Abstract
Purpose
Elucidating subchondral bone remodeling in preclinical models of traumatic meniscus injury may address clinically relevant questions about determinants of knee osteoarthritis (OA).
Methods
Studies on subchondral bone remodeling in larger animal models applying meniscal injuries as standardizing entity were systematically analyzed. Of the identified 5367 papers reporting total or partial meniscectomy, meniscal transection or destabilization, 0.4% (in guinea pigs, rabbits, dogs, minipigs, sheep) remained eligible.
Results
Only early or mid-term time points were available. Larger joint sizes allow reporting higher topographical details. The most frequently reported parameters were BV/TV (61%), BMD (41%), osteophytes (41%) and subchondral bone plate thickness (39%). Subchondral bone plate microstructure is not comprehensively, subarticular spongiosa microstructure is well characterized. The subarticular spongiosa is altered shortly before the subchondral bone plate. These early changes involve degradation of subarticular trabecular elements, reduction of their number, loss of bone volume and reduced mineralization. Soon thereafter, the previously normal subchondral bone plate becomes thicker. Its porosity first increases, then decreases.
Conclusion
The specific human topographical pattern of a thinner subchondral bone plate in the region below both menisci is present solely in the larger species (partly in rabbits), but absent in rodents, an important fact to consider when designing animal studies examining subchondral consequences of meniscus damage. Large animal models are capable of providing high topographical detail, suggesting that they may represent suitable study systems reflecting the clinical complexities. For advanced OA, significant gaps of knowledge exist. Future investigations assessing the subchondral bone in a standardized fashion are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the spatio-temporal trajectory of subchondral bone remodeling may provide better insights into osteoarthritis (OA) [27]. In the knee, both menisci complement and protect the osteochondral unit [25]. Meniscus injury is common [20, 32], and meniscus tissue insufficiency and loss are one of the most important causes of knee OA [5,6,7, 21, 49, 52]. The principle of inducing a defined meniscal lesion is applied in many animal models to model OA initiation and development [42, 50]. However, the resulting subchondral bone alterations are incompletely understood [36], in contrast to the cartilage [35]. Though rodents represent by far the most popular model, their small joints limit an accurate topographical recapitulation. Therefore, this systematic review focuses on larger animal models only, analyzing the available knowledge about subchondral bone remodeling applying traumatic meniscal injuries as a standardizing entity. It reports study designs, establishes missing gaps and pinpoints future research directions.
Comparative morphology of the subchondral bone
As its two major parts, the subchondral bone plate and subarticular spongiosa are structurally dissimilar, they need to be considered separately [37]. The human subchondral bone plate is composed of 0.2–0.4 mm thick plates joining together, enclosing pores, extensions of the marrow space and invading vascular channels [16]. Humans have the largest tibial plateau width (~ 7.4 ± 0.5 cm), followed by sheep (~ 5.1 ± 0.1 cm), minipigs (~ 3.9 ± 0.1 cm), rabbits (~ 1.6 ± 0.1 cm), rats (~ 0.7 ± 0.1 cm), and mice (~ 0.3 ± 0.1 cm). Remarkably, the human subchondral bone plate is considerably thinner (0.52 ± 0.11 mm) than in sheep (1.32 ± 0.14 mm), minipigs (0.82 ± 0.17 mm) and similar to rabbits (0.49 ± 0.05 mm) [39]. In these animals, the subchondral bone plate is more compact and less porous [39]. When normalized to the tibial plateau width, the subchondral bone plate in minipig is twofold, and in sheep and rabbits threefold thicker than in humans [39]. Its microstructure (Table 1) relates to the severity of OA [24, 26, 34, 40]. The subchondral bone plate of rabbits and minipigs is most similar to humans, including similar BS/BV, BS/TV, and closed porosity, while BV/TV is higher, and open and total porosity are lower [39]. In sheep, BV/TV is higher, and BS/BV, BS/TV, open and total porosity are lower than in humans [39]. Of note, the specific human topographical pattern of a thinner subchondral bone plate in the region below both menisci is present solely in the larger species (partly in rabbits), but absent in rodents, a clinically highly relevant fact to consider when designing animal studies examining structural (subchondral) consequences of meniscus damage.
The more porous subarticular spongiosa of sheep, minipigs and rabbits is also more dense and complex than humans, reflected by higher BV/TV, Tb.N, BS/TV, and lower Tb.Sp [39]. For example, the BV/TV of sheep, minipig and rabbit is ~ twofold higher, the Tb.N of sheep and rabbit is ~ twofold, minipig is ~ threefold higher, and the Tb.Sp of sheep is 0.3-fold, minipig is 0.15-fold, and rabbit is 0.4-fold lower than in humans [39]. Structural differences between the lateral and medial tibial plateau trabecular structure as in humans exist in sheep and minipigs [39], but are largely absent in rabbits [39]. These differences include in the medial subregions higher BV/TV (humans, sheep, minipigs), BS/TV (sheep), Tb.Th (humans, minipigs, rabbits), Tb.N, DA and Conn.Dn (sheep), and lower Tb.Pf (humans, sheep), and Tb.Sp (humans) compared to lateral [39]. In contrast, a weaker medial trabecular structure is reflected in a lower BS/BV (humans, minipigs), BS/TV (minipigs), Tb.N (rabbits), FD (sheep, minipigs), Conn.Dn (minipigs), and higher SMI (rabbits), and Tb.Sp (sheep) in the medial subregions compared to lateral [39].
