showed consistency, validity and responsiveness to assess quality of life in individuals with OAB symptoms [10].
To date, there is no instrument adapted and validated to Brazilian Portuguese that specifically assesses the impact of OAB symptoms on quality of life. A validated instrument will increase the knowledge of health professionals, incorporating the individual’s point of view in the evaluation and treatment plan. The purpose of the present study was to adapt and validate the Brazilian Portuguese version of the ICIQ-OABqol.
Methods
The proposal for this observational cross-sectional study was presented to the Ethics Committee of the Pontifical Catholic University of Minas Gerais–PUC-Minas, and the study was approved, following the ethical and legal precepts (CAAE register no. 2.482.999).
Description of the ICIQ-OABqol
The ICIQ-OABqol is a self-administered questionnaire of the ICIQ platform (International Consultation on Incontinence Questionnaire) Module [9] developed by Coyne et al. [10]. It received a grade A recommendation because of its robust psychometric validation for investigation of the impact of OAB symptoms on the quality of life of men or women [9]. The questionnaire has 28 questions divided into four domains: sleep, social interaction, concern and coping with symptoms. The first 27 questions have 6 alternative answers (none of the time: 1; a little of the time: 2; some of the time: 3; a good bit of the time: 4; most of the time: 5; all of the time: 6), while question 28 investigates the impact of urinary symptoms on the individual’s daily routine, rating it on a scale of 0 (no impact) to 10 (severe impact) [10, 11].
Translation, cross-cultural adaptation and validation of the ICIQ-OABqol
The process of translation, synthesis and back-translation of the ICIQ-OABqol was carried out in 2006 by the Mapi Research Institute along with a team of Brazilian professionals. The institute performed this process for several languages, including Brazilian Portuguese, following the reference of the Manual for Patient-Reported Outcomes (PRO) [12, 13].
After permission had been given by the authors and delivery of the translated version of the questionnaire, the process of cross-cultural adaptation of the ICIQ-OABqol followed the methodology described by Beaton et al. [14]. The validation process was performed using the criteria established by Terwee et al. [15] and by the international standards of the ICIQ Module platform [9], which were also used in the original article by Coyne et al. [10].
Cross-cultural adaptation and content validation for the ICIQ-OABqol
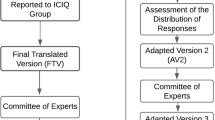
The questionnaire was fully evaluated by a committee of bilingual experts [16], composed of seven physicians and six physiotherapists. At this stage, both translations of the ICIQ-OABqol, the original English version and the version translated into Portuguese, were made available through an online platform and forwarded independently to each member of the committee of experts, accompanied by a letter of invitation. The document contained instructions for the interviewer and the questions of the ICIQ-OABqol in both versions. Each member of the committee was asked to evaluate the relevance of all items in the translation of the questionnaire. In case of disagreement with any of the terms or expressions in the document, members were instructed to suggest changes to improve clarity [16]. The level of agreement by experts with the translated questions of the ICIQ-OABqol was assessed by the concordance percentage (CP) and the content validity index (CVI). We considered a minimum 90% CP [17] and a minimum 0.8 CVI as adequate [18]. The CVI measures the proportion of experts that agree with a given aspect of an instrument and respective items. CVI uses a Likert scale with scores ranging from 1 to 4 in which 1 = item not equivalent; 2 = item needs major revision to be considered equivalent; 3 = item is equivalent but it needs minor changes; 4 = item is clearly equivalent. At the end of this stage, the first consensus was reached on the Portuguese version of the instrument, resulting in the first adapted version (V1-initial).
Pre-test
Subsequently, to identify issues with comprehension of the existing items by the population to which it is intended, the initial version of the questionnaire (V1-initial) was applied to 30 participants with symptoms of OAB, of both sexes, aged between 18 and 70 years [14]. Participants were recruited from the Clinical Center of Physiotherapy and Speech Therapy of the Pontifical Catholic University of Minas Gerais, after confirmation of the OAB symptoms using the International Consultation on Incontinence Questionnaire Overactive Bladder (ICIQ-OAB), an instrument that has already been validated for the Brazilian Portuguese language by Pereira et al. [19]. The ICIQ-OAB investigates the urinary symptoms related to OAB. The individuals who agreed to participate in the interview signed the Informed Consent Form and received instructions on the validation process. The pre-test phase allowed for adjustments of the items that presented the most issues with comprehension, generating the second adapted version (V2-pre-final).
