Abstract
Introduction and hypothesis
The suburethral sling procedure has been widely used as the first-line treatment for stress urinary incontinence (SUI) in women. Although the success rate is high, difficult urination and urine retention can occur in a small portion of patients. A transvaginal sling incision can solve this problem but recurrent SUI may occur. This study investigated the long-term outcomes of women who underwent the pubovaginal sling (PVS) procedure and subsequent transvaginal sling incision for urethral obstruction.
Methods
We retrospectively reviewed the voiding conditions of women who underwent transvaginal sling incision owing to bladder outlet obstruction after the PVS procedure over the past two decades. Urodynamic study was performed before and after each operation. The patients’ Global Impression of Improvement (PGI-I) and quality of life index (QoL-I) due to urinary symptoms were used for outcome evaluation.
Results
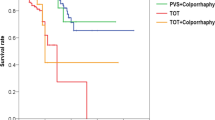
Among 405 women who underwent PVS procedure, 14 (3.5%) underwent subsequent transvaginal sling incision. The main symptoms were severe dysuria, followed by urinary retention or severe wound discomfort. The average interval between the two operations was 147.6 ± 353.6 days (range 3~1,344). The mean follow-up time after sling incision was 91.1 ± 50.7 months. At follow-up, 12 patients (85.7%) could maintain urinary continence whereas 2 had urgency incontinence. Ten patients (71.4%) were satisfied with their quality of life postoperatively.
Conclusions
Transvaginal sling incision is effective for urethral obstruction after PVS procedure. Voiding dysfunction after PVS could be resolved via sling incision. Most patients could maintain urinary continence and reported good satisfaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of stress urinary incontinence (SUI) in female patients is high. The condition is caused by a loss of urethral support, resulting from pelvic support structure damage after childbirth or pelvic surgery. The main symptom consists of uncontrollable urine leakage during actions that increase abdominal pressure such as coughing, sneezing, and lifting [1].
Many different treatment options have been applied to patients with SUI, including conservative treatment, behavioral management, pelvic floor muscle exercises, medications, and surgery. The mid-urethral sling procedure is considered the gold standard of first-line surgical treatment options for female SUI; the procedure has been used worldwide over the past decade. Among the different sling materials, synthetic slings have been developed and widely used owing to minimal patient morbidity, high efficacy, and technical simplicity [2].
Unfortunately, any surgical procedure can lead to complications. Patients who have undergone the suburethral sling procedure may experience postoperative voiding dysfunction because of urethral obstruction or pelvic floor non-relaxation. In the event that acute urinary retention (AUR) does occur, transvaginal urethrolysis or sling incision is indicated. In past studies, the rate of urinary retention following use of the mid-urethral sling procedure to treat female incontinence has been estimated at 1–10% [2]. Interestingly, not all patients who undergo reverse surgery experience a recurrence of SUI. This phenomenon suggests that a sling incision may have effects on the continence mechanism and voiding condition other than direct physical compression of the urethra.
Although the suburethral sling procedure is widely used to treat female SUI and the outcomes and complications of the procedure have been widely discussed, very few studies have discussed the overall conditions of patients before and after such surgery, especially among patients undergoing both the suburethral sling procedure and a transvaginal sling incision. As such, we report herein long-term follow-up clinical and urodynamic results of women who underwent the pubovaginal sling procedure (PVS) and subsequent transvaginal sling incision because of urethral obstruction.
Materials and methods
Patients
We retrospectively reviewed the medical and surgical records of all women with SUI, as determined by video urodynamic study, who received anti-incontinence surgery and subsequent transvaginal sling incision in a tertiary teaching hospital during the period 1991–2015. The study was approved by the Institutional Review Board and Ethics Committee of the Buddhist Tzu Chi General Hospital (approval number: TCGH 105–154-B).
A detailed history was taken and physical examinations were performed for all patients. All patients were diagnosed with iatrogenic bladder outlet obstruction (BOO) after sling surgery based on their clinical symptoms and video urodynamic evaluations. Conservative treatment including temporary catheterization and urethral dilatation were performed before sling incision, but had failed to be effective. A transrectal ultrasound of the bladder and urethra (TRUS-B) and an assessment of lower urinary tract symptoms were conducted before and after each operation. The results of each video urodynamic study included the given patient’s cystometric bladder capacity (CBC), maximum detrusor pressure (Pdet), maximum flow rate (Qmax), and post-void residual volume (PVR). The presence or absence of intrinsic sphincter deficiency (ISD), bladder neck hypermobility, cystocele, detrusor overactivity (DO), and detrusor underactivity (DU) during urodynamic study was also recorded.
