Abstract
Purpose
Studies investigating the influence of comorbidities on patient-reported outcomes after acute Achilles tendon ruptures (ATR) are lacking. In this study, the aim was to investigate the effect of comorbidity and medical treatment on the patient-reported outcome measure Achilles tendon total rupture score (ATRS).
Methods
The study was performed as a registry study from the Danish Achilles tendon Database (DADB). In the DADB, ATRS was registered at baseline (prior to rupture), at 3–6 month, 1-year and 2-year follow-ups. The outcomes were ATRS at follow-up and the change in ATRS from baseline to follow-up. Variables of interest were diabetes, hypertension, rheumatic disease and treatment with orally administrated corticosteroids. Linear mixed-effects models including all follow-up time points in the same model were used adjusting for sex, age group, treatment (operative or non-operative) and the investigated comorbidities.
Results
Data were collected from 2012 to 2019. Two thousand and four patients with ATR were included. Patients with the investigated comorbidities and treatment with orally administrated corticosteroid scored 10.6–19.1 points lower in mean ATRS at baseline (prior to rupture) compared to patients without the respective disease or treatment. At follow-up, patients with diabetes (mean difference, [95% CI]) (− 6.2, [− 11.7; − 0.8]; P = 0.03) and patients in treatment with orally administrated corticosteroids (− 10.9, [− 16.2; − 5.7]; P < 0.01) had a statistically significantly worse ATRS than patients without the respective disease. However, change in ATRS from baseline to follow-up was not affected. Hypertension and rheumatic disease did not affect ATRS at follow-up but had a positive effect on change in ATRS (4.3, [0.5; 8.1]; P = 0.03) and (12.0, [5.0; 19.9]; P < 0.01), respectively. No other statistically significant differences were found.
Conclusion
This study showed that patients with diabetes, hypertension, rheumatic disease and patients in treatment with orally administrated corticosteroids had a lower ATRS at baseline (prior to the rupture) when compared to patients without the respective disease or treatment. Diabetes and treatment with orally administrated corticosteroids did negatively affect ATRS at follow-up, but none of the investigated comorbidities or treatment with orally administrated corticosteroids did negatively affect change in ATRS from baseline to follow-up.
Level of evidence
Level III.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The latest reported incidence of acute Achilles tendon rupture (ATR) is 31–35 per 100.000/year with an increasing trend in the past decades [13, 19]. The injury causes sick leave and can potentially lead to permanent functional deficits [24, 29]. Due to the consequences of ATR, optimization of the treatment is needed. The injury is commonly debated and the primary focus of the discussion has been an assessment of operative versus non-operative treatment [11, 23]. No treatment consensus has yet been established, which has resulted in new research strategies focusing on different methods to individualize the treatment based on factors, such as patient characteristics and the morphology of the rupture [2, 15, 18]. For individualizing treatment, it is of interest to investigate treatment outcomes for subgroups of patients based on variables, such as sex, age, comorbidity and activity level.
It has been suggested that sex and age influence patient-reported and clinical outcomes after ATR, however, the results are conflicting [3, 6, 25, 27]. Olsson et al. found that higher body mass index (BMI) was a strong predictor of more limitations [25] and Hillam et al. showed that obesity had an increased association with wound dehiscence in operatively treated patients [16]. Diabetes, hypertension, smoking, treatment with orally administrated corticosteroid and female sex have been shown to increase the risk of surgical site infections following ATR [5, 9]. Studies investigating the influence of comorbidities on functional and patient-reported outcomes at follow-up are lacking.
In this study, the aim was to investigate the influence of diabetes, hypertension, rheumatic disease and treatment with orally administrated corticosteroids on Achilles tendon total rupture score (ATRS) after ATR. It was hypothesized that comorbidity or treatment with orally administrated corticosteroids would negatively affect treatment outcome. This knowledge will help medical professionals identify patients in risk of an unsatisfactory outcome and allow for better information of patients.
Materials and methods
Trial registration
Institutional review board (IRB) approval was given by the Ethical Review Board of the Capital Region of Denmark April 23 2020, registration no. H-20028216. Approval from the Danish Data Protection Agency of the Capital Region of Denmark was given March 11 2020, registration no. P-2020-238.
Design
The study was performed as a registry study based on data from the Danish Achilles tendon Database (DADB).
Population
Data extraction from DADB was performed January 1, 2020. The study population was defined as patients registered in DADB before August 1, 2019 to allow for a minimum of 6 months of follow-up.
