Abstract
Introduction and hypothesis
There is a difference of opinion in the literature as to whether pelvic organ prolapse (POP) is a direct cause of female sexual dysfunction (FSD). Sexual function in women is negatively impacted by the presence of urinary symptoms. Thus, sexual dysfunction (SD) might be improved, unchanged, or worsened by pelvic floor surgery.
Methods
In this study, we observed SD and impact of surgical intervention on female sexual function (FSF) using a validated Prolapse/Urinary Incontinence Sexual Questionnaire Short Form (PISQ-12) in women undergoing surgery for POP with or without urinary incontinence. Two hundred women were recruited and followed up at 6 and 12 months postoperatively.
Results
Sexual function (SF) as measured by the PISQ-12 improved after surgery irrespective of the nature of surgery or the patient’s past gynaecology history. Improvement in SF was seen by 6 months (97 patients) postsurgery (P < 0.05), after which (at 12 months; 80 patients) no further change was observed. Improved SF was associated with better patient satisfaction postoperatively.
Conclusions
Sexual function improved in women following surgery for POP with or withour urinary incontinence, irrespective of the nature of surgery and the patient’s past gynecologic history. Results of this study will assist when counselling women with POP with or without urinary incontinence regarding treatment options.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Female sexual dysfunction (FSD) is a common problem, with data from the National Health and Social Life Survey showing that 43 % of women aged 18–59 years are experiencing some form of FSD [1]. The aetiology of FSD is multifactorial, with hormonal, psychological, anatomical, vascular and neurogenic elements all being possible factors [2].
Pelvic organ prolapse (POP) and urinary incontinence (UI) are major health burdens to 41–65 % of women. A large population study suggests that the prevalence of stage 3 or 4 prolapse is in the range of 2–11 % [3, 4]. An epidemiological study reported UI to affect up to 41 % of women. [5]. At least one in three parous women undergo at least one surgery for these conditions by the age of 80 years [6]. Women with POP and/or UI are at a higher risk of sexual dysfunction [7,8,9,10,11] compared with those without.
Traditionally, pelvic floor surgeons have assessed the outcome of vaginal repair surgery by the degree of restoration of normal pelvic anatomy. Increasingly, however, the effect of prolapse surgery upon a woman’s sexual function (SF) is being used as an outcome measure of for success [12, 13], especially since the introduction of vaginal mesh repairs for prolapse [14]. There is a difference of opinion in the literature, however, as to whether POP is a direct cause of FSD. It may not be the prolapse itself but the associated coital incontinence that predicts sexual dysfunction. Likewise, it appears that vaginal anatomy per se is not an independent factor in the aetiology of FSD: neither vaginal calibre, nor length, nor atrophy, nor menopausal status have a direct influence on the presence of FSD [22].
Materials and methods
In this study, we aimed to assess the incidence of FSD in a group of sexually active women with stress urinary incontinence (SUI) and/or POP who were awaiting surgical management. The secondary aim was to determine whether vaginal surgery for prolapse or UI leads to alteration in SF and then compare SF in patients undergoing POP or UI surgery alone with POP and UI surgery combined.
Study population
The study was coordinated from the Department of Urogynaecology at the South Glasgow University Hospital in their established Urogynaecology and Pelvic Floor Dysfunction Research Unit. All women undergoing any type of POP repair and/or UI surgery were invited to participate: 200 women were recruited through the urogynaecology clinics across the service over a 12-month period from June 2011 to May 2012. All gave written informed consent to be involved in the study. Inclusion criteria were: (1) women on the waiting list for surgical repair of POP, UI, or both, and (2) women sexually active in the past 6 months and expect to remain so postoperatively. Exclusion criteria were: (1) women younger than 18 years, (2) women unable to understand the information leaflet and (3) women unable to complete the questionnaire.