Surgical procedures: pubovaginal sling procedure
All the patients underwent a retropubic PVS procedure using the same type of polypropylene mesh (tailored from a 30*30 cm mesh, PROLENE™, PML1; Ethicon); each procedure was performed by the same surgeon (HCK) using a modified technique from McGuire and Lytton [3]. This surgical procedure has been reported previously [4] and its anatomical and functional results have also been assessed [5]. Long-term results for the surgery have also been reported, including the finding that durable therapeutic outcomes can be achieved with this procedure [6]. In brief, the sling was placed with minimal tension beneath the proximal urethra through a subepithelial tunnel made by two longitudinal vaginal incisions at both vaginal sulci. The ends of the sling were pulled outside the lower suprapubic transverse incision by a long Kelly clamp at about 2.5 cm from the midline. The vaginal wounds were closed with 3–0 Vicryl suture. Cystoscopy was performed to check for any perforation of the bladder wall and the urethral condition after sling placement. The patient was asked to cough and perform straining at a bladder volume of 300 ml. If urine leaked, a slight adjustment of the sling tension to achieve no leakage was then performed. The operation ended after insertion of a 14Fr Foley catheter and vaginal gauze for wound compression. The Foley catheter and vaginal gauze were removed the next morning and the patient was allowed to void spontaneously.
Surgical procedures: transvaginal sling incision
For each patient, uroflowmetry and PVR were routinely checked several times before discharge from the hospital. If a patient had a Qmax <15 ml/s, PVR > 200 ml, with or without symptoms of severe dysuria, micturition pain, incomplete emptying, or urinary retention, intermittent catheterization was performed. If the voiding condition did not improve at the 3rd postoperative day or during the follow-up period after PVS, a video urodynamic study was performed to differentiate between true urethral obstruction and poor relaxation of the pelvic floor muscles after the PVS procedure. Once urethral obstruction had been confirmed by video urodynamic study (high voiding pressure > 22 cmH2O, low flow rate < 12 ml/s, and a narrow urethra in voiding cystourethrography), a transvaginal sling incision was advised.
The patient was prepared for the transvaginal sling incision in the same manner as for the PVS procedure. A 2-cm anterior vaginal wall incision at the midline was made and the suburethral sling beneath the urethra was easily identified if the procedure was performed within 2 weeks of the PVS procedure (Fig.1). If the procedure was performed more than 3 months after the PVS procedure, progressive dissection of the fibrotic tissues was necessary to reach the sling. The sling and fibrotic band were cut at the midline with sharp dissection until the sling was divided and the urethra was easily mobilized. The postoperative care was the same as that following the PVS procedure described above.
Follow-up
Patients received postoperative follow-up consisting of a regular direct interview at an out-patient clinic. A postoperative video urodynamic study was also performed 3–6 months after the sling incision procedure. The Patient’s Global Impression of Improvement (PGI-I, subdivided to very much improved, much improved, minimally improved, and no change) and quality of life index due to urinary symptoms (QoL-I, captured from the International Prostate Symptom Score), which range from 0 to 6, were used for outcome evaluation.
Statistical analysis
The urodynamic parameters were compared using the one-way RM-ANOVA for longitudinal comparison. A p value of <0.05 was considered to indicate statistical significance. All analyses were performed using SPSS for Windows, version 18. The uroflowmetry parameters from a group of 234 patients who had successful long-term outcome after PVS were selected for comparison of the baseline parameters of the patients who needed sling incision.
Results
We retrospectively reviewed the records for a total of 405 women who underwent the PVS procedure over the past two decades. Among these patients, only 14 (3.5%) received a subsequent transvaginal sling incision after the PVS procedure. Among these 14 patients, the main reason for undergoing the subsequent transvaginal sling incision was dysuria (100%, n = 14), followed by urine retention (57.1%, n = 8), and severe wound discomfort (7.1%, n = 1). The average patient ages at the time of the PVS procedure and sling incision were 59.6 ± 7.0 and 60.0 ± 7.0 years respectively.
