Abstract
Purpose
The purpose of this study was to perform a systematic review of randomized controlled trials comparing the results of matrix-induced chondrogenesis with other therapies for local chondral lesions of the knee.
Methods
A systematic search for randomized controlled trials (RCT) about matrix-induced chondrogenesis for focal chondral lesions in the knee was performed according to the PRISMA guidelines. Data source was PubMed central, EMBASE and Google scholar.
Results
Five articles could be included, whereas two originated from the same study group. Three studies compared matrix-induced chondrogenesis to microfracture (MFx) only. One trial compared AMIC® to collagen-covered autologous chondrocyte implantation (ACI-C). One study assessed the improvements given by the combination of AMIC® with bone marrow aspirate concentrate (BMAC). In three studies, clinical improvements compared to baseline were seen at 2-year postoperation, irrespective of the technique used. After 5 years, one trial showed better results for the AMIC® group compared to MFx, including MRI defect filling. One study showed also good results after AMIC® with faster recovery for patients with AMIC® + BMAC 12 months postoperatively.
Conclusion
Results of RCTs comparing matrix-induced chondrogenesis with other treatment options showed that matrix-induced chondrogenesis is a valid and safe cartilage repair option for small- to medium-sized cartilage defects of the knee. This one-stage surgical technique presents a good alternative for patients.
Level of evidence
I.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The hyaline articular cartilage has an important function for the joints of the musculoskeletal system, as it enables the transmission of forces and at the same time guarantees the smooth movement of the joint partners. For this reason, cartilage damage can lead to a severe functional restriction and, depending on the size, may result in osteoarthritis in the long term. Various surgical methods are currently available for the treatment of local cartilage damage [2]. The selection of the appropriate procedure is primarily based on the size and depth of the defect as well as age and degree of activity of the patient [10]. In the case of chondral damage, according to the working group “Clinical Tissue Regeneration” of the German Society of Orthopaedics and Trauma (DGOU), the indication for autologous cartilage cell transplant is seen from a defect size of more than 2.5 cm2. Microfracture (MFx) is recommended in the case of a defect smaller than 2.5 cm2 or in patients with low to moderate activity level [10, 23]. Both techniques, MFx and autologous chondrocyte transplantation (ACT), have drawbacks. One disadvantage of MFx is the inferior quality of the tissue, which might lead to unfavorable long-term results [21]. The disadvantage of ACT is that this procedure has to be carried out in two stages (1. arthroscopic removal of tissue samples and 2. open reimplantation of the matrix populated with chondrocytes).
To improve the results after MFx, matrix-based chondrogenesis has been developed in recent years as one-stage surgical technique which should combine the advantages of both procedures. The matrix is intended to offer the cells three-dimensional growth conditions to promote the differentiation of progenitor cells towards chondrocytes. Furthermore, it offers the cells protection against mechanical overload.
There are different matrices available for this procedure. The most popular option is a type I/III collagen membrane (Chondro-Gide®, Geistlich Pharma). The surgical technique with the Chondro-Gide® membrane is known as autologous matrix induced chondrogenesis (AMIC™). Other matrices consist of hyaluronic acid (chondrotissue®, BioTissue; Hyalofast®, Anika). The stem cells in matrix-supported chondrogenesis can derive from two different sources: 1. from the subchondral bone opened by microfracture or drilling or 2. from a source remote from the joint (e.g., iliac crest aspirate).
Animal experiments have confirmed the principle of matrix-based chondrogenesis [7, 8]. In a preclinical study in sheep, the implantation of a cell-free PGA hyaluronic acid matrix (chondrotissue®, BioTissue) after MFx showed significant improvement in regeneration compared to classic treatment with MFx alone [8].
The clinical experience with this new procedure has been positive so far and various studies reported encouraging results [11, 19, 25, 27]. Most of these studies were case series and cohort studies. In the last few years, however, various controlled randomized studies (RCT) on this method have been published.
Aim of this systematic review was to analyze RCTs of patients with small to medium-size chondral lesions of the knee treated with matrix-induced chondrogenesis. Further objective was to find out if this method is associated with any side effects. Regarding the outcome, it was hypothesized that matrix-induced chondrogenesis is a valid and safe cartilage repair option for focal cartilage defects of the knee.
