Abstract
Introduction and hypothesis
There is considerable variation worldwide on how the assessment of voiding function is performed following midurethral sling (MUS) surgery. There is potentially a financial cost, and reduction in efficiency when patient discharge is delayed. Using our current practice of two normal void and residual (V&R) readings before discharge, the aim of this retrospective study was to evaluate the likelihood of an abnormal second V&R test if the first V&R test was normal in order to determine if a policy of discharge after only one satisfactory V&R test is reasonable.
Methods
Data from 400 patients who had had MUS surgery with or without other procedures were collected. Our unit protocol included two consecutive voids of greater than 200 ml with residuals less than 150 ml before discharge. The patients were divided into the following groups: MUS only, MUS plus anterior colporrhaphy (AR) plus any other procedures (MUS/AR), and MUS with any non-AR procedures (MUS+).
Results
Complete datasets were available for 335 patients. Once inadequate tests (low volume voids <200 ml) had been excluded (28% overall), the likelihood of an abnormal second V&R test if the first test was normal was 7.1% overall, but 3.6% for MUS, 11.5% for MUS/AR and 8.6% for MUS+.
Conclusion
The findings in the MUS-only group indicate that it is probably safe to discharge patients after one satisfactory V&R test, as long as safety measures such as ‘open access’ are available so that patients have unhindered readmission if problems arise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The assessment of voiding function following midurethral sling (MUS) surgery varies worldwide. This variation may have an impact on resource utilization and risk of infection from recatheterization. With the continuing imperative to reduce overall costs, ensuring that patients who are listed as day cases are discharged on the same day secures maximum tariff. This is reflected in the Health Resource Group (HRG) awards in a healthcare system such as the National Health Service (NHS) in the UK. Unintended overnight stays can block beds and reduce efficiency. A single voiding test, if satisfactory, may help ensure that patients admitted as planned day cases are indeed discharged on the day of operation.
Recent data on current practice in the UK obtained via an online survey of members of the British Society of Urogynaecologists (response rate 31%) [1] revealed a variation in both postoperative monitoring and the critical thresholds used to determine retention. The survey revealed that 10% of surgeons do not do the procedure as a day case, 15% routinely leave a catheter in situ postoperatively (no detail given on rates of regional anaesthesia), and more than 30% of the latter leave the catheter in situ for at least 24 h. Two void and residual (V&R) tests were used by 69% of respondents, one V&R test by 16.5% and five V&R tests by 11.8%. Although most respondents measured voided volume (95.2%) and residual volume (91.3%), some measured only one of these. Ultrasonography was used to determine the postvoid residual by 87.3% of respondents, with 60.7% using a residual of 100 ml as the threshold and 30.8% using 150 ml as the threshold.
Practices also vary widely in the USA. In 2010, an email survey of clinicians with 618 respondents with complete datasets revealed that 20.6% routinely discharged their patients with a self-retaining catheter in situ for 1–3 days and a further 1.9% for more than 3 days. A further 2.9% used a suprapubic catheter routinely. Voiding assessment methods were reported as variable and included postvoid residual check, subjective patient reporting and uroflowmetry [2].
In addition there has been interest, mainly in the USA, in a single retrograde filling V&R test. Trial failure rates varied from 7.8% in a heterogeneous group of patients with prolapse and MUS surgery [3] to 20% in patients with MUS surgery only [4]. The data are conflicting, as one would expect the addition of prolapse surgery particularly anterior colporrhaphy (AR) to increase the risk of voiding difficulty [5, 6]. The advantage of a retrograde filling test is that the test is standardized and easy to perform. The disadvantage is that patients with larger bladder capacities may not have adequate sensation with 300 ml to initiate a normal void. This may explain the findings of Barr et al. [4]. Natural filling has the disadvantage that patients may attempt to void before the bladder is full, and therefore the test is of poor exclusion value, and time is wasted. However, patients may achieve bladder volumes closer to their capacity and therefore a true assessment is achieved.
There are few data on the consistency of these tests. The discrepancy between the first and second test was 7% in the spontaneous filling group of a small crossover randomized controlled trial comparing retrograde and natural filling [7]. Consistency with retrograde filling was not measured. This study included a heterogeneous group of patients undergoing pelvic floor and continence surgery. In another study looking at two consecutive retrograde filling V&R tests in a similar heterogeneous group, the likelihood of an abnormal second test if the first test was normal was 16.4% [8]. Our unit policy is for two consecutive sets of natural filling V&R tests to be normal prior to discharge. As in most units, this policy is in place for historical rather than evidence-based reasons. There are currently no good data on whether a single natural filling test is able to ensure adequate voiding function. We therefore set out to test the hypothesis that a single V&R test is sufficient to confirm the integrity of postoperative voiding function. If this were demonstrated, it would enable the relative safety of a policy of discharging patients on the basis of a normal first V&R test to be assessed.