The average interval between PVS and sling incision was 147.6 ± 353.6 (3~1,344) days. Seven (50%) of the patients received the sling incision within 1 month of undergoing the PVS procedure; only 1 had the sling incision operation more than 1 year after undergoing the PVS procedure. The mean follow-up time after transvaginal sling incision was 91.1 ± 50.7 (range 13 to 190) months. At the most recent follow-up visit, 12 (85.7%) patients were still free from urinary incontinence, 2 patients (14.3%) had persistent urgency urinary incontinence but not SUI. Table 1 shows the demographics.
All patients were older than 50 years and post-menopausal at the time of undergoing the PVS procedure. Thirteen of the patients (92.9%) were multi-parous and 3 of them had experienced pregnancy and subsequent transvaginal delivery more than five times. Seven (50%) of the patients had also previously undergone pelvic surgery, including hysterectomy. The average body mass index of the patients was 27.9 ± 3.6 kg/m2, which was the same as that of the patients who did not require sling incision.
The detailed video urodynamic parameters of the patients are listed in Table 2. We found that the Qmax, voided volume, and voiding efficiency were significantly lower after the PVS procedure, but recovered significantly to the baseline after sling incision. The PVR, maximum Pdet and BOO index all showed no significant difference, but still improved after sling incision. Interestingly, the bladder function also improved after sling incision and most of the patients were very satisfied with their voiding and bladder condition after the sling incision operation.
The patients who need sling incision for the BOO after PVS had a lower Qmax (17.5 ± 11.0 ml/s), greater PVR (92.6 ± 155.3 ml) and lower voiding efficiency (0.75 ± 0.31) at baseline (Table 2). These uroflowmetry parameters are significantly different from a group of 234 patients who had successful long-term outcome after PVS (Qmax 22.9 ± 12.2 ml/s, PVR 31.3 ± 66.9 ml, VE 0.89 ± 0.21, all p < 0.05).
The PGI-I results showed that 10 of the patients (71.4%) had improved substantially, 3 (21.4%) had unchanged symptoms, and 4 (28.6%) were unsatisfied with their quality of life postoperatively. The reasons for these unsatisfactory outcomes include persistent urgency urinary incontinence (14.3%, n = 2), urinary frequency (7.1%, n = 1), and persistent dysuria (7.1%, n = 1).
Discussion
This study revealed that the transvaginal sling incision is a safe and efficient treatment for solving voiding problems while also carrying a low risk of recurrent urinary incontinence. This procedure could be performed at any time postoperatively after BOO has been proven.
There are many different types of suburethral sling widely used in the treatment of female SUI. Numerous techniques and sling materials have been prescribed, including autologous and allograft fascia and synthetic meshes. A successful suburethral sling placement requires a balance between the inhibition of urine leakage and the preservation of efficient voiding. It is mandatory to provide efficient urethral support during increased abdominal pressure and not to affect normal voiding. To date, there is no general consensus regarding a standard technique for determining an appropriate sling tension. A tension-free suburethral sling had been advocated to avoid obstruction, but mechanical BOO can still occur [7].
Previous studies have reported that transient urine retention is common after a suburethral sling procedure and that most patients return to normal voiding after a few days of observation. The risk of permanent urinary retention has been reported to be only 1–2% [7, 8]. The most obvious symptoms of iatrogenic BOO after sling surgery are bladder fullness and an inability to void. Other common symptoms include dysuria, weak stream, straining to void, frequency, urgency, urgency incontinence, recurrent urinary tract infection, and large PVR. However, not all patients present with typical symptoms and may be delayed in diagnosis. A detailed history taking, physical examination, cystoscopy, and urodynamic study are usually required to evaluate the urethral obstruction [9]. A urodynamic urethral obstruction can be defined as a Pdet greater than 20 cmH2O and a Qmax of less than 15 ml/s [10]. For the patients in this study, the average Pdet was 23.1 cmH2O and the average Qmax was only 6.6 ml/s after the PVS procedure. Moreover, many patients had experienced urinary retention.