Approval of the cross-cultural adaptation process
After completion of the second version, the committee of experts was consulted again to compare the two versions (V1-initial and V2-pre-final) to verify whether the questionnaire was adapted appropriately. Thus, the final version (V3-final) was generated as part of the last stage of the cross-cultural adaptation process. After ensuring semantic, idiomatic, experiential and conceptual equivalence of the ICIQ-OABqol instrument in Brazilian Portuguese, the final version was then named ICIQ-OABqol_portuguese.
Validation of the ICIQ-OABqol_portuguese questionnaire
To validate the instrument, individuals with OAB symptoms were selected at the Clinical Center of Physiotherapy and Speech Therapy of the Pontifical Catholic University of Minas Gerais. We recruited individuals with symptoms in the lower urinary tract in the bladder-filling phase (urgency, frequency or nocturia), assessed using the ICIQ-OAB [19]. The exclusion criteria were: confirmed pregnancy, individuals with neurologicaldiseases, cognitive alterations, and inability to read or speak in Brazilian Portuguese. Discontinuation criteria were: individuals who did not fully participate in the proposed activities in both interviews.
Sample size was calculated as proposed by Terwee et al. (2007), that is, multiplying the number of items in the instrument by 4 [15] (ICIQ-OABqol: 28 items × 4 = 112 participants). To ensure adequate sample size, we recruited 121 participants to account for possible loss to follow-up. Data from 118 participants were analyzed, after dropouts (n = 3). Those who agreed to participate in the interview signed the informed consent form and received instructions for the validation process.
The process was performed through an online platform (Google Forms electronic form), where both ICIQ-OAB and ICIQ-OABqol_portuguese were made available [19]. Participants answered both questionnaires on two occasions, the first interview and second interview, with a minimum interval of 15 days between them [9].
Data analysis
Data collected from the questionnaires in both interviews were organized on a spreadsheet (Microsoft EXCEL 2010®) and analyzed using the STATA (version 12.0) software programs and R Project for Statistical Computing (version 3.5). The Bland-Altman plot was analyzed and developed in STATA and the remaining statistical analysis in R.
Internal consistency and intra-observer reliability were analyzed. Inter-observer reliability was not analyzed as the questionnaire is self-administered, which minimizes a possible influence of interviewers.
Internal consistency was analyzed with Cronbach’s alpha coefficient statistical test to investigate the degree of interrelation between the items of the ICIQ-OABqol_portuguese [15, 20]. Appropriate values were those > 0.7 (p ≤ 0.05).
Confirmatory factor analysis (CFA), using the Kaiser-Meyer-Olkin test (KMO), was also analyzed, and close values in the test-retest were considered acceptable [21].
The intra-observer reliability (agreement of the responses after both interviews) of the ICIQ-OABqol_portuguese instrument was evaluated using a two-way, random, single-measure intraclass correlation coefficient [(ICC (2,1)] and significance when p ≤ 0.05. Values > 0.7 were considered suitable and confirmed homogeneity of the measures [22].
Pearson’s correlation coefficient was used to analyze the direct correlation between the domains of the ICIQ-OABqol_portuguese questionnaire and the specific questions of the ICIQ-OAB. Additionally, the Bland-Altman scatter plot was used to describe the agreement between the two interviews considering the average scores of each domain [23].
Results
The study included 121 participants with OAB symptoms identified using the ICIQ-OAB questionnaire [19]. Three participants who did not answer the questionnaire at retest were excluded from the study, leaving 118 individuals with a mean age of 42.46 (± 17.47) years, of which 105 were female (89%) and 13 male (11%).
The sample consisted mostly of individuals who declared themselves as white (69.5%), with at least 12 years of education (83.9%), single (39.3%) and family income of one to two times the minimum wage (29.9%). The most prevalent symptoms were nocturia (68.6%) and frequency (60.2%), followed by urinary urgency (58.5%).