Furthermore, there have been numerous studies [8,9,10,11] that have examined risk factors for abnormal voiding function after MUS surgery using logistic regression, but the results have been conflicting. We therefore planned to examine the ability of predefined independent risk factors to predict the normality of the second V&R test to inform a policy of discharge after one normal V&R test.
Materials and methods
Patients
We reviewed the notes of 400 consecutive patients who had undergone MUS surgery with or without additional procedures between 2007 and 2011 from two hospitals within one NHS Trust as part of a large retrospective audit examining morbidity. Research and Development Committee approval was obtained to analyse the data for publication in the medical literature, but Ethics Committee approval was not required as this was an analysis of historical anonymized data. Patients receiving either a transobturator or a retropubic MUS and those undergoing concomitant prolapse surgery were included. All patients had general anaesthesia for the procedure and local anaesthetic infiltration intraoperatively at the vaginal incision site. Demographic, urodynamic and outcome data were collected when available.
Patients who had MUS-only surgery were not catheterized and natural bladder filling commenced immediately after surgery. Patients who had MUS surgery together with prolapse surgery had a self-retaining catheter inserted along with a vaginal pack for between 4 and 20 h (at the clinician’s discretion), after which it was removed and filling and the first voiding test were started.
The unit policy for the postoperative voiding test was as follows. From theatre (MUS-only surgery) or from removal of the self-retaining catheter (MUS and additional operations), the bladder was allowed to fill naturally. When the patient had a normal sensation of wanting to void, she voided into a container and the volume was measured. The residual bladder volume was measured by a bladder ultrasound scan as soon as possible after the void and within 15 min. The bladder was allowed to fill naturally again and a further V&R test recorded as above. We considered a residual of less than 150 ml to be normal if associated with a voided volume of 200 ml or more (thus ensuring adequate bladder filling, and therefore a representative test). This protocol was in place for historical reasons, but has been used by others [11]. If the voided volume was 300 ml or more, then a residual of up to half the voided volume was accepted (for example, if the voided volume was 400 ml, a residual of up to 200 ml residual would be accepted). Voiding tests were continued until the patient had two consecutive normal V&R tests or a decision made by the clinician (along with the patient) to discharge the patient with a self-retaining catheter for 2–7 days, with a view to a further test without the catheter. If this failed, the patient was discharged for a further 5–7 days (at the clinician’s discretion) with a self-retaining catheter. Patients with voiding problems beyond this time frame were taught clean intermittent self-catheterization or offered surgical release of the tape.
Analysis
As this was a retrospective study using a predefined dataset, no power calculation was performed. The primary outcome of this retrospective study was to determine the likelihood of an abnormal second V&R test if the first test was normal. The secondary outcome was to study the effect of concomitant surgery on postoperative voiding function. The patients were therefore divided into three groups: (1) MUS only, (2) MUS with AR plus any other operation (MUS/AR), (3) MUS plus any operation other than AR (MUS+). The rationale for this categorization was that AR may contribute to voiding dysfunction due to suture tension, haematoma or oedema [6]. An abnormal voiding test was defined as: low-volume void only (LVV; i.e. with a normal residual), a high residual (HR), or both HR and LVV.
Data were analysed and expressed as ratios and percentages, and where appropriate sensitivity and specificity were determined. Groups were compared using the chi-squared test and Fisher’s exact test. Multivariate regression was performed to identify individual factors and procedures associated with an abnormal second V&R test after inadequate tests (LVV) had been removed from the dataset. Backward stepwise regression was undertaken by removing the least significant variable, one at a time. Significant factors and clinically relevant factors were used to construct a prediction model for an abnormal second V&R test. Stata 14 software (StataCorp LP; College Station, TX) was used for statistical analysis.
Results
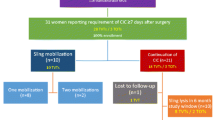
A total of 335 patient records had complete first and second voiding test datasets. In 314 there was additional information. Demographic, procedure and outcome data are shown in Table 1. Operative procedures performed in addition to MUS are shown in Table 2, and the early voiding data in each of the three groups are shown in Table 3. There were no significant differences in the prevalence of LVVs among the three groups .