After full evaluation of the given patients, the diagnosis of iatrogenic BOO was established. Further treatment depends on the preferences of both the patient and surgeon. Nonsurgical therapies include intermittent catheterization, indwelling urethral catheterization, medical management, biofeedback therapy, and urethral dilation. Actually, these treatments may not be classified as definite treatments for BOO after the suburethral sling procedure, as some of them can only treat urinary retention rather than urethral obstruction. As such, they should be considered only in patients with mild obstruction or in those who are worried about recurrent incontinence after surgical intervention. Medical managements such as the use of alpha-blockers and pelvic floor exercises usually do not release an obstruction at all. Repeated urethral dilation is one possible nonsurgical procedure for loosening the sling mechanically and decreasing urethral resistance. However, previous studies have indicated that it can only provide short-term and limited effects without sufficient clinical evidence [2, 11].
Patients with urinary retention or moderate to severe urinary symptoms may require a surgical intervention to ensure the permanent correction of BOO after the suburethral sling procedure. The relevant surgical options can be divided into sling loosening, sling incision, sling excision, and extensive urethrolysis. Sling loosening is helpful immediately after the suburethral sling procedure before tissue ingrowth has occurred. Sling incision and excision consist of direct disruption of the sling to release the external urethral compression. These procedures are very fast and effective in terms of releasing iatrogenic BOO, but may cause recurrent SUI or lower urinary tract symptoms [12, 13]. Urethrolysis is defined as the mobilizing of the urethra; the sling itself is usually excised during the operation. Urethrolysis can be performed transabdominally or transvaginally, but is most often performed transvaginally [14]. A previous study reported an 84% success rate for the procedure, with recurrent SUI occurring in 19% of cases [15]. However, Ulrich et al. found that women who undergone tape division or excision have a lower quality of life because of higher subjective SUI rates [16].
After sling incision, all the patients in our study were free from recurrent SUI, but 2 had urgency incontinence. This result was surprising but not unexpected. Actually, the PVS provides support to the urethra from both below and bilaterally on both sides. The supportive mechanism is not only caused by the sling itself, but also by fibrosis and adhesion to the surrounding tissues. Sling incision or urethrolysis is the standard procedure when the suburethral sling creates BOO. Although in the short term urinary continence can be maintained in most patients, some may have recurrent urinary incontinence after long-term follow-up. Based on the results of this study, if the sling incision was made at the midline without wide dissection or urethrolysis, the remaining sling could still provide enough support to prevent SUI, even after a mean follow-up period of 91 months. Figure 1 shows the relative position of the sling and urethra before and after sling incision. Even if part of the sling is removed, the bilateral sling ends remain adhered to the endopelvic fascia and still provide enough support to prevent SUI.
Bladder function changes are found in both male and female patients after long-term BOO. The condition affects both bladder storage function and bladder contractility [17, 18]. Patients may present with DO or DU due to BOO after the suburethral sling procedure. In our study, we had several patients with DO and DU after the PVS, but these issues were generally resolved after sling incision. In addition to BOO caused by direct urethral compression, other mechanisms may affect bladder function, including local inflammation and pelvic floor afferent nerve hyperactivity.
The patients who need sling incision for the BOO after PVS had a lower Qmax, greater PVR, and lower voiding efficiency at baseline. These urodynamic parameters are significantly different from a group of 234 patients who had successful long-term outcome after PVS. These data implied that these patients may have a poor relaxation of the pelvic floor during voiding. Therefore, patients may have a greater chance of developing voiding dysfunction after PVS.
In this study, 3 patients experienced both SUI and dysuria after the PVS procedure, but these symptoms were also resolved after sling incision. Because the suburethral sling increases urethral resistance artificially, when a sling causes BOO, the patient may still leak urine when the bladder is extremely full. It is also possible that a tight suburethral sling might increase local inflammation, which could in turn induce pelvic floor muscle hypertonicity and cause urethral sphincter incompetence when the bladder is full. Therefore, if patients cannot urinate spontaneously because of urethral sling obstruction, they may also leak urine in addition to urinary retention. Once the sling tension was adjusted after sling incision, the inflammation of the pelvic floor muscles was reduced and urethral sphincter tonicity returned to normal; therefore, the patients could void smoothly without SUI.
The PVS procedure is a retropubic anti-incontinence technique similar to TVT. Before the advent of TVT, PVS was the most common procedure for female SUI. The therapeutic mechanism and adverse events are the same as for TVT. When urethral obstruction occurs, transvaginal sling incision is the standard procedure for releasing sling tension and resuming spontaneous voiding. The conclusion of this study is valid for all suburethral slings.
