Abstract
Purpose
The present study was to analyze graft failure rates of hamstring tendon (HT) autografts with a cut-off graft diameter of 8 mm or 7 mm, and compare clinical outcomes between augmented small HT with an allograft and non-augmented relatively large HT in single-bundle anterior cruciate ligament reconstruction (ACLR).
Methods
A literature search of PubMed, EMBASE, and the Cochrane Library was performed based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) guidelines. Studies to assess graft failure of autologous HT ACLR were reviewed, and graft failure rates with a cut-off graft diameter of 8 mm or 7 mm were further extracted. Clinical comparative studies of ACLR between augmented small HT with an allograft and non-augmented relatively large HT autografts were also included. Results are presented as risk ratio (RR) for binary data and weighted mean difference for continuous data with 95% confidence intervals (CI).
Results
Nine studies with 2243 knees were included. Four studies examined the effect of HT autograft diameter on graft failure and five studies assessed clinical outcomes of allograft augmentation to small HT autografts. No significant difference was noted in graft failure with a cut-off diameter of 8 mm. No significant difference was found between diameters > 7 and ≤ 7 mm, but a significant difference was observed between diameters ≥ 7 and < 7 mm (RR = 0.49; 95% CI 0.26–0.92, I2 = 0%, P = 0.03). A trend towards increased risk of graft failure was noted for allograft-augmented HT compared with non-augmented HT autografts (RR = 0.43; 95% CI 0.18–1.02, I2 = 0%), but no significant differences were noted in IKDC, Lysholm, and Tegner scores between these groups.
Conclusion
The present study did support the use of 7 mm as a reference for cut-off diameter for small HT autografts, but not allograft augmentation to small HT autografts. These findings would guide clinical application of small HT autografts in single-bundle ACLR.
Level of evidence
IV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hamstring tendon (HT) autograft remains one of the most popular graft choices in anterior cruciate ligament reconstruction (ACLR). When performing autologous HT ACLR, small HT autografts are frequently encountered due to the variability in patients’ hamstring sizes or iatrogenic damage during graft harvest. Graft diameter has a considerable effect on graft failure. The exact graft diameter needed to avoid failures is not absolutely clear and could depend on other factors [8], but every 0.5-mm increase in diameter could result in a 0.82–0.86 times lower likelihood of revision surgery among HT grafts from 7 to 10 mm [18, 19]. A recent survey showed that there is no consensus on the minimum acceptable HT autograft size between 6 and 9 mm [12]. The cut-off diameter that defines a diminutive graft, most commonly cited as 7 mm or 8 mm, has been a matter of debate in the literature [4].
Clinically, augmentation of an allograft to small HT autografts to create a hybrid graft is a popular solution to this problem. This solution is theoretically advantageous to increase graft size and maintain autograft tissue, and it avoids the morbidity of additional autograft harvest. However, clinical outcomes have been controversial. With the variance in the reference diameter of small HT autografts to augment, some authors have reported that ACL reconstruction with a hybrid graft has a similar rupture rate and clinical outcomes to those achieved using a hamstring autograft [10, 11], whereas others reported that hybrid grafts may have an increased risk of the revision ACLR [3, 5, 20].
The purpose of the present systematic review and meta-analysis was to (1) analyze graft failure of HT autografts with a cut-off diameter of 8 or 7 mm; (2) compare graft failure and functional outcomes between the augmented small HT with an allograft and non-augmented relatively large HT autografts in single-bundle ACLR. It was hypothesized that there would be significant differences in graft failure with different cut-off diameters for HT autografts, but no significant difference in graft failure and functional outcomes between augmented and non-augmented HT autografts. Significant findings in the present study could clarify the appropriate cut-off diameter for small hamstring autografts and the debate about subsequent allograft augmentation in single-bundle ACLR.
Materials and methods
Literature search
Two independent reviewers performed the literature search based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines through the databases of PubMed, EMBASE, and Cochrane Library on 1 August 2018, using the following free text words: anterior cruciate ligament, ACL, hamstring, hybrid, augmentation, size, diameter, revision, and failure (the detailed search strategy can be seen in the Appendix, available in the online version of this article).