Methodology
This was a prospective observational study. Ethical approval was obtained from West of Scotland Ethics Committee (reference 10/S0709/69; 16/03/2011). Patient demographics and details about their surgical procedure(s) were obtained from hospital records. Women complaining of symptomatic POP and/or SUI who were on the waiting list for POP surgery with or without UI surgery were recruited at their preoperative assessment visit or during hospital admission for their procedure. Consenting participants completed the preoperative questionnaire, which included primary and secondary outcome measures prior to surgery. At 6 and 12 months after surgery, the baseline questionnaire and a questionnaire designed by our unit (“Appendix 1”) were sent by post, along with a stamped addressed envelope, to be completed at home and returned. Women who did not respond within 2 weeks were sent a reminder letter and questionnaire and were contacted by telephone if there was no response after a further 2 weeks.
Primary outcome
Primary outcome was to assess incidence of sexual dysfunction using a condition-specific, validated, quality of life (QoL) assessment tool, the Prolapse/Urinary Incontinence Sexual Questionnaire Short Form (PISQ-12) score [10, 15].
Secondary outcome
Secondary outcome was change in PISQ-12 and the International Consultation on Incontinence Questionnaire–Vaginal Symptoms (ICIQ-VS) scores between baseline and 6 and 12 months after surgery. We also assessed UI symptom distress and its impact on QoL at 6 and 12 months using the Urogenital Distress Inventory Short Form (UDI-6) and the Incontinence Impact Questionnaire (IIQ-7). Patient satisfaction with surgery was measured using a study-specific, nonvalidated instrument (’Appendix 1”) at 6 and 12 months.
Analysis
We tabulated descriptive statistics, reporting baseline demographics and clinical characteristics with means and standard deviations (SDs) or medians and interquartile ranges (IQRs), as appropriate. A paired t test was used to compare baseline and 6- and 12-month scores, and analysis of variance (ANOVA) to test for differences between groups and for postoperative satisfaction levels. Data were analysed in SPSS version 19, and a 5 % level of significance was used throughout.
Results
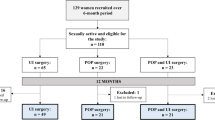
Two hundred women were recruited, of whom 180 (90 %) returned completed baseline questionnaires. At 6 months, 97 (48.5 %) patients returned completed questionnaires and 87 (43.5 %) were returned at 12 months (Fig. 1).
Mean patient age was 54.4 years [standard deviation (SD) 10.1]. All women except three were parous, with a median parity of two (range 0–5); 121 (67.2 %) women were postmenopausal, of whom 15 (8.3 %) were on hormone replacement therapy (HRT) at the time of surgery. A significantly higher proportion of women who had surgery for prolapse were menopausal (chi-square = 9.412, df = 2, P = 0.009). Most women who had surgery for POP (98 %) had stage ≥2 prolapse (Table 1).
Thirty-seven (19.5 %) women had POP prior surgery, with most having conventional prolapse surgery without mesh. Four (2.2 %) patients had a mesh graft, and two (1.1 %) had both conventional surgery and mesh graft. Seventeen (9.5 %) patients had a previous UI procedure, and 116 (68 %) had no documented urinary symptoms (Table 1). One hundred and thirty women underwent POP surgery, 29 UI surgery and 21 both POP and UI surgery (Table 2).
Sexual function
Overall mean baseline PISQ-12 score was 30.54 (SD 6.55). There was no statistically significant difference in baseline PISQ-12 regarding age, parity, menopausal status or previous POP and/or UI surgery(Table 3). There was also no statistically significant difference in baseline PISQ-12 between women awaiting POP surgery only, UI surgery only, or both (Table 4).
Mean PISQ-12 score for all women increased (improved) significantly from baseline to 33.4 (SD 7.36) at 6 and nonsignificantly to 33.5 (SD 7.40) at 12 months (Table 3). Improvement was not significantly different between groups having POP surgery, UI surgery or both (ANOVA: F = 2.266, df = 2, P = 0.109). It was also not influenced by any of the above-mentioned demographic characteristics. Improvements in UDI-6, VS and IIQ-7 scores from baseline to 6 months were statistically significant but not from 6 to 12 months (Table 4). No significant difference was seen between surgery types (no mesh, vaginal mesh for prolapse, mesh for UI, abdominal mesh for prolapse) regarding change in PISQ-12 score from baseline to 6 months (ANOVA F = 1.463, df = 3, P = 0.230); specifically, there was no difference between women having prolapse surgery with and without mesh (mean difference −0.99, standard error 1.49, P = 0.510).