Table 4 shows the voiding data for each group after patients with LVVs (inadequate tests) had been removed, including the sensitivity/specificity of the first V&R test for predicting a second normal test. Overall, the likelihood of an abnormal second V&R test if the first test was normal was 7.1%. This value was lower (3.6%) in the MUS-only group than in the MUS/AR group and the MUS+ group (11.5% and 8.6%, respectively; Table 4).
The following variables were found to be significant predictors of the second V&R test result: operation group (MUS, MUS/AR, MUS+; p = 0.037), concomitant operations such as anterior repair (p = 0.010), and first V&R test result (p = 0.000). Other variables individually were not significant predictors: sacrospinous fixation (p = 0.632), vaginal hysterectomy (p = 0.483), any vault procedure (p = 0.184), sacrohysteropexy/sacrocolpopexy (p = 0.054), preoperative V&R test result (p = 0.884) and age (p = 0.139). However, examining all the factors together in a backward stepwise logistical regression, only the first V&R test result retained significance throughout (p = 0.000). Operative group did not retain significance in the regression model, but operation type was retained in the final model because of its clinical relevance.
Longer term outcomes are shown in Table 5, although these data are incomplete. The number of postvoid residual tests in individual patients ranged from 2 to 14, with a mode of 2 and a median of 3: 74 patients had 4–6 V&R tests, 25 had 7–11 V&R tests and 1 had 14 V&R tests. Bladder injury occurred in two patients as a result of concomitant vaginal hysterectomy, and an indwelling catheter was left in situ for 12 and 14 days. There were no bladder injuries related to MUS insertion. Three patients had voiding difficulty at 3 months having had no voiding difficulty immediately postoperatively (the reasons for not presenting earlier were not recorded). Recurrence of stress incontinence occurred in 15 patients up to 8 years after surgery.
Discussion
Patients undergoing many minor and intermediate gynaecological procedures such as hysteroscopy, endometrial ablation, cystoscopy and laparoscopy, do not need, other than routine, postoperative assessments prior to discharge and therefore their discharge is relatively predictable. Most clinicians require an assessment of voiding prior to discharge following MUS insertion. If two voiding tests are performed with natural filling, then the time to discharge can be less predictable with a proportion of patients inevitably needing to stay overnight. This affects patient flow through the healthcare system, jeopardizing maximum use of beds, and reducing efficiency. This is particularly pertinent if some of the V&R tests are unsatisfactory, as in our dataset (28%), and therefore repeated voiding tests are required to provide adequate information for a decision to discharge.
Voiding dysfunction is defined by the International Urogynaecological Association and the International Continence Society as an abnormally slow and/or incomplete micturition, diagnosed by symptoms and urodynamic investigations [12]. Changes in flow are expected after MUS surgery [13] and therefore many patients will have voiding dysfunction after MUS surgery according to the above definition.
The rationale for a voiding test after MUS surgery is to ensure that the patient is emptying her bladder satisfactorily most of the time, to avoid over-distension and recurrent infections. Some studies have shown that consecutive voiding tests are inconsistent [14], and therefore suggest the need for a repeat test to ensure the bladder is emptying consistently. However, if a HR is not persistent, then over-distension is not likely to occur without symptoms [9]. A threat to renal function in women with HR would be extremely unlikely because of the low-pressure female system. Although the literature on women is lacking, data from men suggest that there is no specific threshold of residual that predisposes to infection [15, 16].
There are very few consistent data in the literature to provide guidance on best practice in the management of patients after MUS surgery. Both methods of assessment and volume thresholds used for voiding tests vary enormously. A small randomized controlled trial demonstrated that retrograde filling (300 ml) had better accuracy (composite score of sensitivity and specificity) than natural filling, although natural filling provided better sensitivity [7]. This study however, focused on a heterogeneous group of patients undergoing pelvic surgery (not primarily looking at MUS patients) and made the assessment on day 1 following removal of the indwelling catheter.