Materials and methods
Search details
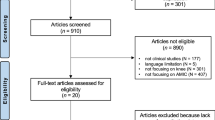
Between May 15, 2020 and July 30, 2020, a systematic literature search was carried out in various databases (PubMed, MEDLINE, EMBASE, Scopus, Google scholar) to identify peer reviewed articles about matrix-induced chondrogenesis of the knee according to the PRISMA guidelines. The PRISMA statement consists of a 27-item checklist and a four-phase flow diagram [14, 22]. Matrix-induced chondrogenesis of the knee was defined as one-step surgical procedure for repair of local cartilage defects using a matrix without cultivation of chondrocytes. Prior to that, the study was registered at PROSPERO, an international database of prospectively registered systematic reviews [4]. For this systematic review, different keywords were utilized: autologous matrix-induced chondrogenesis (AMIC), cell-free collagen type I matrix, polymer-based implant/matrix, collagen-covered microfracture and matrix-augmented bone marrow stimulation. When a study of interest was found, related articles were searched. After identifying those articles, all references were screened for additional relevant publications (Fig. 1).
Inclusion and exclusion criteria
The following inclusion criteria were applied:
-
randomized controlled trial;
-
trials reporting clinical outcome after autologous matrix-induced chondrogenesis in the knee;
-
comparison of at least two treatment techniques;
-
English language reports;
-
publication in a peer reviewed journal.
All criteria should have been satisfied for inclusion in this systematic review.
All papers qualified for inclusion were read by the reviewers and checked for one of the following exclusion criteria:
-
number of patients less than 20;
-
Jadad score ≤ 3.
In case of implementation of at least one exclusion criterion the study was excluded.
Two reviewers (WP, KK) performed the initial study identification, secondary study screening and final determination of eligibility and study inclusion. Both reviewers were also involved in the analysis of the articles.
Analysis
If two separate studies with the same authors and intervention as well as the same patient collective revealed a different follow-up, both publications were analyzed separately if a different follow-up or different outcome measures were reported but both publications were counted as one clinical trial. For the analysis, also the appendices of the included study and publications of the study design were deconstructed.
After extraction of all studies’ data, a brief tabular narrative of each investigation was presented. Data of this tables included (1) first author and year of publication, (2) number of study centers, (3) country, (4) study conducted, (5) number of patients, (6) mean age, (7) matrix used, (8) fixation technique, (9) outcome scores, (10) study design and level of evidence, (11) follow-up and (12) defect size (Table 1). Additional tables were added to illustrate the scores for quality assessment (Table 2) as well as the results (Tables 3, 4).
Study quality and limitations
Each article was analyzed for limitation and bias by all reviewers. For the quality assessment, information has been extracted from the original article, from published appendices or study protocols. Study quality has been analyzed with the Jadad score [15] and with the Coleman methodology score [5].
Primary and secondary endpoints
Primary endpoint of this systematic review was the group difference in the patient reported outcome measures (PROM) of the treatment as reported in the studies in comparison to the control group. Secondary endpoints were the results of postoperative MRI and other clinical scores such as the Lysholm or ICRS score which include ratings by examiner and patient.
Results
Search results and study design
The search results are shown in Fig. 1. Detailed information about the study designs is provided in Table 1. Out of 1480 articles of one step matrix augmented cartilage repair, many had to be excluded due to duplicate publications or missing failure rate.
A total of five articles were identified that reported about results after matrix augmented cartilage repair [1, 9, 12, 13, 29]. Two articles were about one clinical trial with different follow-up [1, 29]. Therefore, four randomized controlled studies on single-stage matrix-assisted chondrogenesis could be analyzed in this systematic review.
One trial compared a microfracture covered by type I/III collagen membrane (AMIC®) to only microfracture (MFx) [1, 29]. One study compared cartilage repair by microfracture and a membrane made of a composite of polyglycolic (PGA) and hyaluronic acid (HA) (chondrotissue®) to MFx alone [13]. In this study the PGA/HA membrane was soaked in autologous plasma. One trial compared AMIC™ to collagen-covered autologous chondrocyte implantation (ACI-C) [9]. One study assessed the improvements given by the combination of AMIC® with bone marrow aspirate concentrate (BMAC) [12]. All trials used arthroscopy and/or a mini-arthrotomy as approach to the defect size of the knee [1, 9, 12, 13, 29]. The matrix used was fixated either with sutures, glue or resorbable pins. The size of the cartilage defect varied with a maximum of 10 cm2 [1] with either one or two defects femoral and/or patellar.