Eligibility and study selection
Two reviewers screened the titles and abstracts of the retrieved papers, and selected relevant studies for full review on the basis of the following inclusion criteria. To address the first purpose, studies were clinical case series of ACLR with HT autografts, and graft failure could be further assessed with a cut-off graft diameter of 8 mm or 7 mm. To address the second purpose, studies were comparative studies with clinical outcomes of small HT augmented with an allograft and non-augmented relatively large HT autografts. For both purposes, graft failure was mainly defined as need for a revision procedure. Patients who were International Knee Documentation Committee (IKDC) grade C or D, or had magnetic resonance imaging (MRI) evidence of graft tear, were considered to be failures. Studies that included patients with a minimum mean or median age of 18 years, and a minimum mean follow-up of 24 months were included. Animal studies, anatomic studies, biomechanical studies, abstracts, case reports, technical notes, reviews, and letters were excluded.
Data extraction
To extract data from the papers, a predefined form was used to collect the information about study characteristics (authors, publication year, study design, and level of evidence) and patient demographic data (number of subjects, sex, age, and follow-up time) (Tables 1, 3). The graft failure criteria and rate, and graft failure per graft diameter for HT graft are presented in Table 2. Clinical outcomes such as graft failure rate, IKDC score, Lysholm score, and Tegner activity score were included between the augmented HT with an allograft and non-augmented HT autografts (Table 4).
Methodological quality assessment
Methodological quality for non-randomized studies concerning the risk of bias was assessed with the Newcastle–Ottawa Quality Assessment Scale [22]. This is a nine-point scale where studies with 7–9, 5–6, 4, and 0–3 points were graded as very good, good, satisfactory, and unsatisfactory, respectively. Any disagreements between the authors were resolved through discussion or review by the third author. Potential publication bias was evaluated with a funnel plot.
Statistical analysis
Review Manager Version 5.3 (The Cochrane Collaboration) was used to estimate the overall pooled effect size for each outcome. Statistical heterogeneity among the studies was assessed using I2, with the values 25%, 50%, and 75% considered low, moderate, and high, respectively, and Cochrane’s Q statistic was used to assess heterogeneity. The meta-analysis was performed with a random-effects model. Results are presented as risk ratio (RR) for binary outcomes and weighted mean difference (WMD) for continuous outcomes with 95% confidence intervals (CI). A P value < 0.05 was considered statistically significant.
Results
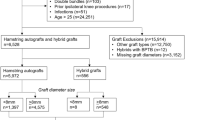
The literature search identified 1479 relevant items. Of these, 611 items were duplicated in the databases. After screening the title and abstract, 850 items were excluded, because they were not relevant to the purpose of the present study. After a thorough full-text review of 18 articles, 9 articles were excluded. For the first purpose, two studies did not describe clinical follow-up of ACLR with HT autografts, two studies did not further assess graft failure rate with a cut-off graft diameter of 8 mm or 7 mm, and one study had a follow-up period of less than 24 months. For the second purpose, two studies described the application of planned hybrid graft but not to augment small HT autografts, and two studies included patients younger than 18 years. Ultimately, nine studies were included for data extraction. Four studies were about the effect of HT graft diameter on graft failure [13, 15, 21, 23], while five studies described clinical outcomes of allograft augmentation to small HT autografts (Fig. 1) [3, 5, 10, 20, 25].
Quality of the included studies
A total of nine studies were included. Seven studies were level 3 retrospective cohort studies and two studies were level 4 case series. Total Newcastle–Ottawa Quality Assessment Scale scores were ≥ 7 points (range 7–9). These results indicated a low risk of bias. The symmetric distribution of studies within the funnel indicates that there was no small study publication bias (Fig. 2).
The effect of HT autograft diameter on graft failure
Cut-off graft diameter of 8 mm
The graft failure rate could be further compared between graft diameter > 8 and ≤ 8 mm in all four studies. A total of 1911 knees were included (593 knees with a diameter > 8 mm and 1318 knees of diameter ≤ 8 mm). No significant difference was noted in graft failure between these two groups (RR = 0.93, 95% CI 0.33–2.56, I2 = 58%) (Fig. 3a). Three studies with 1648 knees (955 knees with a diameter ≥ 8 mm and 693 knees of diameter < 8 mm) compared graft diameters ≥ 8 and < 8 mm, with no significant differences observed (RR = 0.99, 95% CI 0.27–3.62, I2 = 61%) (Fig. 3b).