Pattern of subchondral bone changes
Structural subchondral bone alterations are important characteristics of both early and advanced stages of OA. At onset, primary osteoporotic changes occur [10], besides early degenerative cartilage changes [15, 25, 36, 43, 45]. Subchondral bone plate porosity increases, and BMD, trabecular volume, and complexity of the trabecular structure decrease [15, 43]. These changes may be caused by microdamage of the trabeculae due to altered load [17, 28].
After the initial bone loss, still in early OA, subchondral sclerosis (increase in trabecular volume and complexity), as well as osteophytes occur [25, 43]. Later, pronounced abnormalities of bone shape and cysts appear [25].
Literature search results
(a) PubMed search
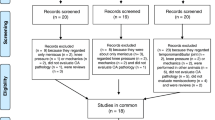
An initial PubMed search was performed on 15.04.2023 with the terms “(osteoarthritis) AND ((meniscus) OR (meniscal) OR (meniscectomy))” yielded 5367 results, including several studies not reporting any subchondral bone data (Fig. 1). When the search was refined as “(subchondral bone) AND (osteoarthritis) AND ((meniscus) OR (meniscal) OR (meniscectomy))”, it resulted in 521 papers (9.7% of the original search). In the detailed analysis only those studies were included where OA was evoked by traumatic tear or injury of the meniscus, surgical destabilization of the meniscus (DMM), or total or partial meniscectomy. To avoid any supplementary factors leading to additional instability such as ligament transection (e.g. anterior cruciate ligament), such combined methods were excluded (Fig. 1). Papers were also excluded if full text was unavailable, if they were reviews, not in English language, subchondral bone or appropriate controls not reported, or meniscal injury not applied to induce OA. The paper selection consequently was further reduced after reading their abstracts (n = 222), and full text (n = 152 papers). When human (n = 9), mouse (n = 96) and rat (n = 25) studies were excluded, n = 23 studies remained eligible for detailed systematic evaluation (only 0.4% of all papers reporting meniscal injury).
(b) Evaluation of the abundance of studies
The first animal study fulfilling the inclusion criteria was published in 1993 [3]. The abundance of eligible papers was constantly low afterwards (n = 0–6 in 4-year intervals) (Fig. 2a).
Summary of the n = 23 papers evaluating the subchondral bone in animal models of OA induced by meniscal injuries. a Histogram showing the number of eligible papers in 4-year intervals and the most important subchondral bone b evaluation methods and c parameters most frequently reported. Note that the cumulative percentages within the graphs may not be equal to 100% due to some studies reporting multiple techniques or parameters. d Reported time points in all of the studies (total n = 23), expressed as percentage of the average life span [41, 42] of the species. Dots indicate study termination time points corresponding to the displayed percent range. Papers reporting multiple time points are presented with multiple dots on the figure. CT computed tomography; DXA Dual Energy X-ray Absorptiometry; IHC immunohistochemistry; MRI magnetic resonance imaging; SCBP subchondral bone plate
(c) Evaluation of the study designs
In the finally selected 23 papers, four main types of induced meniscal damage were described: (1) total meniscectomy, (2) partial meniscectomy, (3) meniscal transection or tear (MMT; the medial collateral ligament [MCL] is transected in small animals and left intact in large ones, and the pars intermedia is transected at its narrowest point, but no parts of the meniscus are removed), and (4) DMM by “meniscal release” (the anterior root is transected, but no parts are removed).
(d) Evaluation of study methods
The most common method to evaluate subchondral bone structure was histology, used in 44% of the studies (n = 10). Micro-CT was used only in 39% of all studies (n = 9), although it represents the gold-standard method for directly evaluating bone microstructure [9], with excellent reproducibility and accuracy [8] (Fig. 2b). When the occurrence of applying a combined evaluation protocol including microcomputed-tomography (micro-CT) or histology, paired with dual energy X-ray absorptiometry (DXA), biochemistry or gross pathology of the joint was examined, histological evaluation was mostly used alone (40% of the total n = 23 studies), or in combination with gross pathology (30%), or DXA (30%). Micro-CT was used alone in 30% of all studies, and in combination with gross pathology in 9%. Histological evaluation was mostly applied for reporting subchondral bone plate thickness, but several studies also used it to evaluate subchondral trabecular microstructure, despite the strong limitation of the stereologic analysis of a few 2-dimensional (2D) sections assuming plate-like underlying structure [8]. Many of such studies did not identify significant differences between treatment groups [23, 33]. Overall, the most frequently reported bone parameters were BV/TV, BMD, presence of osteophytes, Tb.Th, thickness of the subchondral bone plate, and Tb.N (Fig. 2c). Larger animal studies only reported early and mid-term time points (Fig. 2d).