The Danish Achilles tendon Database (DADB)
The DADB was founded in April 2012. Eleven orthopedic departments in Denmark register data from patients with ATR. The purpose of the database is to monitor the quality of treatment for patients with ATR and to collect data for epidemiological research. At inclusion in DADB, the hospitals register data, such as civil registration number, sex, age, comorbidities, prodromal symptoms, case history, treatment regime and complications. Additionally, treatment outcomes are registered up to 2 years after injury.
Data are collected at 5 time points: (1) At inclusion, (2) 3–4 months or 6 months after rupture (listed as 3–6 months further on), (3) 1 year after rupture, (4) 2 years after rupture and (5) if a complication arises (e.g. re-rupture). From medio 2018, the second time point was changed from 3–4 to 6 months after rupture, because functional evaluation of the patients is more applicable 6 months after rupture.
Each hospital in DADB has its own treatment procedure and rehabilitation regime. In general, non-operative treatment has been the most popular treatment option for the hospitals, which has resulted in a lower number of operative treated patients registered in DADB.
Data entered at registration in DADB are found to have a high validity ranging between 83 and 100%. Completeness of registered patients was 77% of patients eligible for registration [7].
Outcomes
The Achilles tendon total rupture score (ATRS) is a validated patient-reported outcome measure developed to evaluate limitations after ATR [12, 22]. A clinically relevant difference in ATRS is considered to be 10 points [4, 22, 26]. Lately, an instruction manual for accurate use of ATRS has been developed and published [14]. The data used in the present study were collected prior to the development of the manual.
ATRS was registered in DADB at baseline, 3–6 months of follow-up, 1-year follow-up and 2-year-follow-up. To obtain the baseline ATRS, patients were asked to recall the situation in the week prior to the rupture.
Primary outcome
The primary outcome was the ATRS at time of follow-up.
Secondary outcome
The secondary outcome was the change in ATRS from baseline to follow-up.
Variables
The variables of interest were:
Diabetes: No differentiation was made between type I and II diabetes or severity of the disease.
Hypertension: No differentiation was made between severity of hypertension.
Rheumatoid arthritis or other rheumatic diseases: No differentiation was made between different types of rheumatic diseases.
The comorbidities were registered by asking the patient to answer “yes” or “no” to if they have the respective comorbidity.
Treatment with oral administration of corticosteroids (further listed as corticosteroids). This was registered by asking the patient to answer “yes” or “no” to if they currently are, or during the past 6 months, have been in treatment with orally administrated corticosteroids.
The patients can be in more than one group. Patients with more than one comorbidity were not grouped together.
Investigated confounding variables were:
Sex (man or woman), type of treatment (operative or non-operative) and age divided into three age groups; < 35 years, 35–65 years and > 65 years. Age was divided into groups because of the possibility of a non-linear association to ATRS.
Statistical analysis
Descriptive baseline characteristics were reported for the investigated comorbidities and for treatment with orally administrated corticosteroids. Mean ATRS ± SD and median ATRS with inter quartile range (IQR) were reported at baseline, 3–6 months of follow-up, 1-year follow-up and 2-year follow-up for sub-groups based on the variables of interest and the confounding variables.
Primary analysis
The mean difference in ATRS at follow-up was investigated using linear mixed-effects model including all follow-up time points in the same model. The fixed effects were the variables of interest (comorbidities and treatment with orally administrated corticosteroids), the confounding variables (sex, type of treatment and age group) and time. The random effects were patient ID and hospital.
Secondary analysis
The mean difference in change in ATRS from baseline to follow-up was investigated using a similar model.
The residuals of the models were analyzed using Q–Q plots. The level of significance was set at P < 0.05. No Bonferroni corrections were applied. Analyses were conducted using R 3.2.2 (R Foundation for statistical computing, Vienna, Austria). No sample size calculation was performed prior to the initiation of the study.
Results
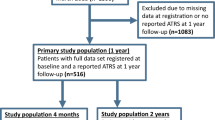
Two thousand, five hundred and fifty-eight patients were registered in DADB from 2012 to 2019. Of those 75 were excluded due to prior rupture of the contralateral Achilles tendon, 45 due to re-rupture and three due to both (in total 123 patients). Four hundred and thirty-one patients were excluded due to missing baseline data. The remaining 2004 patients were included in the study population and the analyses (Fig. 1). Descriptive baseline characteristics are shown in Table 1.