Cut-off graft diameter of 7 mm
The graft failure rate could be compared between graft diameters > 7 and ≤ 7 mm in three studies. A total of 1648 knees were included (1300 knees with a diameter > 7 mm and 348 knees with a diameter ≤ 7 mm), and no significant difference was noted in graft failure rate between the two groups (RR = 0.64, 95% CI 0.38–1.10, I2 = 0%) (Fig. 4a). Three studies with 1648 knees (1513 knees with a diameter ≥ 7 mm and 135 knees with a diameter < 7 mm) compared a graft diameter ≥ 7 and < 7 mm, and a significant difference in graft failure was noted (RR = 0.49, 95% CI 0.26–0.92, I2 = 0%, P = 0.03) (Fig. 4b). This finding indicates that HT autografts with a diameter < 7 mm had double the risk of graft failure compared with a diameter ≥ 7 mm.
Clinical outcomes of small HT autograft with allograft augmentation
There were no significant differences in age (WMD = − 0.71, 95% CI − 2.29 to 0.87, I2 = 0%) and sex (RR = 1.12, 95% CI 0.89–1.40, I2 = 0%) between allograft-augmented and non-augmented HT autografts. A graft diameter of less than 8 mm was the most popular criterion for allograft augmentation to small HT autografts [5, 10, 20], with a graft diameter less than 7.5 mm referred to in one study [3], and a restored insertion site less than 60% of the cross-sectional area was used in another study [25]. Four-strand HT was used in all studies except one with an allograft augmentation to HT, or semitendinosus or gracilis tendon only [5]. After allograft augmentation, graft diameter was significantly larger in allograft-augmented HT autografts than non-augmented HT autografts (WMD = − 0.80, 95% CI − 1.29 to 0.32, I2 = 88%, P = 0.001) (Table 4).
Graft failure
All five studies reported the graft failure rate in the augmented small HT autografts and non-augmented large HT autografts. A total of 332 knees were included (145 knees in the augmented HT group and 187 knees in the non-augmented HT group). Although no significant difference was noted between these two groups, the present analysis demonstrated a trend towards increased risk of graft failure among the allograft-augmented small HT autografts (RR = 0.43, 95% CI 0.18–1.02, I2 = 0%) (Fig. 5).
IKDC score
All five studies reported the IKDC score in the augmented HT graft and non-augmented HT graft groups. A total of 332 knees were included (145 knees in the augmented group and 187 knees in the non-augmented group). No significant difference was noted in IKDC score between these two groups (WMD = 2.03, 95% CI − 1.87 to 5.93, I2 = 81%) (Fig. 6a).
Lysholm score
Among the five studies, three studies reported the Lysholm score in the augmented HT graft and non-augmented HT groups. A total of 183 knees were included (94 knees in the augmented group and 89 knees in the non-augmented group). No significant difference was noted in Lysholm scores between the two groups (WMD = 5.77, 95% CI − 0.72 to 12.27, I2 = 80%) (Fig. 6b).
Tegner activity score
Among the five studies, two studies reported the Tegner activity score in the augmented HT graft and non-augmented HT graft groups. A total of 125 knees were included (65 knees in the augmented group and 60 knees in the non-augmented group). No significant difference was noted in Tegner activity score between the two groups (WMD = 0.04, 95% CI − 0.27 to 0.35, I2 = 0%) (Fig. 6c).
Discussion
The most important finding of the present study was that HT autografts with a diameter < 7 mm have significantly higher graft failure rates than those with a diameter ≥ 7 mm. The present study did support the use of grafts with a 7-mm cut-off diameter for small HT autografts. No significant difference was found in graft failure of HT autografts with a cut-off diameter of 8 mm. Augmentation of small HT autografts with an allograft tended to have an increased risk of graft failure compared with non-augmented relatively large HT autografts. Therefore, augmentation to small HT autografts with an allograft was not supported in single-bundle ACLR in the present study.
The present analysis showed that a graft diameter of 8 mm was not the cut-off graft diameter for small HT autografts or the criterion for subsequent allograft augmentation. This is contrary to the results of a previous review that showed a 6.8 times higher risk of graft failure if the graft diameter was ≤ 8 mm [4]. However, the present results were consistent with large-volume population-based cohort studies. From a Swedish national knee ligament register on 11,339 patients in single-bundle ACLR with HT autografts, no significant difference was found in the incidence of revision between an HT graft diameter < 8 and > 8 mm [1]. In a retrospective cohort analysis of 786 single-bundle ACLR with HT autografts, no significant difference was found in the incidence of revision between a graft diameter ≤ 8 and ≥ 8 mm [23].