Subchondral bone changes caused by meniscus damage in different larger animal models
(a) Guinea pigs
Partial medial meniscectomy and “meniscectomy” (with unclear definition, probably rather involving MMT) protocols were used in four studies (Table 2). In the partial medial meniscectomy model, at 1 month, BMD decreased [47], and subchondral bone plate thickness [46] and BV [46, 47] were unchanged. At 3 months, subchondral bone plate thickness [46] and medial BMD [47] increased (BV unchanged), indicating subchondral bone plate sclerosis at mid-term. In a (probably) MMT model, at 12 weeks, osteophytes [18], decreased trabecular BMD [18, 19], BV/TV [18, 19], Tb.Th [18, 19], and increased Tb.Sp [18, 19], SMI [18, 19], and Tb.Pf [19] were reported, indicating a loss of trabecular bone. Of note, in the Hartley guinea pigs, a commonly used outbred strain of short haired albino guinea pigs, the usage of appropriate, age-matched, sham operated controls is exceedingly important due to the commonness of spontaneous OA.
In sum, meniscal damage at 3 months in guinea pigs resulted in increased subchondral bone plate thickness and loss of trabecular bone (Table 2, Fig. 3a–c), similarly to other early/mid-term OA models.
Numbers and ratios of studies reporting the directions of changes of the individual bone microstructural parameters at different time points in multiple species. Stacked column diagrams showing OA-related changes of the bone microstructural parameters following a guinea pig medial meniscal transection (MMT) at 12 weeks and guinea pig destabilization of the medial meniscus (DMM) at b 1 and c 3 months, rabbit DMM at d 8, and at e 13–40 weeks, f dog DMM at 12 weeks, and sheep partial meniscectomy at g 6 weeks and h 6 months, and total meniscectomy at (i) 3, (j) 6, (k) 9 months. Numbers in columns show the number of studies evaluated. BMD or TMD bone or tissue mineral density; BV/TV percent bone volume; Conn.Dn connectivity density; SCBP th. subchondral bone plate thickness; Tb.N trabecular number; Tb.Pf trabecular pattern factor; Tb.Sp trabecular separation; Tb.Th trabecular thickness
(b) Rabbits
Studies examining the consequences of total and partial medial meniscectomy, or of anterior medial or lateral root tears did not report major subchondral bone changes (Table 3, Fig. 3d, e). At 2 weeks after total meniscectomy, subchondral bone plate BMD decreased [1]. At 3 weeks, a bone remodelling score was unchanged, scintimetric uptake increased [22]. Between 3 and 8 weeks, regional bone blood flow (tibial plateau, femoral condyles) increased [2]. At 4 and 8 weeks, subchondral bone plate BMD decreased [1].
In contrast, beginning at 12 weeks, subchondral bone plate BMD [1] did not differ from sham operated knees. At 13 weeks, osteophyte developed [38] and the subchondral bone plate became thicker (peripheral regions, medial tibial plateau) [23], while BMD [38], total subchondral bone and trabecular BV/TV [23], trabecular BS/TV [23], BS/BV [23], Tb.Th [23], and Tb.N [23] were unchanged. At 24 weeks, unchanged subchondral bone plate BMD [1] was found. At 25 and 40 weeks, the bone structure was similar to the 13 weeks time point, with osteophytes [38], increased subchondral bone plate thickness in the peripheral regions of the medial tibial plateau [23], unchanged BMD [38], subchondral bone and trabecular BV/TV [23], trabecular BS/TV [23], BS/BV [23], Tb.Th [23], and Tb.N [23].
In sum, total meniscectomy induced only minor changes in the subchondral bone, including decreased subchondral bone plate BMD and increased bone blood flow at the earlier time points (2–8 weeks). Osteophytes developed in the later phase after 12 weeks, while BMD and trabecular structure were unchanged.
After partial meniscectomy, no cysts, osteophytes, or sclerosis, and unchanged bone structure [14] were reported at 2–10 weeks. After DMM, at 8 weeks, increased [53] or unchanged [48] BV/TV, decreased Tb.N [53], decreased lateral Tb.N after medial meniscal root tear [48], decreased medial Tb.N after lateral root tear [48], unchanged Tb.Th [48, 53], unchanged trabecular and subchondral bone plate BMD [48], increased lateral Tb.Sp after medial root tear [48], and increased medial Tb.Sp after lateral root tear [48], were reported. At 12 weeks, BV/TV increased [51].
In sum, DMM induced only minor changes of the subchondral bone after 8–12 weeks, including increased BV/TV, and trabecular bone loss mostly in the compartment opposing the operated compartment. The fact that no characteristic major microstructural changes were detected (Fig. 3d, e) might be due to the limited sensitivity of the applied 2D detection methods applied. In order to achieve better comparability with human and other animal data, more short- and long-term studies with sensitive 3D detection methods such as micro-CT are needed.