Other outcomes
Improvement in UDI-6 and IIQ-7 scores from baseline to 6 months was significantly different between the three surgery groups (ANOVA F = 15.9, df = 2, P < 0.005 and F = 17.9, df = 2, P < 0.005, respectively). There was significantly more improvement in both UDI and IIQ scores for women who had prolapse surgery alone compared with UI surgery alone or combined UI and prolapse surgery. Improvement in VS scores from baseline to 6 months was not significantly different between groups (ANOVA F = 1.757, df = 2, P = 0.178).
Relationship with patient satisfaction
Seventy-seven (83 %) women at 6 months and 60 (78 %) at 1 year reported being either satisfied or very satisfied with their surgical outcome. Being satisfied (not satisfied/satisfied/very satisfied) was significantly associated with improvement in PISQ-12 scores (ANOVA F = 5.915, df = 2, P = 0.004). Improvements in UDI-6 (ANOVA F = 4.293, df = 2, P = 0.017) and VS scores (ANOVA F = 3.771, df = 2, P = 0.025) at 6 months from baseline were also statistically significantly associated with patient satisfaction; however, improvement in IIQ-7 was not (ANOVA F = 1.618, df = 2, P = 0.204).
Discussion
Surgery for prolapse has a role in reconstructing the local anatomy and alleviating some symptoms but does not necessarily ensure optimal SF, which might be improved [15,16,17], remains unchanged [8, 18] or worsened [19] after repair. Improvement could also be due to emotional amelioration due to the cessation of incontinence [20, 21]. Most papers, however, only report on SF as a secondary finding. Most are retrospective in nature, and only a few have involved the use of a validated SF questionnaire [22]. The prospective studies are either small with 3–6 months’ follow-up [8] or used non-condition-specific questionnaires. Our study has large numbers with 1-year follow-up and used a validated condition-specific SF questionnaire with SF as the primary outcome.
Several retrospective and prospective studies have used either nonvalidated SF questionnaires [8, 17], self-designed questionnaire or telephonic conversation. Recently, condition-specific validated sexual health questionnaires have been developed. At the beginning of this trial, the PISQ-31 (including the PISQ-12) was the only validated condition-specific (prolapse and UI) female SF questionnaire available. Other validated condition-specific questionnaires used to assess SF following pelvic floor surgery [e.g. Kings Health Questionnaire (KHQ), ICIQ-VS] are QoL questionnaires that include few questions addressing SF but assess overall impact of POP and/or UI surgery on QoL. We therefore chose to use (the short form) PISQ-12 questionnaire. However, we appreciate that it only discriminates between women with and without sexual dysfunction accompanied by POP and UI and may not be optimal to detect SD following treatment, as also concluded by Roos et al. [23]. We also understand that PISQ represents the positive effects of surgery well but does not reflect its possible negative effects on SF [16].