A more recent retrospective study [4] examining retrograde fill voiding assessment in patients who had undergone MUS-only surgery revealed that 80% of patients had a successful void, defined as at least 200 ml voided volume from an instillation of 300 ml. In this study, patients were catheterized postoperatively, and after 1 h in the recovery room, 300 ml of sterile water was instilled and the patient voided. Those who failed the voiding test were recatheterized with an indwelling catheter and discharged, with the instruction to remove the catheter themselves the following morning and only re-present if concerned. Others have used retrograde filling of 250 ml [17], 300 ml [8, 18] or maximum bladder capacity [19] before a void. The use of patient-reported force of stream postoperatively compared with preoperatively has also been used as a voiding test, a force less than 50% representing a failed voiding test [20]. In a prospective study including 114 patients, the patients rated their force of stream on a 12-point visual analogue scale following a 300 ml retrograde fill. Of 114 patients, 105 passed the voiding test including 14 with residuals in the range 152–427 ml. Patients were advised on symptoms to look for. The primary outcome measure was an unscheduled visit to the office or emergency room. No patients needed to attend the office or emergency room, but 5.3% had a urinary tract infection before or during follow-up.
The advantage of retrograde filling is that the test is consistent, as a specific volume is instilled. It is quick to perform, can be done by the nursing staff, and the postvoid residual does not need to be measured by ultrasonography or catheter. The volume voided is simply subtracted from the volume instilled. The disadvantage, however, is that for patients with a larger bladder capacity, 300 ml may not provide adequate sensation to initiate a normal void. This may in part explain why the failure rate in the study by Barr et al. [4] was 20%. Natural filling has the disadvantage that patients may void before capacity, either due to increased sensation postoperatively or in their eagerness to get the test done and be discharged. The test may then be of poor exclusion value and therefore invalid.
The variation in practice is considerable: several studies have used two residuals with an acceptable volume below 100 ml [6, 21, 22], and others have used a single residual of 100 ml [9, 10]. One retrospective study of 100 patients who underwent MUS surgery with or without concomitant prolapse surgery used V&R test thresholds of at least 200 ml and 150 ml or less, respectively, for an acceptable test. Although this indicates that patients were discharged after one satisfactory V&R test, the authors found a 14% voiding failure rate. However, it is not possible to ascertain whether there were any inadequate tests (i.e. patients with LVV), and if present whether these were considered test failures or excluded from the analysis. Also there was a slightly higher proportion of test failures in the concomitant prolapse repair group, but this was not statistically significant [11]. In on study, a catheter was routinely left in situ until midnight of the day of surgery [23], and in another a catheter was routinely left in situ until the morning after surgery (day 1) [6]. In one study an unacceptable residual was defined as 20% of the self-voided volume [23]. When the MUS surgery was accompanied by prolapse repair, the voiding tests were started after removal of the self-retaining catheter on day 1 [11], day 2 [6, 10] to day 5 [6].
Many studies have examined predictors of a failed voiding test using logistic regression. Some studies showed that pre-operative maximum flow rate [8, 24] and concomitant prolapse surgery [6, 25] are independent risk factors, although this is not consistent throughout all studies. We have also previously reported a study in a small group of patients showing that the use of preoperative flow rate can enhance the sensitivity of the first V&R test as a test of voiding [26]. In our cohort, however, not all patients had a uroflow or urodynamic voiding assessment in accordance with Clinical Guideline 40 of the National Institute for Health and Care Excellence (https://www.nice.org.uk/guidance/cg40). Long-term voiding difficulty (at 6 months) has been reported to occur in up to 8% of patients [10] and more recently in 2.4% of patients [27], but there are some data suggesting that maximum flow rates and residuals will improve with time [14]. In our study, post-operative voiding difficulty occurred in 1.8% of patients at 3 months.
Our data suggest that with the addition of prolapse surgery, and particularly AR, there is a nonsignificant trend towards an increase in the risk of having an abnormal second V&R test when the first was normal, and of having two consecutive abnormal V&R tests. Most of these patients would have had the pack and catheter removed on day 1, and they will have been booked for an overnight stay in any case. This slow return to normal voiding has previously been identified in the literature [6, 25]. The number in this group, however, seems to be falling as there is a trend to uncouple continence and prolapse surgery, to some extent influenced by morbidity following mesh surgery and its perception amongst patients.