(c) Dogs
Partial and total meniscectomy and meniscal destabilization were examined in 2 studies (Table 4, Fig. 3f) [31, 33]. Both partial and total meniscectomy at 16 weeks evoked non-significantly increased percentage bone area, besides largely similar cartilage damage, and evidence of meniscal repair [31]. DMM at 12 weeks resulted in increased medial tibial plateau subchondral bone plate thickness [33], unchanged trabecular BV/TV [33] and Tb.Th [33] besides medial compartment cartilage pathology [33].
Thus, data from dogs are scarce and analyses only based on 2D histological sections which have limited accuracy compared to true 3D analyses with micro-CT [8]. Furthermore, time points covered only the 12–16 week period corresponding to early/mid stage OA, when no extensive alterations of the subchondral bone were observed. To allow for a detailed comparison with human and other animal data, more shorter- and longer-term studies are necessary.
(d) Minipigs
Only one study described purely meniscus-related OA changes of the subchondral bone [4] (Table 5). In Yucatan minipigs, at 1 month after DMM, cartilage contact area decreased and concentrated at the cartilage-cartilage region [4], deep BV/TV decreased [4], and superficial Tb.Th increased [4]. At 3 months, contact area, deep BV/TV, and superficial Tb.Th all became normal [4]. These changes might be due to an early transient loss of smaller trabeculae caused by increased loads, which is consistent with other early OA models [15].
Longer duration studies are needed to examine whether the minipig DMM model shows similar late OA subchondral bone sclerosis as humans.
(e) Sheep
A detailed systematic review analyzed meniscectomy-induced OA in sheep [50], although the focus was not on subchondral bone alterations and no studies before 2010 were reported. Surgical OA induction was achieved via total medial (n = 2 studies) [3, 12], total lateral (n = 3 studies) [11, 13, 29], and anterior partial medial (n = 2 studies) [43, 44] meniscectomy, complete transection of the medial pars intermedia (i.e. MMT; n = 1 study) [12], and DMM (n = 1 study) [12] (Table 6).
After total (medial or lateral) meniscectomy, at 3 months, osteophytes [12, 13, 29], increased subchondral bone plate thickness [12, 29], and unchanged BMD [29] were observed. At 6 months, osteophytes [3, 11, 13], increased subchondral bone plate thickness [13], decreased operated (lateral) and increased contralateral (medial) compartment BMD [11], and thicker operated and thinner contralateral compartment subchondral bone plate [11] were detected. At 9 months, subchondral bone plate thickness [29], BMD [29] increased and osteophytes developed [29]. Thus, osteophytes and increased subchondral bone plate thickness are characteristic of both the relatively early and more advanced stages of OA, while BMD first decreased at mid-term, and then increased at a more advanced stage.
At the anterior subregions after anterior partial medial meniscectomy, at 6 weeks, small osteophytes, increased subchondral bone plate porosity, unchanged subchondral bone plate thickness, decreased subchondral bone plate BMD; distinct loss and thinning of subchondral trabeculae were observed besides developing cartilage damage [43]. In other subregions of the medial tibial plateau, such changes were minor and the subchondral bone plate unchanged [43]. At 6 months, subchondral bone plate thickness increased, its porosity and BMD decreased, and large osteophytes occurred in the anterior subregions. The entire medial tibial plateau exhibited a strong loss of subchondral trabeculae (decreased BMD, and Tb.N, and increased Tb.Sp, and Tb.Th) [43, 44]. These data reveal a progressive loss of subchondral trabeculae, starting below the location of the meniscal injury at early and mid-term OA, reflected in disrupted correlations of microstructural osteochondral parameters.
Total meniscectomy, MMT and DMM all induced largely similar cartilage damage at 3 months [12]. Differences included more anterior and focal cartilage lesions in DMM versus more widespread lesions. Osteophyte formation and subchondral bone plate thickness increased after total meniscectomy and MMT [12].
In sum, early and mid-term ovine OA development was observed in the studied 1.5–9 months’ time-frame. It is characterized by a local deterioration of the subchondral bone with osteophyte development, increased subchondral bone plate thickness, loss of bone volume and trabeculae, and decreased mineralization affecting primarily the compartment with compromised meniscal integrity, mostly independently of the applied technique (Table 6, Fig. 3g–k). Importantly, studies resembling human late OA by examining such ovine subchondral bone changes with longer follow-up time (several years) are noticeably lacking.
Discussion
The most important finding is the spatio-temporal pattern of subchondral bone remodeling: Changes in the subarticular spongiosa occur shortly before those of the subchondral bone plate. These early alterations involve a degradation of the trabecular elements, reduction of their number, loss of bone volume and reduced mineralization. Soon thereafter, the previously normal subchondral bone plate becomes thicker. Its porosity first increases, then decreases. Other essential conclusions are that: (1) Only early or mid-term time points were presented. (2) Larger joint sizes allow reporting higher topographical details. (3) The most frequently reported bone parameters were BV/TV (61%), BMD (41%), osteophytes (41%) and subchondral bone plate thickness (39%). (4) Subchondral bone plate microstructure is not comprehensively characterized. (5) Microstructure of the subarticular spongiosa is well described.