The current result showing that HT autografts with a diameter < 7 mm have double the risk of graft failure compared with a graft diameter ≥ 7 mm in single-bundle ACLR may support the use of a graft diameter of 7 mm as the cut-off diameter for small HT autografts. In the previous studies, controversial results were found in the HT autograft failure rate between a diameter < 7 mm and ≥ 7 mm, because the rate of graft size less than 7 mm was reported to be relatively low (2.3–11.2%) [21, 23]. In the present analysis, a total of 135 ACLRs with a graft diameter < 7 mm were analyzed (a rate of 8.2%), and a significantly higher risk of graft failure was found with a graft diameter < 7 mm compared with a graft diameter ≥ 7 mm.
The present results support the biomechanical cut-off diameter of small HT autografts being 7 mm. Several biomechanical studies have tested the maximum failure load of HT grafts with a series of diameters. For HT grafts with a diameter of 6, 7, and 8 mm, Boniello et al. reported a mean load to failure of 2359 (1567–3126) N, 3263 (2288–4292) N, and 3908 (2874–4910) N, respectively [2]. Schimoler et al. reported that the mean load to failure was 2891 (2681–3264) N, 3653 (3366–3936) N, and 4129 (3367–4894) N, respectively [17]. Farmer et al. reported their results of 1990 ± 302 N, 2179 ± 685 N, and 3074 ± 781 N, respectively, for those same graft diameters [7]. With increasing graft diameter, there was a corresponding increase in maximum tensile strength and cross-sectional area [2, 17]. With 1-mm increments in diameter, the greatest percentage gain in strength was seen with the 6–7-mm increment, whereas the lowest strength gain was seen with the 8–9-mm increment [2]. Compared with the quoted values of the native ACL of 1,725N to 2,160N [14], HT grafts with a 8 mm diameter could provide sufficient strength for ACLR, and some concerns still remain with grafts that are 6 mm in diameter. HT grafts with a 7-mm diameter have a slightly stronger strength for ACLR and further studies are needed.
Although a graft diameter of 7 mm could be used as a reference for small HT autografts, patient characteristics should be also considered in ACLR. Conte et al. found that graft diameter was significantly correlated with at least one parameter of patient characteristic, such as age, sex, height, and body mass index [4]. Height was the most common correlation. Park et al. reported that gracilis diameters had the strongest association with gender, while semitendinosus and four-strand graft diameters had the strongest associations with height [15]. Native ACL insertion size and HT graft diameter both have a correlation to patient characteristics, perhaps, suggesting that some patients with a small HT graft may have a small native ACL insertion size and do not need a large graft [4, 9].
The present result that small HT autografts augmented with an allograft to create a hybrid graft tended to have an increased risk of graft failure may not support the clinical allograft augmentation to small HT autografts. In the present study, the graft failure rate was 11% (16/145) for the augmented HT autografts compared with 4.8% (9/187) for the non-augmented HT autografts. With the graft diameter less than 8 mm to augment, Wang et al. reported that failure rate tended to be greater in the hybrid graft group (14.3%) than in the autograft group (3.4%) [20]. Darnley et al. reported that revision surgery was 18.5% for the hybrid graft compared with 7.4% for the autograft [5]. Referring to the graft diameter less than 7.5 mm to augment, Burrus et al. reported that the failure or compromise rate was 37.9% in the hybrid graft compared with 6.9% for the autograft [3]. With an autograft diameter of less than 7 mm, Pennock et al. reported a 30% graft failure for the augmented group and 5% for the non-augmented group [16].
The present study showed no significant differences in IKDC, Lysholm, and Tegner scores between the augmented HT and non-augmented HT autografts. Burrus et al. reported a significantly higher failure or compromise rate in the hybrid graft compared with the autograft [3], and significantly lower associated Lysholm and IKDC scores in the hybrid graft. Darnley et al. [5] and Leo et al. [10] reported no statistically significant differences in ACL graft failure and a similar patient-reported outcome score. The results of functional outcomes were consistent with the outcomes of graft failure and subsequent knee laxity. Wang et al. reported that the postoperative KT-1000 side-to-side difference was 3.5 ± 2.0 mm for the augmented graft compared with 2.5 ± 1.0 mm for the non-augmented graft, and significantly lower mean Lysholm and subjective IKDC scores were observed in the augmented graft group [20]. Xu et al. found that the KT-1000 side-to-side difference was 1.8 ± 0.6 mm for the hybrid graft and 1.6 ± 0.5 mm for the HT autografts, and no significant differences were noted in IKDC, Lysholm, and Tegner scores between these two groups [25].