Of all the patients in our series, 28% had LVV in at least one of the two tests. The reason for this is unclear. There were no significant differences in the risk of LVV among the three groups. During the immediate postoperative period, the bladder may be a little more sensitive because of instrumentation. Patients may respond to an early sensation to void in their eagerness to be discharged and likewise nursing staff may encourage early voiding in their eagerness to discharge patients. Our criteria may have been too stringent. A LVV is essentially uninterpretable and therefore the test is repeated, resulting in time lost waiting for natural filling, and inefficiency in the system. Retrograde filling in theatre immediately after surgery in patients in whom a self-retaining catheter is not required, with a volume of approximately 350 ml (or just short of capacity as determined by inspection of the bladder diary), may result in a reduced LVV. There is some evidence that backfill-assisted voiding is associated with fewer voiding test failures [28]. Additionally, in those patients who require a second V&R test, an ultrasound scan before voiding may be useful, and if the bladder volume is significantly less than capacity, then encouraging the patient to defer the void may result in a reduced incidence of a LVV and a wasted test. The residual can then be calculated from the prevoid volume minus the voided volume, and so a postvoid residual scan may not be required. This requires further evaluation.
As with many retrospective studies, this study had some limitations. We did not have longer term data for all patients, but our objective was to determine the likelihood of an abnormal second V&R test when the first was normal. We were therefore not able to relate this to long-term outcomes. Additionally, the MUS used in this study were predominantly of the obturator type and therefore applicability of the findings to the retropubic type is uncertain. To obtain the benefits of using a single test, clinicians would need to ensure that all the voiding tests were adequate (no LVV). This is only likely to be achieved by scanning patients before voiding to ensure adequate filling, or retrograde filling in theatre or postoperatively with a volume more than the threshold required the V&R test. This change in practice needs further evaluation.
As 69% of respondents to a UK survey of urogynaecologists [1] used two V&R tests, we feel our findings based on a large sample size contribute to the current literature and demonstrate that as long as the patient’s bladder is adequately filled before the test, a single normal V&R test is all that is necessary in patients who have undergone MUS-only surgery. Those clinicians currently employing a single test can therefore be reassured that a single test is adequate. Those using a more stringent protocol (for example, a residual of less than100 ml) can also be reassured, accepting that there are likely to be more test failures and that the sensitivity is likely to increase, but the specificity decreases with these thresholds.
We have therefore revised our own V&R test protocol (Appendix 1). With such a change in policy we accept that a very small number of patients may develop symptoms of retention, and therefore it is important to warn patients, counsel them regarding double voiding and symptoms of retention and infection, and offer open access to the Gynaecology Department. Open access allows patients unhindered, direct access to the clinic or ward for assessment without first having to go to the Accident and Emergency Department or to their general practitioner.
References
Bray R, Garagasole A, Cartwright R, Digesu A, Fernando R, Khullar V. Bladder care post mid urethral sling placement: a survey of recent practice [abstract]. Int Urogynecol J. 2015;26(11):1701–2.
Swartz M, Vasavada S, Goldman H. Perioperative management of patients undergoing sling surgery: a survey of US urologists. Urology. 2010;76(2):314–7.
Kleeman S, Goldwasser S, Vassallo B, Karrem M. Predicting post operative voiding efficiency after operation for incontinence and prolapse. Am J Obstet Gynecol. 2002;187(1):49–52.
Barr SA, Thomas A, Potter A, Melick CF, Gavard JA, McLennan MT. Incidence of successful voiding and predictors of early voiding dysfunction after retropubic sling. Int Urogynecol J. 2016;27:1209–14.
Wang R, Won S, Haviland MJ, Von Bargen E, Hacker MR, Li J, et al. Voiding trial outcome following pelvic floor repair without incontinence procedures. Int Urogynecol J. 2016;27:1215–20.
Chung SM, Moon YJ, Jeon MJ, Kim SK, Bai SW. Risk factors associated with voiding dysfunction after anti-incontinence surgery. Int Urogynecol. 2010;21(12):1505–9.
Geller EJ, Hankins KJ, Parnell BA, Robinson BL, Dunivan GC. Diagnostic accuracy of retrograde and spontaneous voiding trials for postoperative voiding dysfunction: a randomized controlled trial. Obstet Gynecol. 2011;118(3):637–42.
Wheeler TL, Richter HE, Greer WJ, Bowling CB, Redden DT, Varner RE. Predictors of success with postoperative voiding trials after a mid urethral sling procedure. J Urol. 2008;179(2):600–4.
Duckett JR, Patil A, Papanikolaou NS. Predicting early voiding dysfunction after tension-free vaginal tape. J Obstet Gynaecol. 2008;28(1):89–92.
Dawson T, Lawton V, Adams E, Richmond D. Factors predictive of post-TVT voiding dysfunction. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(11):1297–302.