Out of the 5367 identified meniscus-related OA studies, only 521 (9.7%) mentioned subchondral bone in its title or abstract, and out of them only 23 (0.4%) fulfilled the criteria to report subchondral bone characteristics in the surgical protocol solely based on meniscal damage in guinea pigs, rabbits, dogs, minipigs, and sheep. Data on dogs and minipigs were scarce with only a few published studies. For dogs, this might be due to the public perception being companion animals, the often complex ethical approval processes, and their difficult and costly management [41]. Minipigs require specialized husbandry and food, and they are considerable less docile than sheep, and the miniature strains, more suitable for OA research than large agricultural pigs, are possibly less widely available in some countries [41]. No data from horses or goats were identified. Some large animal studies reported their results in high topographic details, usually examining only 1 or 2 time points. In rabbits, contrastingly, 3–5 end points, covering a broader time scale from early to mid-term / late OA were sometimes reported. Only a low percentage (0.4%) of studies report subchondral bone characteristics. The number of such studies did not change recently for the examined larger species. Among them, rabbits are most frequently used (39%). OA is induced mostly in the medial compartment (87%), in a unilateral study design (61%), in the right (57%) knee, by total meniscectomy (48%). Micro-CT was only selected in 39% of the studies (histology: in 44%), although it is the most capable and recommended [8] method to analyze bone structure at high detail.
Eligible work presented only early or mid-term time points of OA development. Studies reporting more severe damage in the region below a meniscus lesion confirm the spatio-temporal pattern of subchondral bone remodeling induced by a meniscus injury [43, 44]. At the site of the injury, osteophytes appear relatively soon. Remarkably, changes in the subarticular spongiosa appear slightly before subchondral bone plate alterations, as ovine data suggests. These early alterations are characterized by a degradation of the trabecular elements and reduction of their number (decreased Tb.N), loss of bone volume (mostly decreased trabecular BV/TV, increased Tb.Pf and Tb.Sp), and reduced mineralization (BMD, TMD). Soon afterwards, the previously normal subchondral bone plate becomes thicker. Its porosity, a parameter negatively associated with sclerosis, first in-, then decreases (Fig. 4).
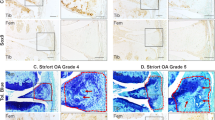
Summary of the reported subchondral bone microstructural changes in early/mid-term OA. a 3-dimensional reconstructed micro-CT model of the subchondral bone plate and subarticular spongiosa showing the generally evaluated microstructural parameters. Representative safranin-O/fast-green stained histological sections of the medial tibial plateau of b a normal sheep and c a sheep 6 months after partial medial meniscectomy [43]. Arrowheads point to characteristic subchondral bone microstructural alterations described in multiple animal models in early / mid-term OA, including (1) increased subchondral bone plate porosity, and degradation of the trabecular elements with (2) increased trabecular separation, and (3) reduction of their number, loss of bone volume and reduced mineralization. BMD bone mineral density; BS/BV bone surface-to-volume ratio; BS/TV bone surface density; BV/TV percent bone volume; Conn.Dn connectivity density; DA degree of anisotropy; FD fractal dimension; SMI structure model index; Tb.N trabecular number; Tb.Pf trabecular pattern factor; Tb.Sp trabecular separation; Tb.Th trabecular thickness
Methodological issues were also identified (Table 7). A detailed, quantitative 3D microstructural assessment of the subchondral bone is still not a general practice (performed in ~ 1/5 of the studies), limiting our knowledge about subchondral remodeling. Although cartilage analyses are relatively well standardized, similar methodical guidance of bone evaluation is absent. Such data would be needed to allow for clinically relevant and comparable conclusions in distinct model species and time-points. A standardization of the evaluation techniques and the reported parameters could be achieved for example by using the gold-standard micro-CT, reporting a minimum parameter set of BV/TV, Tb.Th, Tb.Sp, and Tb.N of the subchondral trabecular bone (as recommended in the classical paper of Bouxsein et al.) [8], together with subchondral bone plate thickness and osteophytes. These parameters can be reliably and accurately detected also in patients with clinical CT [40], allowing for a direct comparisons with the animal models. Still, only 22% of all examined studies report these 4 recommended trabecular parameters, and only 9% of all studies present the extended parameter set including subchondral bone plate thickness and osteophytes.
Definition of analysis volumes of interests (VOI) also needs standardization, by constantly separating the subchondral bone plate from the trabecular bone VOIs. Yet, many studies reported them together, even though separation is possible in all examined species [39]. This appears especially important because simultaneous different direction of numerical changes in the two bone regions, observed commonly in various models (e.g. an increased BV/TV in the subchondral bone plate and decreased BV/TV in the subchondral trabecular bone), would result in apparently unchanged total subchondral bone parameters, complicating to reveal existing structural alterations and possibly resulting in false conclusions.