Overall, the results showed that patients with diabetes, hypertension, rheumatic disease and patients in treatment with orally administrated corticosteroids had a lower baseline ATRS compared to patients without the respective disease or treatment. Diabetes and treatment with corticosteroids negatively affected ATRS at follow-up, but none of the investigated comorbidities or treatment with orally administrated corticosteroid did negatively affect change in ATRS from baseline to follow-up (Table 3).
Baseline ATRS
At baseline, women scored 4.8 points lower in ATRS compared to men. Patients older than 65 years scored 10.4 points lower in ATRS compared to patients between 35 and 65 years (Table 2). Patients with the investigated comorbidities and treatment with orally administrated corticosteroid scored 10.6–19.1 points worse in baseline ATRS compared to patients without the respective disease or treatment (Tables 2, 3).
ATRS at follow-up
Patients with diabetes had a statistically significantly lower ATRS at follow-up compared to patients without diabetes (mean difference, [95% CI]) (− 6.2, [− 11.7; − 0.8], P = 0.03). Treatment with orally administrated corticosteroid negatively affected ATRS at follow-up compared to patients not in treatment with orally administrated corticosteroid (− 10.9, [− 16.2; − 5.7]; P < 0.01). Hypertension and rheumatic disease were not statistically significantly associated with ATRS at follow-up (Table 3).
Change in ATRS from baseline to follow-up
Hypertension (4.3, [0.5; 8.1], P = 0.03) and rheumatic disease (12.0, [5.0; 19.9], P < 0.01) had a statistically significantly positive effect on change in ATRS from baseline to follow-up compared to patients without the respective disease. Diabetes and treatment with orally administrated corticosteroids were not statistically significantly associated with change in ATRS from baseline to follow-up.
Discussion
This study showed that patients with diabetes, hypertension, rheumatic disease and patients in treatment with orally administrated corticosteroids had a lower ATRS at baseline (prior to the rupture) when compared to patients without the respective disease or treatment. Diabetes and treatment with orally administrated corticosteroids did negatively affect ATRS at follow-up, but none of the investigated comorbidities or treatment with orally administrated corticosteroids did negatively affect change in ATRS from baseline to follow-up.
Patients with the investigated comorbidities and patients in treatment with orally administrated corticosteroid had a clinically relevant lower baseline (prior to rupture) ATRS ranging between 10.6 and 19.1 compared to patients without the respective comorbidity or treatment. These baseline differences were not adjusted for confounders. Patients older than 65 years had approximately 10 points lower baseline (prior to rupture) ATRS compared to patients younger than 65 years and women had approximately five points lower baseline (prior to rupture) ATRS compared to men (Table 2). The patients with comorbidities were older and the proportion of women was higher than in the general population (Table 1). Because the differences in baseline ATRS between patients with and without comorbidity were not adjusted for potential confounders, the causality cannot be determined, meaning that the lower baseline ATRS for patients with comorbidities might partly be a result of higher age and a higher representation of women or vice versa. Diabetes seems to be associated with structural abnormalities in the Achilles tendons and hypertension to be associated with tendinopathy [1, 17]. The finding of a lower baseline ATRS for patients with comorbidity is new and has not yet been reported in the literature. When evaluating the ATRS at follow-up, the lower baseline ATRS should be taken into consideration if the comorbidities are unequally distributed between the compared groups.
The lower ATRS at follow-up for patients with diabetes and patients in treatment with orally administrated corticosteroids compared to patients with hypertension and rheumatic disease could be because diabetes and orally administrated corticosteroids have a more serious effect on the tendon tissue and the healing [10, 28], but it might also be because of the severity of the comorbidities for the general health. The patients registered with diabetes and the patients in treatment with orally administrated corticosteroids might consist of higher proportions of patients with severe underlying chronic disease compared to the patients registered with hypertension. It is likely that severe disease will cause less effective rehabilitation and, therefore, a worse treatment outcome. The patients registered with rheumatic disease are highly diverse, which makes it difficult to compare the results from this group with others.
No studies have yet reported results of the influence of diabetes, hypertension, rheumatic disease and treatment with orally administrated corticosteroids on patient-reported and functional outcomes. The lack of studies investigating this might be explained by the relatively low prevalence of comorbidity in patients with ATR, which implies, that a large study population is needed to address this subject. In the present study population, 3% of the patients had diabetes, 16% had hypertension, 4% had rheumatic disease and 4% of the patients were in treatment with orally administrated corticosteroids. Additionally, many of the randomized clinical trials investigating treatment outcomes after ATR exclude patients with specific comorbidities from the studies, which results in limited data on patient-reported and functional outcomes on these patients [20, 21, 30]. Databases, such as the DADB, where every patient with ATR is registered and followed, can provide unique knowledge of subgroups of patients with ATR.