Regarding the obstetric history of the 105 women participating in the study, 45.72% were nulliparous and 54.28% had at least one pregnancy. Among the women who had children, 24.56% were multiparous. Regarding the type of delivery, 61.40% had vaginal delivery, 22.81% had cesarean section, and 15.79% had both vaginal delivery and cesarean section.
Cross-cultural adaptation
The mean CP found was 80% (Fig. 1), which allowed for all questions to be retained; however, changes were needed to improve the comprehension of questions by the target population.
The CVI calculation was then performed (Table 1), as ten items received a score > 0.98 and were not modified by the committee of experts (V2-pre-final). However, after the pre-test, grammatical adaptation was considered necessary, such as the insertion of words in sentences or the replacement of terms by their synonyms. That decision was based on the analysis of comprehension of questions by the interviewees. To facilitate understanding, the expression “Seus sintomas urinários” (Did your urinary symptoms) was added to the beginning of questions 3 to 27. These modifications were carried out by the authors of the study (V3-final: ICIQ-OABqol_portuguese).
Instrument validation
Internal consistency
The ICIQ-OABqol_portuguese presented satisfactory internal consistency with a Cronbach’s alpha coefficient of 0.88, calculated with data from the first interview.
Construct validity
Construct validity of the ICIQ-OABqol_portuguese, assessed by the confirmatory factor analysis and KMO test, showed that the domains had adequate data adjustment and are in accordance with the domains of the original questionnaire developed and validated by Coyne et al. [10] (Table 2).
Reliability
Strong agreement was observed in the analysis of the domains of the ICIQ-OABqol_portuguese based on the first and second interviews, which were conducted 19.68 (± 6.98) days apart. Table 3 presents the reliability analysis (test-retest).
Criterion validity
The correlation between the ICIQ-OABqol_portuguese and ICIQ-OAB was analyzed using Pearson’s correlation coefficient and represented by the r-value, considering the first and second interviews (Table 4).
Additionally, the Bland-Altman plot was used to verify agreement, considering the distance between the average scores from the first and second interviews for each domain of the ICIQ-OABqol_portuguese (Fig. 2).
Discussion
The ICIQ-OABqol_portuguese is the translated, cross-culturally adapted and validated version of an instrument capable of specifically assessing the quality of life in individuals with OAB symptoms, whether or not associated with urinary incontinence, in four domains: sleep, social interaction, concern and coping with symptoms.
In Brazil, the shortage of formal, objective and reliable instruments designed to collect information in both scientific studies and clinical practice in various fields has favored the increasing use of international instruments. However, it is necessary to analyze the relevance of a translated international instrument considering the clinical context and the appropriateness of the questionnaire regarding the culture of the target population [17]. Cross-cultural adaptation requires the same methodological rigor used in the design of a new instrument to maintain reliability and validity [13, 14].
The ICIQ-OABqol is an instrument that evaluates quality of life and has more items than the ICIQ-OAB, which has already been validated in Brazilian Portuguese, with specific questions to evaluate OAB symptoms. Nevertheless, there was a high correlation between both questionnaires. The results for internal consistency of the ICIQ-OABqol_portuguese showed strong consistency according to Streiner [20]. The results also showed a high correlation between the instrument’s items according to the values described by Shrout et al. [22], similar to the original study by Coyne et al. [10].
The AFC confirmed the instrument’s dimensions with satisfactory factor loading for the ICIQ-OABqol_portuguese. The KMO test presented very good adjustment, as the domains’ values were high and close to each other, according to the values described in the literature [23]. Based on the findings of this study, the ICIQ-OABqol_portuguese questionnaire showed satisfactory results in its psychometric measurements compared with the original study by Coyne et al. [10].
Although OAB symptoms are more prevalent in the elderly population [3] and have similar prevalence between men and women, participants in the present study were young adults and mostly women, which can be justified by the characteristics of clients where the study took place. However, this discrepancy can be considered a limitation of our study. The most prevalent symptom found in the analyzed population was nocturia, and the highest Pearson correlation coefficient was for the domain “sleep” from the nocturia questions of the ICIQ-OABs, followed by the domain “concern.” Individuals who wake up and get up during the night to urinate have poor sleep quality, impacting their quality of life, compromising their well-being during the day and favoring the development of psychological and somatic diseases [24].