Study quality and limitations
Only RCTs were included in this systematic review. Quality assessment of the studies with the Jadad and the Coleman methodology score is shown in Table 2. The Jadad score ranges from 3 to 4 points. The modified Coleman methodology score ranges between 98 and 107.
Varus or valgus malalignment was excluded in all studies. Only two trials differentiated the outcome measures into primary and secondary endpoints [9, 13]. Blinding was an issue for all trials as a matter of the study design: the surgeons performing the operation could not be blinded as well as the patients if there was a difference between treatment groups if either arthroscopy or arthrotomy was performed. Just one study used arthroscopy only and could, therefore, be described as single-blinded [13].
Primary outcome measure
Primary outcome measure in this systematic review was the outcome in PROMs of the treatment group in comparison to the control group. Several distinct outcome scores were used in the different studies (Table 1).
KOOS
The Knee Injury and Osteoarthritis Outcome Score (KOOS) was used in three RCTs [9, 12, 13]. In none of these studies with a follow-up of 2 years a group difference between matrix augmented chondrogenesis and the control group was observed.
In the study of Fossum et al., comparing AMIC® and ACI-C, the mean KOOS improved from baseline for both groups AMIC® and ACI-C at both follow-ups after 1 and 2 years postoperative. The mean delta for all KOOS subscales at 2 years was higher in the AMIC group, but the difference was not statistically significant [9].
The same was observed in the trial by de Girolamo et al., where AMIC® plus MFx was compared with AMIC® plus bone marrow aspirate concentrate (BMAC). In this study the KOOS increased in both groups without a group difference [12].
The study by Glasbrenner et al. compared a matrix of PGA/HA and MFx with MFx alone with a 2-year follow-up. In this study, there was also a significant improvement over time in both groups concerning the KOOS without a group difference [13].
Cincinatti score
In the trial comparing MFx and AMIC® with MFx alone a modified Cincinnati score was used comparing results after 2 and 5 years, respectively [1, 29]. At 2-year postoperatively, mean Cincinnati scores increased significantly from baseline values for all groups (MFx, sutured or glued AMIC®) without significant difference between the groups [1]. After 5 years, a significant decrease was observed in the MFx group, whereas the scores improved further for the AMIC® group [29].
IKDC
The objective International Knee Documentation Commitee Form (IKCD) was assessed in two trials [12, 13].
In one study, the score improved for both groups AMIC® as well as AMIC® + BMAC 6 months after surgery. Later, patients treated with the standard AMIC® technique did not show further improvements, whereas patients in the AMIC® + group improved constantly until the 24-month follow-up with significant difference to the pre-operative status for both groups [12].
The other trial showed gradual increase of the IKDC score in both groups treated either with MFx or AMIC®. A significant increase from baseline was only documented at weeks 54 and 108 for both treatment groups but not between groups at all follow-up time points [13].
Pain/VAS score
Pain level was evaluated in all RCTs [1, 9, 12, 13, 29].
Compared with the pre-operative state, pain was rated as less severe at 1 and 2-year postoperation for both the AMIC® and the MFx group [1]. After 5 years, AMIC® treated patients still reported a very low pain level, whereas pain increased in the MFx group, but with no statistical significant difference [29].
Comparing AMIC® with AMIC® + BMAC, VAS score significantly improved 6 months after operation in both groups [12]. At the 12-month follow-up, patients treated with AMIC® + demonstrated a significantly lower pain score with respect to the AMIC® group. This difference was no longer detectable at the 24-month follow-up and a minimum level of pain remained up to 100 months of follow-up for both groups [12].
Also, for AMIC® and ACI-C, the mean VAS score improved from baseline at both follow-ups 1- and 2-year postoperation, but the findings were not significant [9].
Another RCT showed no significant difference in terms of pain intensity between groups treated either with AMIC® or MFx at each follow-up. Nevertheless, as compared with the preoperative situation, patients reported better pain relief at 6, 12, 54, and 108 weeks after treatment with m-BMS in contrast to MFx [13].
36-item short form health survey
Evaluating the 36-Item Short Form Health Survey in one study, there was no significant difference in the outcome between patients treated with m-BMS or MFx. Treatment with MFx did not show a significant improvement in any of the subcategories of physical health. The m-BMS group showed a significant increase in social functioning and emotional role limitations. In contrast, MFx led to a significant increase in only emotional role limitations after 54 and 108 weeks [13].