In our study population, mean PISQ-12 score was 30.54 (SD 6.55) with the maximum possible score of 48. Although a range of scores for this instrument has not yet been established to classify SD severity, we believe our findings indicate that women enrolled in our study displayed a significant decrement in SF before POP and/or UI surgery, consistent with several prior studies that found reduced SF in women with UI and/or POP or both [7, 24, 25]. Baseline PISQ-12 in our study appears to be comparable with that reported by Brubaker et al. in the Stress Incontinence Surgical Treatment Efficacy (SISTEr) trial (mean 30.54) [26] but lower than that reported by Glavind et al. (mean 35.3) [26]. This might be due to different baseline characteristics or patient population. We found statistically significant improvement in PISQ 12 score from baseline (30.54) to 6 months (33.45); other studies either have much smaller number of patients and shorter follow-up. In two different prospective study by Glavind et al. [26] with short term follow-up after prolapse surgery (n = 81), reported baseline PISQ12 was 35.2, with postop improvement with positive difference of 3.0 (SD 3.8). Brubaker et al. [26] reported significant improvement in PISQ-12 scores from 31.6 (SD 6.85) to 36.85 (SD 5.89). A long-term study by Lindquist et al. in which 63 patients were followed for 4 years after tension-free vaginal tape insertion (n = 44) reported baseline mean PISQ-12 of 33.8, which improved postoperatively [27]. A prospective study by Thakar et al. [16], in which 46 women were followed for 4 months postsurgery, showed significant improvement in SF after POP and UI surgery. Srikrishna et al. [17] recruited 52 sexually active women and followed them for 2 years using the Golombok Rust Inventory of Sexual Satisfaction (GRISS) and KHQ questionnaires, concluding that SF improved following surgery for POP with or without UI procedure. Results of the above two studies are not comparable, as they used different questionnaires. A study by Paul et al. [8] in which 51 patients were followed for 6 months found that SF as measured by the Female Sexual Function Index (FSFI) and sexual frequency were unchanged following vaginal surgery for POP with or without UI surgery, despite improvement in the prolapse stage and incontinence symptoms. Weber et al. [15] reported that SF and satisfaction improved or did not change in most women after surgery for prolapse and/or UI. Rogers et al. [15] reported mixed results, with improved SF in 68 % of women and worsened in 32 % using two validated condition-specific questionnaire (PISQ-12 and IIQ-7) preoperatively and 3 and 6 months after surgery in 102 women with a mean age of 47 years. Similar to our study, they observed no differences in total SF scores between women who underwent POP and UI surgery and those who had only one or the other. We found statistically significant improvement in PISQ-12 score from baseline to 6 months with a positive score of 2.91; however, the minimum clinically important difference is not yet determined. We observed positive improvement in SF scores by PISQ-12. Sloan et al. proposed that a change greater than half the SD of the preintervention score is a conservative estimate of a clinically meaningful effect size when using QoL questionnaires. [28]. After 6 months, we observed no further improvement in PISQ=12 scores, suggesting that any improvement due to surgery is generally seen within the first 6months; however, the effect does appear to be maintained up to 1 year. Other studies using self-designed, nonvalidated questionnaire or much smaller numbers [15] have shown stability in SF outcomes over the follow-up period. These results and our findings suggest that assessment at 6 or 12 months is unlikely to be significantly different and the 6-month follow-up can be used for comparison.
Success of surgery was defined as patient satisfaction. In all three subgroups in our study, improvement in PISQ-12 was observed in women with successful surgery and hence associated with patient satisfaction. Improvement in SF was strongly influenced by the outcome of surgery: i.e., patient satisfaction. Patients were not satisfied either because of no symptom improvement or new-onset symptoms like SUI. Patients who reported improvement in UDI 6 and VS scores also reported high satisfaction with surgical outcome. We believe the presence or absence of urinary symptoms—rather than surgical technique—may define SF [25].
Patient numbers in each surgical subgroup were too small to conclude whether one technique/surgery improves SF more than the others. We saw significant improvement in all groups with or without incontinent and hence can conclude that SF improvement after surgery is due not only to improvement in UI scores but also to amelioration of POP symptoms. We found significant improvement in UDI and IIQ-7 in the POP-only group (no UI surgery) and in VS score in the UI-only group (no prolapse surgery). This may have contributed to improvemed PISQ-12 score postoperatively in both instances [26]. In a cohort of 1267 sexually active women, Tok et al. [29] found that women with POP had lower SF scores due to fear of UI during intercourse and also avoided sexual intercourse due to POP. It is therefore understandable that correcting POP, improving body image and ameliorating symptoms should lead to improvement in SF.
We found improved PISQ-12 scores postoperatively irrespective of technique or type of surgery performed for UI. Improvement in UI scores/symptoms was associated with patient satisfaction. Whilst our cohort size may have been too small to assess differences in surgical techniques, our findings are consistent with other authors. Brubaker et al. [25] (SISTEr trial) concluded SF improves after successful surgery for UI irrespective of type/technique of UI surgery or POP with or without concomitant POP surgery.