Other studies have investigated ATRS at follow-up in different subgroups of patients with ATR. Silbernagel et al. showed that women had nine points lower ATRS at 1-year follow-up [27] and Aujla et al. found a mean difference in ATRS, at a minimum of 6 months after injury of 9.7 points in favor of the men [3]. The finding of a difference between the sexes in ATRS at follow-up was confirmed in a recently published study by our research group, that showed a lower ATRS of 9.4 points for women at 1-year follow-up [8]. However, they also showed that women had a 4.3 points lower ATRS at baseline compared to men and that the difference between men and women in ATRS from baseline to 1-year follow-up was not statistically nor clinically relevant [8]. The lower ATRS at follow-up for women might, therefore, partly be explained by a lower baseline ATRS. The results from the present study support this explanation by showing that men had a mean ATRS 7.2 points higher than women at 1-year follow-up, whereas the difference between men and women in change in ATRS from baseline to 1-year follow-up only was 2.4 points in favor of the men without adjustment for potential confounders [8]. Increasing age has also been suggested as a risk factor of poorer treatment outcome; however, the results are conflicting [3, 8, 25]. Olsson et al. found that higher body mass index (BMI) was a strong predictor of more symptoms after ATR [25] which makes BMI a possible confounder when investigating the influence of comorbidities.
The study was limited by a risk of selection bias due to loss to follow-up. Eighty-one percentage of the patients had registered ATRS at 3–6 months of follow-up, 69% had registered ATRS at 1-year follow-up and 42% had registered ATRS at 2-year follow-up. However, the risk of selection bias was probably low as descriptive data at baseline and at the follow-up time points showed no difference in the ratio between patients with and without the investigated comorbidities (Table 2). Moreover, the completeness of registered patients in DADB was 77% [7]. Though the completeness is close to 80% which normally is considered as satisfying, a completeness lower than 100% might result in selection bias.
The statistical models investigating the associations were adjusted for sex, age group, type of treatment, the investigated comorbidities and treatment with orally administrated corticosteroid, however, other medical treatments, other relevant comorbidities, BMI and smoking have not been included in the models and could potentially be confounders influencing the results. Additionally, the models have not been adjusted for rehabilitation regime. If the patients with comorbidity differed from the patients without comorbidity regarding time in bandage and/or when weight-bearing was allowed, it might affect the results. The potential confounders not adjusted for implies that the associations found in the present study do not necessarily have a causal relation. The follow-up time point was changed from 3–4 to 6 months in 2018, which potentially have an influence on the results.
Another limitation which might lower the validity of the diagnoses was that the registration of comorbidities was based on reporting from the patients without confirmation of the diagnoses in the patient records. Finally, the variation in severity of a comorbidity was not considered, for example, the patients with hypertension could either be normotensive or hypertensive at the time of the study dependent on their medical treatment and the severity of the hypertension. Subdivisions of the comorbidities might give clearer results, but would also lead to a substantial reduction in sample size.
In a clinical perspective, the study enables the clinician to identify patients with diabetes and patients in treatment with orally administrated corticosteroids as a population in risk of an unsatisfactory outcome. Furthermore, the study allows for better information of the patient and alignment of expectations concerning the outcome.
Conclusion
This study showed that patients with diabetes, hypertension, rheumatic disease and patients in treatment with orally administrated corticosteroids had a lower ATRS at baseline (prior to the rupture) when compared to patients without the respective disease or treatment. Diabetes and treatment with orally administrated corticosteroids did negatively affect ATRS at follow-up, but none of the investigated comorbidities or treatment with orally administrated corticosteroids did negatively affect change in ATRS from baseline to follow-up.