Secondary outcome
Secondary outcome criteria were the defect filling on MRI, other clinical scores or adverse effects.
MRI
Three studies assessed the degree of defect filling and integration of the regenerate postoperatively [1, 12, 13, 29].
The quality of the regenerate surface and defect cover was similar for the MFx group as well as the AMIC® group with a trend towards reduced surface quality, but better defect cover in the glued AMIC® group after 1 year [1]. In addition, at 2 year postoperatively, defect filling was largely comparable between the groups without statistical significance [1]. At 5-year postoperation, the defect filling was the lowest in the MFx group compared to AMIC® treated groups [29].
These results are comparable to the RCT of Glasbrenner et al., where MRI revealed progressive defect filling in terms of cartilage repair tissue formation in both treatment.
groups with no significant difference between the m-BMS and MFx at postoperative 108 weeks [13]. However, changes in overall Henderson score were significantly higher at week 12 and week 108 in the m-BMS group as compared with the MFx group [13].
Another RCT also observed an improved surface appearance and MRI signal after 2 years for patients treated with AMIC® + compared to preoperative status 12 months after surgery [12].
Other clinical scores
A modified International Cartilage Repair Society (ICRS) Score was used in one RCT comparing results of AMIC® versus MFx only after 2 and 5 years [1, 29].
With regard to the functional status, all patients rated it as improved at 1 year postoperatively compared to baseline. At 2-year postoperation, only two patients deteriorated from normal to nearly normal (sutured AMIC®). The assessments performed by the surgeon including classification of the affected knee, crepitation and functional status showed no statistical significances between the groups [1].
At 5 years, in the MFx group, two thirds of the patients rated their status as abnormal, whereas in the AMIC® group, severe ranking was only reported by 6–7% of the patients.
Regarding the objective functional status after 5 years, two thirds in the MFx group and 0–7% in the AMIC® groups were rated as abnormal/severely abnormal.
Summarized, almost 100% of the AMIC® treated patients had improved to a normal or nearly normal functional status, whichever assessment was made [29].
The Lysholm score, a validated patient-administered instrument to measure symptoms and function in patients with knee injuries [18], measures activities of daily living and was assessed in two RCTs [9, 12].
Comparing AMIC® with ACI-C after 1 and 2 years, the mean Lysholm score improved from baseline at both follow-ups, with the mean delta Lysholm score being higher in the AMIC® versus the ACI-C group at 2 years, but with no statistical difference [9].
Analyzing the different outcome after AMIC® and AMIC® + , Lysholm score improved in both groups 6 and 12 months after surgery, with significance in favor of the AMIC® + group. After 24 months from surgery both groups presented significant Lysholm score improvements with respect to baseline. At the 60- and 100-month follow-ups, a slight progressive reduction of this score was observed, although AMIC® + patient scores always remained significantly higher compared to baseline [12].
Adverse events and drop-outs
Side effects were analyzed in all RCTs. In all studies, the rate of adverse events in both groups to compare was low [1, 9, 12, 13, 29].
No serious adverse events were reported for the trial comparing AMIC® to MFx alone [1, 29]. After 1 year, one patient receiving glued AMIC® dropped out of the study, because he received a total knee replacement and another patient of the MFx group received ACI after 1 year. Furthermore, 13 adverse events in nine out of 47 patients were mentioned without closer description [29].
Another trial also reported a total knee replacement in two patients 2 years after AMIC® [9]. One patient of the AMIC® group of a further study developed synovitis [12].
One trial reported one severe adverse event each group—one infected haematoma of a knee treated with m-BMS, which was addressed with arthroscopical lavage and intravenous antibiotics, and one instable cartilage regenerate in the MFx group which was, therefore, treated with MACI in the following and excluded from the study [13].
Discussion
The most important finding of the present systematic review is that matrix-based chondrogenesis is a safe and effective procedure for the treatment of local chondral defects in the knee. Five articles about four randomized controlled clinical trials could be included.
In two studies that compared matrix-augmented chondrogenesis with MFx, no difference in the PROMs could be seen after 2 years. After 5 year follow-up, however, the Cincinatti score decreased significantly in the MFx group compared to the AMIC® group [24]. At 5-year postoperation, the defect filling was the lowest in the MFx group compared to AMIC® treated groups [29]. In both studies, the tissue quality determined by MRI was better after cartilage repair with matrix augmented chondrogenesis than after MFx. In the study by Volz et al. defect filling was lower in the MFx group compared to AMIC® treated groups [29]. In this study, the poorer tissue quality after MFx in the MRI correlates with the poorer values in the Cincinatti Score.