In early human and late large animal OA related to traumatic meniscal injuries, structural subchondral data are largely absent that could provide crucial information about the temporal trajectory of changes. As many parameters decrease in early OA below normal, and increase above it in late OA (or vice versa), a direct comparison of the results is not feasible without a clear definition of “early”, “mid-term”, and “late” OA stages within each species. Depending on OA stage, an increase, decrease or no difference of a certain parameter vs. normal is also possible and may reliably mirror the actual disease stage. Combining multiple microstructural parameters may identify a phenotypical fingerprint of each stage of the disease. Thus, detailed longitudinal studies with identical surgical protocols, multiple (early, mid, late) time points, appropriate normal/sham controls, and reliable and exhaustive structural analyses are required in all species.
Limitations include the absence of large animal microstructural data on late OA, complicating the comparability with humans. They are required in the future. High quality longitudinal studies revealing subchondral bone microstructural changes following meniscal injuries are unavailable, but needed to determine which animal model species represents best the human condition most faithfully. By using in vivo micro-CT or multiple termination time points, comparable longitudinal animal data could also be collected. While the meniscus tear is traumatic in nearly all animal models, the more common clinical situation of degenerative tears needs more attention [30]. However, degenerative lesions of the meniscus will be difficult to imitate in animal models.
In sum, changes in the subarticular spongiosa have a short temporal priority over those of the subchondral bone plate. These early alterations involve a degradation of the trabecular elements, reduction of their number, loss of bone volume and reduced mineralization. Soon thereafter, the subchondral bone plate becomes sclerotic; its porosity first increases, then decreases. The specific human topographical pattern of a thinner subchondral bone plate in the region below both menisci is present solely in the larger species (partly in rabbits), but absent in rodents, an important fact to consider when designing animal studies examining subchondral consequences of meniscus damage. Large animal models are capable of providing high topographical detail, suggesting that they may represent suitable study systems reflecting the clinical complexities. Future studies need to assess the subchondral bone in a standardized fashion. Comparative longitudinal studies investigating its microstructure in early, mid-term, and late stages with appropriate normal controls in all larger animal species will allow addressing clinically relevant questions about fundamental determinants of subchondral bone remodeling in knee OA caused by meniscal injuries.
Data and materials availability
All data associated with this study are available in the main text.
References
Anetzberger H, Mayer A, Glaser C, Lorenz S, Birkenmaier C, Muller-Gerbl M (2014) Meniscectomy leads to early changes in the mineralization distribution of subchondral bone plate. Knee Surg Sports Traumatol Arthrosc 22:112–119
Anetzberger H, Thein E, Loffler G, Messmer K (2004) Fluorescent microsphere method is suitable for chronic bone blood flow measurement: a long-term study after meniscectomy in rabbits (1985). J Appl Physiol 96:1928–1936
Armstrong SJ, Read RA, Ghosh P, Wilson DM (1993) Moderate exercise exacerbates the osteoarthritic lesions produced in cartilage by meniscectomy: a morphological study. Osteoarthritis Cartilage 1:89–96
Bansal S, Miller LM, Patel JM, Meadows KD, Eby MR, Saleh KS et al (2020) Transection of the medial meniscus anterior horn results in cartilage degeneration and meniscus remodeling in a large animal model. J Orthop Res 38:2696–2708
Beaufils P, Becker R, Kopf S, Englund M, Verdonk R, Ollivier M et al (2017) Surgical management of degenerative meniscus lesions: the 2016 ESSKA meniscus consensus. Knee Surg Sports Traumatol Arthrosc 25:335–346
Beaufils P, Becker R, Verdonk R, Aagaard H, Karlsson J (2015) Focusing on results after meniscus surgery. Knee Surg Sports Traumatol Arthrosc 23:3–7
Borque KA, Jones M, Cohen M, Johnson D, Williams A (2022) Evidence-based rationale for treatment of meniscal lesions in athletes. Knee Surg Sports Traumatol Arthrosc 30:1511–1519
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25:1468–1486
Burghardt AJ, Link TM, Majumdar S (2011) High-resolution computed tomography for clinical imaging of bone microarchitecture. Clin Orthop Relat Res 469:2179–2193