Abbreviations
- ATR:
-
Acute Achilles tendon rupture
- ATRS:
-
Achille tendon total rupture score
- DADB:
-
Danish Achilles tendon Database
- BMI:
-
Body mass index
- SD:
-
Standard deviation
- IQR:
-
Inter quartile range
- CI:
-
Confidence interval
References
Abate M, Salini V, Antinolfi P, Schiavone C (2014) Ultrasound morphology of the Achilles in asymptomatic patients with and without diabetes. Foot Ankle Int 35:44–49
Amlang MH, Zwipp H, Friedrich A, Peaden A, Bunk A, Rammelt S (2011) Ultrasonographic classification of Achilles tendon ruptures as a rationale for individual treatment selection. ISRN Orthop. https://doi.org/10.5402/2011/869703
Aujla R, Patel S, Jones A, Bhatia M (2018) Predictors of functional outcome in non-operatively managed Achilles tendon ruptures. Foot Ankle Surg 24:336–341
Barfod KW, Hansen MS, Holmich P, Troelsen A, Kristensen MT (2016) Efficacy of early controlled motion of the ankle compared with no motion after non-operative treatment of an acute Achilles tendon rupture: study protocol for a randomized controlled trial. Trials 17:564
Bruggeman NB, Turner NS, Dahm DL, Voll AE, Hoskin TL, Jacofsky DJ, Haidukewych GJ (2004) Wound complications after open Achilles tendon repair: an analysis of risk factors. Clin Orthop Relat Res. https://doi.org/10.1097/01.blo.0000144475.05543.e7
Carmont MR, Zellers JA, Brorsson A, Nilsson-Helander K, Karlsson J, Grävare Silbernagel K (2020) Age and tightness of repair are predictors of heel-rise height after Achilles tendon rupture. Orthop J Sport Med 8:2325967120909556
Cramer A, Hansen MS, Sandholdt H, Jones PK, Christensen M, Jensen SML, Hölmich P, Barfod KW (2019) Completeness and data validity in the Danish Achilles tendon Database. Dan Med J 66:A5548
Cramer A, Jacobsen NC, Hansen MS, Sandholdt H, Hölmich P, Barfod KW (2020) Outcome after acute Achilles tendon rupture is not negatively affected by female sex and age over 65 years. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-020-06003-7
Dombrowski M, Murawski CD, Yasui Y, Chen AF, Ewalefo SO, Fourman MS, Kennedy JG, Hogan MV (2019) Medical comorbidities increase the rate of surgical site infection in primary Achilles tendon repair. Knee Surg Sports Traumatol Arthrosc 27:2840–2851
Egemen O, Ozkaya O, Ozturk M, Sen E, Akan M, Sakiz D, Aygit C (2016) The biomechanical and histological effects of diabetes on tendon healing: experimental study in rats. J Hand Microsurg 04:60–64
Erickson BJ, Mascarenhas R, Saltzman BM, Walton D, Lee S, Cole BJ, Bach BR (2015) Is operative treatment of achilles tendon ruptures superior to nonoperative treatment?: a systematic review of overlapping meta-analyses. Orthop J Sport Med 3:2325967115579188
Ganestam A, Barfod K, Klit J, Troelsen A (2013) Validity and reliability of the Achilles tendon total rupture score. J Foot Ankle Surg 52:736–739
Ganestam A, Kallemose T, Troelsen A, Barfod KW (2016) Increasing incidence of acute Achilles tendon rupture and a noticeable decline in surgical treatment from 1994 to 2013. A nationwide registry study of 33,160 patients. Knee Surg Sports Traumatol Arthrosc 24:3730–3737
Hansen MS, Nilsson Helander K, Karlsson J, Barfod KW (2020) Performance of the Achilles tendon total rupture score over time in a large national database: development of an instruction manual for accurate use. Am J Sports Med 48:1423–1429
Hansen MS, Vestermark MT, Hölmich P, Kristensen MT, Barfod KW (2020) Individualized treatment for acute Achilles tendon rupture based on the Copenhagen Achilles Rupture Treatment Algorithm (CARTA): a study protocol for a multicenter randomized controlled trial. Trials NLM 21:399
Hillam JS, Mohile N, Smyth N, Kaplan J, Aiyer A (2019) The effect of obesity on Achilles rupture repair. Foot Ankle Spec 12:503–512
Holmes GB, Lin J (2006) Etiologic factors associated with symptomatic Achilles tendinopathy. Foot Ankle Int 27:952–959
Hutchison AM, Topliss C, Beard D, Evans RM, Williams P (2015) The treatment of a rupture of the Achilles tendon using a dedicated management programme. Bone Jt J 97-B:510–515
Huttunen TT, Kannus P, Rolf C, Fellander-Tsai L, Mattila VM (2014) Acute Achilles tendon ruptures: incidence of injury and surgery in Sweden between 2001 and 2012. Am J Sports Med 42:2419–2423