In the RCT of Glasbrenner et al., changes in overall Henderson score were significantly higher at week 12 and week 108 in the m-BMS group as compared with the MFx group [13]. In this study, there was no difference in KOOS between the two treatment groups, which might be due to the small number of cases or a too short follow-up period to see any effect in the clinical scores. This is supported by the results of Anders et al. with a follow-up of 2 years, where no difference between groups in terms of clinical improvement was shown [1].
Fossum et al., investigating the results of AMIC® in comparison to ACI-C with KOOS as primary outcome and a 2-year follow-up, detected significantly improved scores in comparison to baseline without distinction within the groups for all assessed scores [9]. In this study, the defect size was roughly the same with 4.9 cm2 in the AMIC® group and 5.2 cm2 in the ACI-C group. Further studies are necessary to find out whether one or the other procedure performs better after a longer follow-up period.
The origin of the repair cells in matrix augmented cartilage repair is currently being debated. With this in mind, the study by Girolamo et al. is of interest, in which the outcome after AMIC® compared to AMIC® enhanced with bone marrow aspirate concentrate (AMIC® +) was examined [12]. After 12 months, patients treated with AMIC® plus bone marrow aspirate showed higher Lysholm scores and lower VAS scores compared to standard AMIC®. In the long term, however, there was no significant difference in the clinical scores between both treatment modalities. It can, therefore, be concluded that the protection of the subchondral bone in the bone marrow augmented group leads to faster recovery after one step matrix augmented cartilage repair. In this study, too, it must be criticized that the number of 24 patients at the last follow-up was probably too low to detect a difference in the clinical scores [12].
All studies excluded patients with varus or valgus malalignment and ligamentary instabilities which might affect the outcome after cartilage repair [20, 28]. It has been shown by Bode et al. that even in deformities of less than 5° varus, high tibial osteotomy (HTO) leads to longer survival rates [3].
A previous systematic review, investigating RCTs on surgical treatments of cartilage defects of the knee, came to the conclusion that on the long term, larger lesions (> 4.5 cm2) treated with cartilage regenerative techniques (ACI/MACI) had better outcomes than with MFx. [6]. Against this background, it seems interesting that the mean defect size in the analyzed studies varies between 0.5 and 10 cm2.
All the studies that were analyzed were controlled randomized studies and, therefore, correspond to the highest level of evidence. Nevertheless, several limitations in the RCTs which could be included in this systematic review could be found. One obvious limitation is the lack of feasible blinding for the surgeon as well as the patients, since in most studies, the matrix augmented chondrogenesis was performed in an open fashion via a small incision, whereas microfracture was performed arthroscopically. Only in the study by Glasbrenner et al. both procedures were performed arthroscopically [13]. However, no blinding was performed in this study either. Another limitation is the small number of patients. This means that there is only limited power to find differences in the clinical scores. Recruitment of patients and selection bias is a well known limitation of RCTs [17]. If eligible patients for the study could have the choice between MFx only and MFx with additional matrix-augmentation, e.g., they would probably prefer the operation pretending “more” treatment. That means that no follow-up of the patients preferring not to enter the study can be done. Selection bias is assumed when the recruitment rate is below 80% [5]. Therefore, findings of studies with a recruitment rate below 80% should only be generalized cautiously [26]. Another limitation of the included RCTs is that no study has carried out systematic evaluations of cartilage biopsies. Histological analyzes provide the best information about tissue quality. A comparison with autologous chondrocyte implantation would be particularly interesting here. It is also of concern that two studies used the Lysholm score as outcome measurement [9, 12]. The Lysholm score was originally developed for the assessment of patients with ligamentous instability. Hence this score might not be the first choice for the evaluation of outcome after matrix-induced chondrogenesis. A further flaw of the included studies is that in no study surgical process quality was controlled. A surgical treatment as variable in a clinical trial is more complex than, e.g., a pharmacological treatment [16, 26]. This is why surgical process quality must be controlled and more effort should be given to describe and standardize the surgical technique, given important surgical details such as the use of tourniquet, the experience of the surgeon and the use of photos or videos for documentation.