The reason for the increased failure rate of hybrid grafts is not completely understood, but some factors may reduce this phenomenon. First, as described above, some patients with a small HT graft may have a small native ACL insertion size and do not need a large graft [4, 9]. Therefore, the graft diameter of the hybrid graft, usually more than 9 mm, may not always be suitable, and we could control the graft diameter for the hybrid graft to some extent. Second, biologic incorporation of allografts was slower than that of autografts [24], so the allograft sandwiched in between the autograft portions may accelerate the hybrid graft incorporation and promote tendon-bone healing. Third, gamma irradiation has a dose-dependent negative effect on allograft strength, with particularly large effects noted at irradiation doses of ≥ 2.5 M rad [6]. Grafts processed with low-dose irradiation or fresh-frozen grafts may yield good results.
Several limitations have been acknowledged in the present study. First, only graft cut-off diameters of 7 mm or 8 mm were analyzed in the present study, because diameters of 7 mm or 8 mm were the most commonly cited references for debate in the literature, and the date of other diameters could not be extracted in most studies. Second, graft failure was mainly defined as ACL revision in all included studies, but IKDC grade C or D, and MRI evidence of graft tear were also included, which makes a little difference in graft failure for all included studies. Third, although there was no consensus on cut-off diameter for small HT autografts, the most commonly cited criterion to augment was a graft diameter around 8 mm in the literature, so the variation in the reference diameter was accepted. Fourth, the level of evidence of retrieved studies was poor, with no level I or II studies included. Larger prospective, and ideally randomized, studies looking at this topic are needed.
Conclusion
A significant difference was found in the graft failure rate between HT autografts with a graft diameter ≥ 7 mm and < 7 mm. The present study did support the use of a graft diameter of 7 mm as a reference for the cut-off diameter for small HT autografts. Augmentation of small HT autografts with an allograft tended to have an increased risk of graft failure compared with non-augmented HT autografts. Allograft augmentation to small HT autografts was not supported in single-bundle ACLR.
References
Andernord D, Bjornsson H, Petzold M, Eriksson BI, Forssblad M, Karlsson J, Samuelsson K (2014) Surgical predictors of early revision surgery after anterior cruciate ligament reconstruction: results from the swedish national knee ligament register on 13,102 patients. Am J Sports Med 42(7):1574–1582
Boniello MR, Schwingler PM, Bonner JM, Robinson SP, Cotter A, Bonner KF (2015) Impact of hamstring graft diameter on tendon strength: a biomechanical study. Arthroscopy 31(6):1084–1090
Burrus MT, Werner BC, Crow AJ et al. (2015) Increased failure rates after anterior cruciate ligament reconstruction with soft-tissue autograft-allograft hybrid grafts. Arthroscopy 31:2342–2351
Conte EJ, Hyatt AE, Gatt CJ Jr, Dhawan A (2014) Hamstring autograft size can be predicted and is a potential risk factor for anterior cruciate ligament reconstruction failure. Arthroscopy 30(7):882–890
Darnley JE, Leger-St-Jean B, Pedroza AD, Flanigan DC, Kaeding CC, Magnussen RA (2016) Anterior cruciate ligament reconstruction using a combination of autograft and allograft tendon: a MOON cohort study. Orthop J Sports Med 4:2325967116662249
DiBartola AC, Everhart JS, Kaeding CC, Magnussen RA, Flanigan DC (2016) Maximum load to failure of high dose versus low dose gamma irradiation of anterior cruciate ligament allografts: a meta-analysis. Knee 23:755–762
Farmer K, Kenney N, Moser M, Conrad B (2013) Biomechanical qualities of small size hamstring grafts: how small is too small? Arthroscopy 29(10):e145–e146
Figueroa F, Figueroa D, Espregueira-Mendes J (2018) Hamstring autograft size importance in anterior cruciate ligament repair surgery. EFORT Open Rev 3(3):93–97