Lantto I, Heikkinen J, Flinkkila T, Ohtonen P, Siira P, Laine V, Leppilahti J (2016) A prospective randomized trial comparing surgical and nonsurgical treatments of acute Achilles tendon ruptures. Am J Sports Med 44:2406–2414
Nilsson-Helander K, Silbernagel KG, Thomee R, Faxen E, Olsson N, Eriksson BI, Karlsson J (2010) Acute achilles tendon rupture: a randomized, controlled study comparing surgical and nonsurgical treatments using validated outcome measures. Am J Sports Med 38:2186–2193
Nilsson-Helander K, Thomee R, Silbernagel KG, Thomee P, Faxen E, Eriksson BI, Karlsson J (2007) The Achilles tendon total rupture score (ATRS): development and validation. Am J Sports Med 35:421–426
Ochen Y, Beks RB, Van Heijl M, Hietbrink F, Leenen LPH, Van Der Velde D, Heng M, Van Der Meijden O, Groenwold RHH, Houwert RM (2019) Operative treatment versus nonoperative treatment of Achilles tendon ruptures: systematic review and meta-analysis. BMJ 7(364):k5120
Olsson N, Nilsson-Helander K, Karlsson J, Eriksson BI, Thomee R, Faxen E, Silbernagel KG (2011) Major functional deficits persist 2 years after acute Achilles tendon rupture. Knee Surg Sports Traumatol Arthrosc 19:1385–1393
Olsson N, Petzold M, Brorsson A, Karlsson J, Eriksson BI, Silbernagel KG (2014) Predictors of clinical outcome after acute Achilles tendon ruptures. Am J Sports Med 42:1448–1455
Olsson N, Silbernagel KG, Eriksson BI, Sansone M, Brorsson A, Nilsson-Helander K, Karlsson J (2013) Stable surgical repair with accelerated rehabilitation versus nonsurgical treatment for acute Achilles tendon ruptures. Am J Sports Med 41:2867–2876
Silbernagel KG, Brorsson A, Olsson N, Eriksson BI, Karlsson J, Nilsson-Helander K (2015) Sex differences in outcome after an acute Achilles tendon rupture. Orthop J Sport Med 3:2325967115586768
Taguchi T, Kubota M, Saito M, Hattori H, Kimura T, Marumo K (2016) Quantitative and qualitative change of collagen of Achilles tendons in rats with systemic administration of glucocorticoids. Foot Ankle Int 37:327–333
Westin O, Svensson M, Nilsson Helander K, Samuelsson K, Gravare Silbernagel K, Olsson N, Karlsson J, Hansson Olofsson E (2018) Cost-effectiveness analysis of surgical versus non-surgical management of acute Achilles tendon ruptures. Knee Surg Sports Traumatol Arthrosc 26:3074–3082
Willits K, Amendola A, Bryant D, Mohtadi NG, Giffin JR, Fowler P, Kean CO, Kirkley A (2010) Operative versus nonoperative treatment of acute Achilles tendon ruptures: a multicenter randomized trial using accelerated functional rehabilitation. J Bone Jt Surg Am 92:2767–2775
Acknowledgements
We would like to thank the following hospitals in Denmark that have contributed data to the Danish Achilles tendon Database; Aalborg Hospital, Køge Hospital, Nykøbing Falster Hospital, Amager-Hvidovre Hospital, Kolding Hospital, Vendsyssel Hospital, Hjørring Hospital, Thy-Mors Hospital, Himmerland Farsø Hospital, Viborg Regional Hospital, Randers Regional Hospital and Slagelse Hospital.
Funding
No funds were received.
Author information
Authors and Affiliations
Contributions
The authors (AC, NCJ, MSH, HS, PH, KWB) designed the study and interpreted the data. AC, HS and KWB made the statistical analysis. AC and KWB wrote the first version of the manuscript. All authors have critically revised the manuscript and have approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
KWB is paid consultant for DJO Nordic. The rest of the authors declare that they have no conflict of interests.
Ethical approval
Institutional review board (IRB) approval was given by the Ethical Review Board of the Capital Region of Denmark April 23 2020, registration no. H-20028216. Approval from the Danish Data Protection Agency of the Capital Region of Denmark was given March 11 2020, registration no. P-2020-238.
Informed consent
Informed consent was obtained from all study participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cramer, A., Jacobsen, N.C., Hansen, M.S. et al. Diabetes and treatment with orally administrated corticosteroids negatively affect treatment outcome at follow-up after acute Achilles tendon rupture. Knee Surg Sports Traumatol Arthrosc 29, 1584–1592 (2021). https://doi.org/10.1007/s00167-020-06371-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-020-06371-0