Burr DB, Gallant MA (2012) Bone remodelling in osteoarthritis. Nat Rev Rheumatol 8:665–673
Cake MA, Read RA, Appleyard RC, Hwa SY, Ghosh P (2004) The nitric oxide donor glyceryl trinitrate increases subchondral bone sclerosis and cartilage degeneration following ovine meniscectomy. Osteoarthritis Cartilage 12:974–981
Cake MA, Read RA, Corfield G, Daniel A, Burkhardt D, Smith MM et al (2013) Comparison of gait and pathology outcomes of three meniscal procedures for induction of knee osteoarthritis in sheep. Osteoarthritis Cartilage 21:226–236
Cake MA, Read RA, Guillou B, Ghosh P (2000) Modification of articular cartilage and subchondral bone pathology in an ovine meniscectomy model of osteoarthritis by avocado and soya unsaponifiables (ASU). Osteoarthritis Cartilage 8:404–411
Calvo E, Palacios I, Delgado E, Ruiz-Cabello J, Hernandez P, Sanchez-Pernaute O et al (2001) High-resolution MRI detects cartilage swelling at the early stages of experimental osteoarthritis. Osteoarthritis Cartilage 9:463–472
Chen Y, Hu Y, Yu YE, Zhang X, Watts T, Zhou B et al (2018) Subchondral trabecular rod loss and plate thickening in the development of osteoarthritis. J Bone Miner Res 33:316–327
Ching K, Houard X, Berenbaum F, Wen C (2021) Hypertension meets osteoarthritis—revisiting the vascular aetiology hypothesis. Nat Rev Rheumatol 17:533–549
Choi BS, Chung J, Kwak J, Han HS (2023) Subchondral insufficiency fracture is a predictive factor of osteoarthritis progression and conversion to arthroplasty in non-surgically treated medial meniscus root tear. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-023-07444-6
Chu JG, Dai MW, Wang Y, Tian FM, Song HP, Xiao YP et al (2017) Strontium ranelate causes osteophytes overgrowth in a model of early phase osteoarthritis. BMC Musculoskelet Disord 18:78
Dai MW, Chu JG, Tian FM, Song HP, Wang Y, Zhang YZ et al (2016) Parathyroid hormone(1–34) exhibits more comprehensive effects than celecoxib in cartilage metabolism and maintaining subchondral bone micro-architecture in meniscectomized guinea pigs. Osteoarthritis Cartilage 24:1103–1112
Deviandri R, Daulay MC, Iskandar D, Kautsar AP, Lubis AMT, Postma MJ (2023) Health-economic evaluation of meniscus tear treatments: a systematic review. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-022-07278-8
Englund M, Roemer FW, Hayashi D, Crema MD, Guermazi A (2012) Meniscus pathology, osteoarthritis and the treatment controversy. Nat Rev Rheumatol 8:412–419
Fahlgren A, Chubinskaya S, Messner K, Aspenberg P (2006) A capsular incision leads to a fast osteoarthritic response, but also elevated levels of activated osteogenic protein-1 in rabbit knee joint cartilage. Scand J Med Sci Sports 16:456–462
Fahlgren A, Messner K, Aspenberg P (2003) Meniscectomy leads to an early increase in subchondral bone plate thickness in the rabbit knee. Acta Orthop Scand 74:437–441
Finnila MAJ, Thevenot J, Aho OM, Tiitu V, Rautiainen J, Kauppinen S et al (2017) Association between subchondral bone structure and osteoarthritis histopathological grade. J Orthop Res 35:785–792
Goldring SR, Goldring MB (2016) Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol 12:632–644
Han X, Cui J, Xie K, Jiang X, He Z, Du J et al (2020) Association between knee alignment, osteoarthritis disease severity, and subchondral trabecular bone microarchitecture in patients with knee osteoarthritis: a cross-sectional study. Arthritis Res Ther 22:203
Hu Y, Chen X, Wang S, Jing Y, Su J (2021) Subchondral bone microenvironment in osteoarthritis and pain. Bone Res 9:20
Huizinga JL, Shah N, Smith SE, Notino A, Kluczynski MA, Jordan K et al (2020) Prevalence of undiagnosed subchondral insufficiency fractures of the knee in middle age adults with knee pain and suspected meniscal tear. Osteoarthr Cartil Open 2:100089
Hwa SY, Burkhardt D, Little C, Ghosh P (2001) The effects of orally administered diacerein on cartilage and subchondral bone in an ovine model of osteoarthritis. J Rheumatol 28:825–834
Jarraya M, Roemer FW, Englund M, Crema MD, Gale HI, Hayashi D et al (2017) Meniscus morphology: Does tear type matter? A narrative review with focus on relevance for osteoarthritis research. Semin Arthritis Rheum 46:552–561
Johnson KA, Francis DJ, Manley PA, Chu Q, Caterson B (2004) Comparison of the effects of caudal pole hemi-meniscectomy and complete medial meniscectomy in the canine stifle joint. Am J Vet Res 65:1053–1060
Kopf S, Beaufils P, Hirschmann MT, Rotigliano N, Ollivier M, Pereira H et al (2020) Management of traumatic meniscus tears: the 2019 ESSKA meniscus consensus. Knee Surg Sports Traumatol Arthrosc 28:1177–1194
Kuroki K, Cook CR, Cook JL (2011) Subchondral bone changes in three different canine models of osteoarthritis. Osteoarthritis Cartilage 19:1142–1149