Conclusion
This systematic review could show that RCTs comparing matrix-induced chondrogenesis with other treatment options indicated that matrix-induced chondrogenesis is a valid and safe cartilage repair option for small- to medium-sized cartilage defects of the knee. This surgical procedure spares a second operation for the patient and will lower the clinical costs compared to autologous chondrocyte implantation. Despite all limitations, the clinical evidence is currently sufficient to further pursue the approach of matrix-based chondrogenesis. It is a safe procedure for the treatment of local chondral defects, which might be superior to pure microfracturing in the medium term. Recent developments concern the use of stem cells in combination with a matrix. Here too, the results so far are promising.
References
Anders S, Volz M, Frick H, Gellissen J (2013) A randomized, controlled trial comparing autologous matrix-induced chondrogenesis (AMIC®) to microfracture: analysis of 1- and 2-year follow-up data of 2 centers. Open Orthop J 7:133–143
Angele P, Niemeyer P, Steinwachs M, Filardo G, Gomoll AH, Kon E, Zellner J, Madry H (2016) Chondral and osteochondral operative treatment in early osteoarthritis. Knee Surg Sports Traumatol Arthrosc 24(6):1743–1752
Bode G, Schmal H, Pestka JM, Ogon P, Südkamp NP, Niemeyer P (2013) A non-randomized controlled clinical trial on autologous chondrocyte implantation (ACI) in cartilage defects of the medial femoral condyle with or without high tibial osteotomy in patients with varus deformity of less than 5. Arch Orthop Trauma Surg 133(1):43–49
Booth A, Clarke M, Dooley G, Ghersi D, Moher D, Petticrew M, Stewart L (2012) The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev 1:2
Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD (2000) Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Scand J Med Sci Sport 10(1):2–11
Devitt BM, Bell SW, Webster KE, Feller JA, Whitehead TS (2017) Surgical treatments of cartilage defects of the knee: systematic review of randomised controlled trials. Knee 24(3):508–517
Erggelet C, Endres M, Neumann K, Morawietz L, Ringe J, Haberstroh K, Sittinger M, Kaps C (2009) Formation of cartilage repair tissue in articular cartilage defects pretreated with microfracture and covered with cell-free polymer-based implants. J Orthop Res 27(10):1353–1360
Erggelet C, Neumann K, Endres M, Haberstroh K, Sittinger M, Kaps C (2007) Regeneration of ovine articular cartilage defects by cell-free polymer-based implants. Biomaterials 28(36):5570–5580
Fossum V, Hansen AK, Wilsgaard T, Knutsen G (2019) Collagen-covered autologous chondrocyte implantation versus autologous matrix-induced chondrogenesis: a randomized trial comparing 2 methods for repair of cartilage defects of the knee. Orthop J Sport Med 7(9):2325967119868212
Gelse K, Angele P, Behrens P, Brucker PU, Fay J, Günther D, Kreuz P, Lützner J, Madry H, Müller PE, Niemeyer P, Pagenstert G, Tischer T, Walther M, Zinser W, Spahn G (2018) Debridement in Focal Cartilage Damage of the kneeSystematical review of the literature and recommendations of the working group clinical tissue regeneration of the German Society of Orthopaedics and Trauma (DGOU). Z Orthop Unfall 156(4):423–435
Gille J, Behrens P, Volpi P, De Girolamo L, Reiss E, Zoch W, Anders S (2013) Outcome of autologous matrix induced chondrogenesis (AMIC) in cartilage knee surgery: data of the AMIC Registry. Arch Orthop Trauma Surg 133(1):87–93
de Girolamo L, Schönhuber H, Viganò M, Bait C, Quaglia A, Thiebat G, Volpi P (2019) Autologous matrix-induced chondrogenesis (AMIC) and AMIC enhanced by autologous concentrated bone marrow aspirate (BMAC) allow for stable clinical and functional improvements at up to 9 years follow-up: results from a randomized controlled study. J Clin Med 8(3):392
Glasbrenner J, Petersen W, Raschke MJ, Steiger M, Castelli C, Verdonk R, Fritschy D, Herbort M (2020) A multicenter randomized controlled trial: matrix-augmented bone marrow stimulation with a polyglycolic acid membrane with hyaluronan vs microfracture alone in local femoral cartilage defects of the femoral condyles. Orthop J Sport Med 8(5):2325967120922938