The Portuguese version of ICIQ-OABqol was adapted and presented high internal consistency and agreement, with moderate correlation, being considered a reliable instrument to investigate the quality of life of individuals of both sexes with symptoms of overactive bladder. The ICIQ-OABqol adaptation for the Brazilian context has provided a versatile instrument that can be applied in any clinical setting and also in scientific research.
Abbreviations
- CP:
-
Concordance percentage
- CFA:
-
Confirmatory factor analysis
- CVI:
-
Content validity index
- ICIQ-OABqol:
-
International Consultation on Incontinence Questionnaire Overactive Bladder Symptoms Quality of Life Module
- ICIQ-OAB:
-
International Consultation on Incontinence Questionnaire Overactive Bladder Symptoms
- ICS:
-
International Continence Society
- ICC:
-
Intraclass correlation coefficient
- KMO:
-
Kaiser-Meyer-Olkin
- PRO:
-
Patient-reported outcomes
- OAB:
-
Overactive bladder
- PUC MINAS:
-
Pontifical Catholic University of Minas Gerais
- UNICAMP:
-
State University of Campinas
References
Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26. https://doi.org/10.1007/s00192-009-0976-9.
Milsom I, Altman D, Cartwright R, Lapitan MC, Nelson R, Sjöström S, Tikkinen KAO. Epidemiology of Urinary Incontinence (UI) and other lower Urinary Tract Symptoms (LUTS), Pelvic Organ Prolapse (POP) and Anal (AI) Incontinence in Abrams P, Cardozo L, Wagg A, Wein A. Incontinence. 6th International Consultation on Incontinence, Tokyo, September, 2016, International Continence Society, 6th edn. (pp 49–54). Bristol: International Continence Society; 2017.
Teloken C, Caraver F, Weber FA, et al. Overactive bladder: prevalence and implications in Brazil. Eur Urol. 2006;49(6):1087–92. https://doi.org/10.1016/j.eururo.2006.01.026.
Soler R, Gomes CM, Averbeck MA, Koyama M. The prevalence of lower urinary tract symptoms (LUTS) in Brazil: results from the epidemiology of LUTS (Brazil LUTS) study. Neurourol Urodyn. 2018;37(4):1356–64. https://doi.org/10.1002/nau.23446.
Cheung WW, Khan NH, Choi KK, et al. Prevalence, evaluation and management of overactive bladder in primary care. BMC Fam Pract. 2009;10:8. https://doi.org/10.1186/1471-2296-10-8.
Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108:1132–9. https://doi.org/10.1111/j.1464-10X.2010.09993.x.
Irwin DE, Milsom I, Kopp Z, Abrams P, Cardozo L. Impact of overactive bladder symptoms on employment, social interactions and emotional well-being in six European countries. BJU Int. 2006;97(1):96–100. https://doi.org/10.1111/j.1464-410X.2005.05889.x.
Abrams P, Kelleher CJ, Kerr LA, Rogers RG. Overactive bladder significantly affects quality of life. Am J Manag Care. 2000;6:S580.
The International Consultation on Incontinence Questionnaire (2019) Bristol Urological Institute. http://www.iciq.net. Accessed 20 Nov 2018.
Coyne K, Revicki D, Hunt T, Corey R, Stewart W, Bentkover J. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Qual Life Res. 2002;11(6):563–74.
Matza LS, Thompson CL, Krasnow J, Brewster-Jordan J, Zyczynski T, Coyne S. Test-retest reliability of four questionnaires for patients with overactive bladder: the overactive bladder questionnaire (OAB-q), patient perception of bladder condition (PPBC), urgency questionnaire (UQ), and the primary OAB symptom questionnaire (POSQ). Neurourol Urodyn. 2005;24(3):215–25. https://doi.org/10.1002/nau.20110.