Lucena HM, Rai H, Siozos C, Tincello DG, Basak S, Giarenis I. Early postoperative voiding dysfunction after insertion of retropubic midurethral tape. Int Urogynecol J. 2016;27:1529–33.
Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4–20.
Dietz HP, Ellis G, Wilson D, Herbison P. Voiding function after tension-free vaginal tape: a longitudinal study. Aust N Z J Obstet Gynaecol. 2004;44:152–5.
Saaby ML, Lose G. Repeatability of post-void residual urine ≥ 100 ml in urogynaecologic patients. Int Urogynecol J. 2012;23(2):207–9.
Brookman-May S, Burger M, Hoschke B, Wieland WF, Kendal F, Gilfrich C, et al. Association between residual urinary volume and urinary tract infection: prospective trial in 225 male patients. Urologe A. 2010;49(9):1163–8.
Brookman-May AS, Hoschke B, Gilfrich C, Braun KP, Kendel F. Post-void residual urine as a predictor of urinary tract infection: is there a cut off value in asymptomatic men? J Urol. 2009;181(6):2540–4.
Lee SY, Lee YS, Lee HN, Choo MS, Lee JG, Kim HG, et al. Transobturator adjustable tape for severe stress urinary incontinence and stress urinary incontinence with voiding dysfunction. Int Urogynecol J. 2011;22(3):341–6.
Ferrante KL, Kim HY, Brubaker L, Wai CY, Norton P, Kraus SR, Shepherd J, Sirls LT, Nager CW; Urinary Incontinence Treatment Network. Repeat post-op voiding trials: an inconvenient correlate with success. Neurourol Urodyn. 2014;33(8):1225–8.
Kim JM, Moon DG, Shin JH, Bae JH, Lee JG, Oh MM. Predictors of voiding dysfunction after mid-urethral sling surgery for stress urinary incontinence. Int Neurourol J. 2012;16(1):30–6.
Ingbar MS, Vasavada SP, Moore CK, Rackley RR, Firoozi F, Goldman HB. Force of stream after sling therapy: safety and efficacy or rapid discharge care pathway based on subjective patient report. J Urol. 2011;185(3):993–7.
Bodelsson G, Henriksson L, Osser S, Stjernquist M. Short term complications of the tension free vaginal tape operation for stress urinary incontinence in women. BJOG. 2002;109(5):566–9.
Huwyler M, Burton C, Renganathan A, Latthe P, Robinson D, Parsons M, et al. Retrospective case modelling to assess the impact of early intervention for voiding dysfunction after retropubic tape. When is it best to intervene? Int Urogynecol J. 2010;21(7):823–7.
Wang AC, Chen MC. The correlation between preoperative voiding mechanism and surgical outcome of the tension-free vaginal tape procedure, with reference to quality of life. BJU Int. 2003;91(6):502–6.
Hong B, Park S, Kim HS, Choo MS. Factors predictive of urinary retention after a tension-free vaginal tape procedure for female stress urinary incontinence. J Urol. 2003;170(3):852–6.
Wang KH, Wang KH, Neimark M, Davila GW. Voiding dysfunction following TVT procedure. Int Urogynecol J. 2002;13(6):353–7.
Ballard P, Shawer S. Anderson C, Afridi G, Khunda A. One normal void and residual is all that is necessary in selected patients who have undergone mid-urethral tape surgery. www.ics.org/Abstracts/Publish/218/000641.pdf
Glavind K, Shim S. Incidence and treatment of postoperative voiding dysfunction after the tension-free vaginal tape. Int Urogynaecol J. 2015;26:1657–00.
Foster RT, Borawski KM, South MM, Weidner AC, Webster GD, Amundsen CL. A randomized controlled trial evaluating 2 techniques of postoperative bladder testing after transvaginal surgery. Am J Obstet Gynecol. 2007;197(6):627.e1–e4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
P. Ballard: Astellas, Pfizer, Boston Scientific sponsored speaker meetings and educational grants.
A Khunda: Astellas, Pfizer, Specialty European Pharma, Boston Scientific sponsored speaker meetings and educational grants. S. Shawer and C. Anderson declare no conflicts of interest.
Appendix 1
Appendix 1
Rights and permissions
About this article
Cite this article
Ballard, P., Shawer, S., Anderson, C. et al. One normal void and residual following MUS surgery is all that is necessary in most patients. Int Urogynecol J 29, 563–569 (2018). https://doi.org/10.1007/s00192-017-3449-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-017-3449-6