Kopf S, Pombo MW, Szczodry M, Irrgang JJ, Fu FH (2011) Size variability of the human anterior cruciate ligament insertion sites. Am J Sports Med 39(1):108–113
Leo BM, Krill M, Barksdale L, Alvarez-Pinzon AM (2016) Failure rate and clinical outcomes of anterior cruciate ligament reconstruction using autograft hamstring versus a hybrid graft. Arthroscopy 32:2357–2363
Li J, Wang J, Li Y, Shao D, You X, Shen Y (2015) A prospective randomized study of anterior cruciate ligament reconstruction with autograft, gamma-irradiated allograft, and hybrid graft. Arthroscopy 31:1296–1302
Magnussen RA, Kaeding CC, Taylor DC (2015) Solutions to small hamstring autograft harvest: a survey of the ACL study group. Curr Orthop Pract 26(1):43–44
Mariscalco MW, Flanigan DC, Mitchell J et al. (2013) The influence of hamstring autograft size on patient-reported outcomes and risk of revision after anterior cruciate ligament reconstruction: a multicenter orthopaedic outcomes network (MOON) cohort study. Arthroscopy 29(12):1948–1953
Noyes FR, Butler DL, Grood ES, Zernicke RF, Hefzy MS (1984) Biomechanical analysis of human ligament grafts used in knee-ligament repairs and reconstructions. J Bone Joint Surg Am 66:344–352
Park SY, Oh H, Park S, Lee JH, Lee SH, Yoon KH (2013) Factors predicting hamstring tendon autograft diameters and resulting failure rates after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 21(5):1111–1118
Pennock AT, Ho B, Parvanta K, Edmonds EW, Chambers HG, Roocroft JH, Bastrom TP (2017) Does allograft augmentation of small-diameter hamstring autograft ACL grafts reduce the incidence of graft retear? Am J Sports Med 45(2):334–338
Schimoler PJ, Braun DT, Miller MC, Akhavan S (2015) Quadrupled hamstring graft strength as a function of clinical sizing. Arthroscopy 31(6):1091–1096
Snaebjörnsson T, Hamrin Senorski E, Ayeni OR et al. (2017) Graft diameter as a predictor for revision anterior cruciate ligament reconstruction and KOOS and EQ-5D values: a cohort study from the Swedish national knee ligament register based on 2240 patients. Am J Sports Med 45(9):2092–2097
Spragg L, Chen J, Mirzayan R, Love R, Maletis G (2016) The effect of autologous hamstring graft diameter on the likelihood for revision of anterior cruciate ligament reconstruction. Am J Sports Med 44(6):1475–1481
Wang HD, Gao SJ, Zhang YZ (2018) Comparison of clinical outcomes after anterior cruciate ligament reconstruction using a hybrid graft versus a hamstring autograft. Arthroscopy 34(5):1508–1516
Webster KE, Feller JA, Leigh WB, Richmond AK (2014) Younger patients are at increased risk of graft rupture and contralateral injury after anterior cruciate ligament reconstruction. Am J Sports Med 42(3):641–647
Wells GA, Shea B, O’Connell D et al (2011) The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in metaanalyses. Ottawa Hospital Research Institute, Ottawa
Wernecke GC, Constantinidis A, Harris IA, Seeto BG, Chen DB, MacDessi SJ (2017) The diameter of single bundle, hamstring autograft does not significantly influence revision rate or clinical outcomes after anterior cruciate ligament reconstruction. Knee 24(5):1033–1038
Wydra FB, York PJ, Johnson CR, Silvestri L (2016) Allografts for ligament reconstruction: where are we now? Am J Orthop (Belle Mead NJ) 45(7):446–453
Xu H, Dong J, Xin D, Zhang J, Kang K, Gao S (2017) Second-look arthroscopic evaluation and clinical outcomes of anatomic anterior cruciate ligament reconstruction with autograft and hybrid graft: a retrospective study. Med Sci Monit 23:5564–5573
Acknowledgements
We thank Peter Mittwede, MD, PhD, from Liwen Bianji, Edanz Editing China (http://www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
There is no funding source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kang, H., Dong, C. & Wang, F. Small hamstring autograft is defined by a cut-off diameter of 7 mm and not recommended with allograft augmentation in single-bundle ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 27, 3650–3659 (2019). https://doi.org/10.1007/s00167-019-05475-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-019-05475-6