Acquadro C, Kopp Z, Coyne K, Corcos J, Tubaro A, Choo MS. Translating overactive bladder questionnaires in 14 languages. Urology. 2006;67(3):536–40. https://doi.org/10.1016/j.urology.2005.09.035.
Koller M, West K. Linguistic validation manual for patient-reported outcomes (PRO) instruments. In: C Acquadro, K Conway, C Girourdet, I Mear. MAPI research trust. Lyon, France. 15. Qual Life Res. 2005;14(7):1791–2. https://doi.org/10.1007/s11136-005-5367-1.
Beaton DT, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross cultural adaptation of self report measures. Spine. 2000;25(24):3186–91.
Terwee CB, Bot SD, Boer MR, Van der Windt DA, Knol DL, Dekker J. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34–42. https://doi.org/10.1016/j.jclinepi.2006.03.012.
Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol. 1993;46(12):1417–32.
Costa NM, Coluci AM. Validade de conteúdo nos processos de construção e adaptação de instrumentos de medidas. Ciênc saude coletiva. 2011;16(7):3061–8. https://doi.org/10.1590/S1413-81232011000800006.
Polit DF, Beck CT. The content validity index: are you sure you know what’s being reported? Critique and recommendations. Res Nurs Health. 2006;29(5):489–97.
Pereira S, Thiel R, Riccetto C, et al. Validação do International Consultation On Incontinence Questionnaire Overactive Bladder (ICIQ-OAB) para a língua portuguesa. Rev Bras Ginecol Obstet. 2010;32(6):273–8. https://doi.org/10.1590/S0100-72032010000600004.
Streiner DL. Starting at the beginning: an introduction to coefficient alpha and internal consistency starting at the beginning: an introduction to coefficient alpha and internal consistency. J Pers Assess. 2003;80(1):99–103. https://doi.org/10.1207/S15327752JPA800118.
Filho DB, Júnior JS. Visão além do alcance: uma introdução à análise fatorial. Opin Pub Jun16(1):160-185. 2010. https://doi.org/10.1590/S0104-62762010000100007.
Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–8. https://doi.org/10.1037/0033-2909.86.2.420.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–10. https://doi.org/10.1016/S0140-6736(86)90837-8.
Coyne K, Schmier J, Hunt T, Corey R, Liberman J, Revicki D. Developing a specific HRQL instrument for overactive bladder. Value Health. 2000;3:141. https://doi.org/10.1016/S1098-3015(11)70554-X.
Acknowledgments
We thank the honorary professors of the Pontifical Catholic University of Minas Gerais (PUC Minas), Patrícia Dayrell and Daniel Câmara Azevedo, for the critical review of the manuscript. We also thank the research subjects, institutions involved and International Consultation on Incontinence Questionnaire (http://iciq.net/) for all their support for this study.
Funding
The present study was carried out with support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)-Financing Code 001, through the State University of Campinas (UNICAMP), as well as support from the Federal University of Alfenas (UNIFAL-MG) and Pontifical Catholic University of Minas Gerais (PUC MINAS).
This study was financed in part by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)-Finance Code 001, through the Postgraduate Program in Surgical Science of the State University of Campinas (UNICAMP), with support from the Federal University of Alfenas-UNIFAL-MG, Fundação de Amparo à Pesquisa do Estado de Minas Gerais-FAPEMIG (PPM-00471-18) and the Pontifical Catholic University of Minas Gerais-PUC MINAS - Brazil.
Author information
Authors and Affiliations
Contributions
• Sílvia Monteiro: Project development, data management, manuscript writing/editing.
• Cássio Riccetto: Project development, manuscript writing/editing.
• Anna Karoline Rocha: Data collection and data analysis.
• Kamila Santos: Data collection and data analysis.
• Ingrid Campos: Data collection and data analysis.
• Tuany Pereira: Data collection and data analysis.
• Simone Botelho: Project development, manuscript writing/editing.
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Monteiro, S., Riccetto, C., Rocha, A.K. et al. The Brazilian Portuguese version of the ICIQ-OABqol: cross-cultural adaptation and reliability. Int Urogynecol J 31, 2507–2514 (2020). https://doi.org/10.1007/s00192-020-04280-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-020-04280-x