The main limitations of the present study are the small case number reviewed, the use of self-reported grading for outcome evaluation, and only 8 patients received video urodynamic study after sling incision. The strength of the study is the sequential urodynamic data before the PVS procedure, before sling incision, and after sling incision. In addition, the differential diagnosis of true urethral obstruction and poor pelvic floor relaxation was made via video urodynamic study, which enabled us to select only patients who had true BOO to undergo sling incision.
In conclusion, transvaginal sling incision is effective for urethral obstruction after the PVS procedure. Both bladder dysfunction and voiding dysfunction after PVS could be resolved via sling incision. Most patients could maintain urinary continence and expressed satisfaction with the procedure. It can thus be concluded that transvaginal sling incision is a safe and efficient treatment in patients experiencing BOO after undergoing the sling procedure, including the fact that such patients need not worry about recurrent SUI.
References
Cox A, Herschorn S, Lee L. Surgical management of female SUI: is there a gold standard? Nat Rev Urol. 2013;10:78–89.
Patel BN, Kobashi KC, Staskin D. Iatrogenic obstruction after sling surgery. Nat Rev Urol. 2012;9:429–34.
Mcguire EJ, Lytton B. Pubovaginal sling procedure for stress incontinence. J Urol. 1978;119:82–4.
Kuo HC. Comparison of video urodynamic results after the pubovaginal sling procedure using rectus fascia and polypropylene mesh for stress urinary incontinence. J Urol. 2001;165:163–8.
Kuo HC. Anatomical and functional results of pubovaginal sling procedure using polypropylene mesh for the treatment of stress urinary incontinence. J Urol. 2001;166:152–7.
Kuo HC. The surgical results of the pubovaginal sling procedure using polypropylene mesh for stress urinary incontinence. BJU Int. 2001;88:884–8.
Nitti VW, Carlson KV, Blaivas JG, Dmochowski RR. Early results of pubovaginal sling lysis by midline sling incision. Urology. 2002;59:47–51.
Song PH, Yoo ES. Five-year outcomes of the transection of synthetic suburethral sling tape for treating obstructive voiding symptoms after transobturator sling surgery. Urology. 2012;80:551–5.
Farrar DJ, Osborne JL, Stephenson TP, Whiteside CG, Weir J, Berry J, et al. A urodynamic view of bladder outflow obstruction in the female: factors influencing the results of treatment. Br J Urol. 1975;47:815–22.
Chassange S, Bernier PA, Haab F, Roehrborn CG, Reisch JS, Zimmern PE. Proposed cut-off values to define bladder outlet obstruction in women. Urology. 1998;51:408–11.
Klutke C, Siegel S, Carlin B, Paszkiewicz E, Kirkemo A, Klutke J. Urinary retention after tension-free vaginal tape procedure: incidence and treatment. Urology. 2001;58:697–701.
Laurikainen E, Kiilholma P. A nationwide analysis of transvaginal tape release for urinary retention after tension-free vaginal tape procedure. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:111–9.
Hong B, Park S, Kim HS, Choo MS. Factors predictive of urinary retention after a tension-free vaginal tape procedure for female stress urinary incontinence. J Urol. 2003;170:852–6.
Foster HE, McGuire EJ. Management of urethral obstruction with transvaginal urethrolysis. J Urol. 1993;150:1448–51.
Goldman HB, Rackley RR, Appell RA. The efficacy of urethrolysis without re-suspension for iatrogenic urethral obstruction. J Urol. 1999;161:196–8.
Ulrich D, Bjelic-Radisic V, Höllein A, Trutnovsky G, Tamussino K, Aigmüller T. Quality of life and objective outcome assessment in women with tape division after surgery for stress urinary incontinence. PLoS One. 2017;27:12:e0174628.
Hsiao SM, Lin HH, Kuo HC. Videourodynamic studies of women with voiding dysfunction. Sci Rep. 2017;7:6845.
Jiang YH, Kuo HC. Video-urodynamic characteristics of non-neurogenic, idiopathic underactive bladder in men—a comparison of men with normal tracing and bladder outlet obstruction. PLoS One. 2017;12:e0174593.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Wu, SY., Kuo, HC. Long-term outcomes of anti-incontinence surgery and subsequent transvaginal sling incision for urethral obstruction. Int Urogynecol J 30, 761–766 (2019). https://doi.org/10.1007/s00192-018-3733-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-018-3733-0