Strengths of our study are its prospective nature and large number of patients, even though not all women completed the questionnaires due to embarrassment. We used a disease-specific, validated questionnaire and followed patients for 1 year postoperatively using multiple validated indices for bladder, POP and SF. We also acknowledge the limitations: There is no established normative data for PISQ-12, and the questionnaire only demonstrates the effect of intervention [10]. As this is an observational study, it is not possible reach a definite conclusion that the intervention led to improvement. No condition-specific questionnaire measuring distress is available at present [16]; the PISQ-12 revised was not available at the time of our study.
POP and/or UI rather than severity or POP subtype may impact SF. We did not perform an objective follow-up for prolapse and cannot comment whether improvement in SF as reported by patients was due to functional improvement rather than being secondary to objective improvement [30]. Srikrishna et al. [17] objectively and found that women with a well-supported pelvic floor were less likely to have sexual dysfunction. We used the validated IIQ-7 and VS questionnaires instead to evaluate the impact of POP and UI on social functioning. This may not exactly represent objective evidence of cure of UI and POP; however, they do assess in a standardised and validated manner patient overall improvement and perception of surgical success and are thus relevant and representative of treatment outcomes for POP and/or UI surgery [15].
Conclusion
We found that SF improves after surgery for POP and UI in most patients. This improvement is strongly positively associated with patient satisfaction with the surgery. Improvement is seen by 6 months and tends to be maintained at 1 year. Our study will help when counselling women who are considering surgery for POP and/or UI regarding improvement in SF.
References
Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537–44. https://doi.org/10.1001/jama.281.6.537.
Raina R, Pahlajani G, Khan S, Gupta S, Agarwal A, Zippe CD. Female sexual dysfunction: classification,pathophysiology, and management. Fertil Steril. 2007;88(5).
Swift SE. Distribution of pelvic organ support in a population of female subjects seen for routine gynaecological health care. Am J Obstet Gynecol. 2000;183(2):277–85.
Swift S, Woodman P, O'Boyle A, Kahn M, Valley M, Bland D, et al. Pelvic organ support study (POSST): the distribution, clinical definition, and epidemiologic condition of pelvic organ support defects. Am J Obstet Gynecol. 2005;192(3):795–806.
Jolleys JV. Reported prevalence of urinary incontinence in women in a general practice. Br Med J (Clin Res Ed). 1988;296(6632):1300–2.
Fialkow MF, Newton KM, Lentz GM, Weiss NS. Lifetime risk of surgical management for pelvic organ prolapse or urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(3):437–40.
Barber MD, Visco AG, Wyman JF, Fantl JA, Bump RC, Continence Program for Women Research Group. Sexual function in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 2002;99(2):281–9.
Pauls RN, Silva WA, Rooney CM, Siddighi S, Kleeman SD, Dryfhout V, et al. Sexual function after vaginal surgery for pelvic organ prolapse and urinary incontinence. Am J Obstet Gynecol. 2007;197(6):622.e1–7.
Salonia A, Zanni G, Nappi RE, Briganti A, Dehò F, Fabbri F, et al. Sexual dysfunction is common in women with lower urinary tract symptoms and urinary incontinence: results of a cross-sectional study. Eur Urol. 2004;45(5):642–8. discussion 648
Rogers RG, Kammerer-Doak D, Villarreal A, et al. A new instrument to measure sexual function in women with urinary incontinence or pelvic organ prolapse. Am J Obstet Gynecol. 2001;184:552.
Handa VL, Cundiff G, Chang HH, Helzlsouer KJ. Female sexual function and pelvic floor disorders. Obstet Gynecol. 2008;111(5):1045–52. https://doi.org/10.1097/AOG.0b013e31816bbe85.
Nappi R, Salonia A, Traish AM, van Lunsen RH, Vardi Y, Kodiglu A, et al. Clinical biologic pathophysiologies of women's sexual dysfunction. J Sex Med. 2005;2(1):4–25.
Althof SE, Dean J, Derogatis LR, Rosen RC, Sisson M. Current perspectives on the clinical assessment and diagnosis of female sexual dysfunction and clinical studies of potential therapies: a statement of concern. J Sex Med. 2005;48:642–9.