Li Y, Liem Y, Dall’Ara E, Sullivan N, Ahmed H, Blom A et al (2021) Subchondral bone microarchitecture and mineral density in human osteoarthritis and osteoporosis: a regional and compartmental analysis. J Orthop Res 39:2568–2580
Madry H, Kon E, Condello V, Peretti GM, Steinwachs M, Seil R et al (2016) Early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 24:1753–1762
Madry H, Luyten FP, Facchini A (2012) Biological aspects of early osteoarthritis. Knee Surg Sports Traumatol Arthrosc 20:407–422
Madry H, van Dijk CN, Mueller-Gerbl M (2010) The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc 18:419–433
Messner K, Fahlgren A, Ross I, Andersson B (2000) Simultaneous changes in bone mineral density and articular cartilage in a rabbit meniscectomy model of knee osteoarthrosis. Osteoarthritis Cartilage 8:197–206
Michaelis JC, Olah T, Schrenker S, Cucchiarini M, Madry H (2022) A high-resolution cross-species comparative analysis of the subchondral bone provides insight into critical topographical patterns of the osteochondral unit. Clin Transl Med 12:e745
Olah T, Cai X, Gao L, Walter F, Pape D, Cucchiarini M et al (2022) Quantifying the human subchondral trabecular bone microstructure in osteoarthritis with clinical CT. Adv Sci. https://doi.org/10.1002/advs.202201692e2201692
Olah T, Cai X, Michaelis JC, Madry H (2021) Comparative anatomy and morphology of the knee in translational models for articular cartilage disorders. Part I Large Anim Ann Anat 235:151680
Olah T, Michaelis JC, Cai X, Cucchiarini M, Madry H (2021) Comparative anatomy and morphology of the knee in translational models for articular cartilage disorders. Part II Small Anim Ann Anat 234:151630
Olah T, Reinhard J, Gao L, Haberkamp S, Goebel LKH, Cucchiarini M et al (2019) Topographic modeling of early human osteoarthritis in sheep. Sci Transl Med 11:6775
Olah T, Reinhard J, Laschke MW, Goebel LKH, Walter F, Schmitt G et al (2022) Axial alignment is a critical regulator of knee osteoarthritis. Sci Transl Med 14:eabn0179
Ondresik M, Azevedo Maia FR, da Silva MA, Gertrudes AC, Dias Bacelar AH, Correia C et al (2017) Management of knee osteoarthritis. Current status and future trends. Biotechnol Bioeng 114:717–739
Pastoureau P, Leduc S, Chomel A, De Ceuninck F (2003) Quantitative assessment of articular cartilage and subchondral bone histology in the meniscectomized guinea pig model of osteoarthritis. Osteoarthritis Cartilage 11:412–423
Pastoureau PC, Chomel AC, Bonnet J (1999) Evidence of early subchondral bone changes in the meniscectomized guinea pig. A densitometric study using dual-energy X-ray absorptiometry subregional analysis. Osteoarthritis Cartilage 7:466–473
Steineman BD, LaPrade RF, Santangelo KS, Warner BT, Goodrich LR, Haut Donahue TL (2017) Early osteoarthritis after untreated anterior meniscal root tears: an in vivo animal study. Orthop J Sports Med 5:2325967117702452
Verdonk R, Madry H, Shabshin N, Dirisamer F, Peretti GM, Pujol N et al (2016) The role of meniscal tissue in joint protection in early osteoarthritis. Knee Surg Sports Traumatol Arthrosc 24:1763–1774
Veronesi F, Vandenbulcke F, Ashmore K, Di Matteo B, Nicoli Aldini N, Martini L et al (2020) Meniscectomy-induced osteoarthritis in the sheep model for the investigation of therapeutic strategies: a systematic review. Int Orthop 44:779–793
Yan F, Zhao X, Duan S, Maimaiti A, Qi Y, Li M et al (2021) High fibular osteotomy ameliorates medial compartment knee osteoarthritis in a rabbit model. J Biomech 128:110734
Zhan H, Liu Z, Wang Y, Chen Y, Teng F, Yang A et al (2023) Radiographic OA, bone marrow lesions, higher body mass index and medial meniscal root tears are significantly associated with medial meniscus extrusion with OA or medial meniscal tears: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc 31:3420–3433
Zhou Z, Deng Z, Liu Y, Zheng Y, Yang S, Lu W et al (2021) Protective effect of SIRT1 activator on the knee with osteoarthritis. Front Physiol 12:661852
Funding
Open Access funding enabled and organized by Projekt DEAL. Funded by the Center of Experimental Orthopaedics.
Author information
Authors and Affiliations
Contributions
HM and TO conceptualized the study; TO acquired data, collected references, and prepared the figures and tables; TO, MC and HM wrote the initial draft. All authors contributed to editing and revising the manuscript, and have approved the submitted version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oláh, T., Cucchiarini, M. & Madry, H. Subchondral bone remodeling patterns in larger animal models of meniscal injuries inducing knee osteoarthritis – a systematic review. Knee Surg Sports Traumatol Arthrosc 31, 5346–5364 (2023). https://doi.org/10.1007/s00167-023-07579-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-023-07579-6