Harris JD, Quatman CE, Manring MM, Siston RA, Flanigan DC (2014) How to write a systematic review. Am J Sports Med 42(11):2761–2768
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17(1):1–12
Johnson TC, Evans JA, Gilley JA, DeLee JC (2000) Osteonecrosis of the knee after arthroscopic surgery for meniscal tears and chondral lesions. Arthroscopy 16(3):254–261
Karpinski K, Müller-Rath R, Niemeyer P, Angele P, Petersen W (2019) Subgroups of patients with osteoarthritis and medial meniscus tear or crystal arthropathy benefit from arthroscopic treatment. Knee Surg Sports Traumatol Arthrosc 27(3):782–796
Kocher MS, Steadman JR, Briggs KK, Sterett WI, Hawkins RJ (2004) Reliability, validity, and responsiveness of the Lysholm knee scale for various chondral disorders of the knee. J Bone Joint Surg 86A(6):1139–1145
Kusano T, Jakob RP, Gautier E, Magnussen RA, Hoogewoud H, Jacobi M (2012) Treatment of isolated chondral and osteochondral defects in the knee by autologous matrix-induced chondrogenesis (AMIC). Knee Surgery, Sport Traumatol Arthrosc 20(10):2109–2115
Lungwitz S, Niemeyer P, Maurer J, Fritz J, Albrecht D, Angele P, Fickert S, Gelse K, Zinser W, Hofmann GO, Spahn G (2019) Degenerative Cartilage Lesions of the Medial Knee CompartmentAssociated Factors, Operative Options, and Preliminary Results from the CartilageRegistry DGOU. Z Orthop Unfall 157(5):515–523
Mithoefer K, Williams RJ, Warren RF, Potter HG, Spock CR, Jones EC, Wickiewicz TL, Marx RG (2005) The microfracture technique for the treatment of articular cartilage lesions in the knee: a prospective cohort study. J Bone Joint Surg 87A(9):1911–1920
Moher D, Liberati A, Tetzlaff JAD (2009) PRISMA flow diagram. Prism statement. . PLoS Med 6(7):e1000097
Niemeyer P, Becher C, Brucker PU, Buhs M, Fickert S, Gelse K, Günther D, Kaelin R, Kreuz P, Lützner J, Nehrer S, Madry H, Marlovits S, Mehl J, Ott H, Pietschmann M, Spahn G, Tischer T, Volz M, Walther M, Welsch G, Zellner J, Zinser W, Angele P (2018) Significance of matrix-augmented bone marrow stimulation for treatment of cartilage defects of the knee: a consensus statement of the DGOU Working Group on tissue regeneration. Z Orthop Unfall 156(5):513–532
Oussedik S, Tsitskaris K, Parker D (2015) Treatment of articular cartilage lesions of the knee by microfracture or autologous chondrocyte implantation: a systematic review. J Arthrosc Relat Surg 31(4):732–744
Pascarella A, Ciatti R, Pascarella F, Latte C, Di Salvatore MG, Liguori L, Iannella G (2010) Treatment of articular cartilage lesions of the knee joint using a modified AMIC technique. Knee Surg Sports Traumatol Arthrosc 18(4):509–513
Petersen W, Achtnich A, Lattermann C, Kopf S (2015) The treatment of non-traumatic meniscus lesions. Dtsch Aerzteblatt Online 112(42):705–713
Schiavone Panni A, Del Regno C, Mazzitelli G, D’Apolito R, Corona K, Vasso M (2018) Good clinical results with autologous matrix-induced chondrogenesis (Amic) technique in large knee chondral defects. Knee Surg Sports Traumatol Arthrosc 26(4):1130–1136
Spahn G, Fritz J, Albrecht D, Angele P, Fickert S, Aurich M, Hofmann GO, Niemeyer P (2017) Coincidence and therapy of dysalignments and degenerative knee cartilage lesionsresults from the german cartilageregistry DGOU. Z Orthop Unfall 155(4):457–467
Volz M, Schaumburger J, Frick H, Grifka J, Anders S (2017) A randomized controlled trial demonstrating sustained benefit of autologous matrix-induced chondrogenesis over microfracture at five years. Int Orthop 41(4):797–804
Funding
There is no funding source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
We hereby confirm that for this review article no studies with human beings were performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karpinski, K., Häner, M., Bierke, S. et al. Matrix-induced chondrogenesis is a valid and safe cartilage repair option for small- to medium-sized cartilage defects of the knee: a systematic review. Knee Surg Sports Traumatol Arthrosc 29, 4213–4222 (2021). https://doi.org/10.1007/s00167-021-06513-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-021-06513-y