Su TH, Lau HH, Huang WC, Chen SS, Lin TY, Hsieh CH, et al. Short term impact on female sexual function of pelvic floor reconstruction with the Prolift procedure. J Sex Med. 2009;6(11):3201–7. https://doi.org/10.1111/j.1743-6109.2009.01399.x.
Rogers RG, Kammerer-Doak D, Darrow A, Murray K, Qualls C, Olsen A, et al. Does sexual function change after surgery for stress urinary incontinence and/or pelvic organ prolapse? A multicenter prospective study. Am J Obstet Gynecol. 2006;195(5):e1–4.
Thakar R, Chawla S, Scheer I, et al. Sexual function following pelvic floor surgery. Int J Gynaecol Obstet. 2008;102:110.
Srikrishna S, Robinson D, Cardozo L, Gonzalez J. Can sex survive pelvic floor surgery? Int Urogynecol J. 2010;21(11):1313–9. https://doi.org/10.1007/s00192-010-1198-x.
Occhino JA, Trabuco EC, Heisler CA, Klingele CJ, Gebhart JB. Changes in vaginal anatomy and sexual function after vaginal surgery. Int Urogynecol J. 2011;22(7):799–804. https://doi.org/10.1007/s00192-011-1386-3.
Helström L, Nilsson B. Impact of vaginal surgery on sexuality and quality of life in women with urinary incontinence or genital descensus. Acta Obstet Gynecol Scand. 2005;84(1):79–84.
Hasse P, Skidstead L. Influence of operations for stress incontinence and/or genital descensus on sexual life. Acta Obstet Gynecol Scand. 1988;67(7):659–61.
Lemack GE, Zimmern PE. Sexual function after vaginal surgery for stress incontinence: results of a mailed questionnaire. Urology. 2000;56:223.
Neill C, Abdel-Fattah M, Ramsay IN. Sexual function and vaginal surgery. Obstet Gynaecol. 2009;11:193–8. https://doi.org/10.1576/toag.11.3.193.27504.
Roos AM, Thakar R, Sultan AH, de Leeuw JW, Paulus AT. The impact of pelvic floor surgery on female sexual function: a mixed quantitative and qualitative study. BJOG. 2014;121(1):92–100. https://doi.org/10.1111/1471-0528.12412.
Weber AM, Walters MD, Schover LR, Mitchinson A. Sexual function in women with uterovaginal prolapse and urinary incontinence. Obstet Gynecol. 1995;85:483.
Brubaker L, Chiang S, Zyczynski H, Norton P, Kalinoski DL, Stoddard A, et al. The impact of stress incontinence surgery on female sexual function. Am J Obstet Gynecol. 2009;200(5):562.e1–7. https://doi.org/10.1016/j.ajog.2008.11.017.
Glavind K. Sexual function in women before and after surgery for pelvic organ prolapse. AOGS. 2015;94:80–5.
Lindquist AS, Glavind K. Long-term follow-up of sexual function in women before and after tension-free vaginal tape operation for stress urinary incontinence. IUJ. 2016;27(10):1571–6. https://doi.org/10.1007/s00192-016-3004-x.
Sloan J, Symonds T, Vargas-Chanes D, Fridley B. Practical guidelines for assessing the clinical significance of health-related quality of life changes within clinical trials. Drug Inf J. 2003;37(1):23–31.
Tok EC, Yasa O, Ertunc D, Savas A, Durukan H, Kanik A. The effect of pelvic organ prolapse on sexual function in a general cohort of women. J Sex Med. 2010;7(12):3957–62. https://doi.org/10.1111/j.1743-6109.2010.01940.x.
Lowenstein L, Gamble T, Sanses TV, et al. Changes in sexual function after treatment for prolapse are related to the improvement in body image perception. J Sex Med. 2010;7:1023.
Funding
Project was funded by the Department of Urogynaecology, South Glasgow University Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Appendix 1
Appendix 1

Rights and permissions
About this article
Cite this article
Tyagi, V., Perera, M., Guerrero, K. et al. Prospective observational study of the impact of vaginal surgery (pelvic organ prolapse with or without urinary incontinence) on female sexual function. Int Urogynecol J 29, 837–845 (2018). https://doi.org/10.1007/s00192-017-3500-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-017-3500-7