Abstract
Purpose
The current study investigated the distribution of hamstrings graft size and body mass index and any potential effect on the risk of revision surgery in a large prospective cohort of patients undergoing ACL reconstruction. More specifically, the aim of the study was to investigate whether larger graft size or smaller BMI would decrease the risk of revision after ACL reconstruction.
Methods
A total of 4029 patients, prospectively registered in the Norwegian Knee Ligament Registry, were included in the study. Univariate Kaplan–Meier survival analyses (with log-rank tests) and the Cox proportional hazard (PH) regression model were applied to compare risk of revision between groups of patients. Mutual adjustment for gender, age, activity at the time of injury and fixation method of the graft was performed.
Results
Graft sizes spanned from 5.5 to 11.0 mm and the median of 8.0 mm was reported in 42% of patients in the cohort. BMI was reported from 15 to 57 with a median of 25. 46% of patients were classified as overweight (WHO standards), while 23% of patients were obese. At a median of 2.5 years after surgery, 150 patients had undergone revision surgery. Although certain effects were seen in the unadjusted analyses, neither graft size (diameter) nor patient BMI did affect the risk of undergoing revision surgery in the adjusted analyses.
Conclusions
Graft size and BMI was not found to be independent risk factors for undergoing ACL revision surgery. In contrast to other studies, graft size of 8 mm or larger did not have a better outcome than smaller graft sizes. A relatively large group of overweight patients undergoing ACL surgery reflects the general increase in weight seen in Western societies. Although the current study differs from previous findings, it might indicate that graft diameter is less important than previously stated.
Level of evidence
Cohort study, II.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anterior cruciate ligament (ACL) reconstruction is amongst the most common procedures in sports medicine. It has been proven successful in restoring knee stability and thereby returning patients to their preinjury activity level [1,2,3]. There is, however, a significant failure rate after surgery that warrants further research aiming to improve outcomes [4,5,6]. Of the most commonly debated factors, believed to affect the rate of failure, is graft diameter used for the reconstruction [7]. Unlike autografts from patellar or quadriceps tendons, which can reliably be harvested to a predetermined graft diameter, hamstrings autografts show a considerable variability in their length and diameter between patients [8,9,10]. These differences can, to a certain degree, be predicted based on anthropometric measures [8, 11], but often the graft size is first given after harvesting has been performed. Therefore, the effect on outcomes due to differences in graft diameter is important knowledge supporting intra-operative decision making.
Biomechanical testing suggest that quadrupled hamstrings autografts have initial strength properties that exceed that of bone–patellar tendon–bone grafts [12, 13] providing a strong enough tissue for replacing the native ACL. The tensile strength is dependent on the diameter of the graft and a certain disagreement exists on what size will provide the sufficient mechanical properties for an ACL reconstruction [13, 14]. Given a reduction in strength over time, and accounting for any disadvantageous effects during graft healing, it has been argued that a larger size graft is beneficial for optimizing outcomes after surgery [7]. Although a certain variety is seen in clinical outcomes, several reports support these basic concepts—suggesting a graft diameter of 8 mm or more should be sought when using hamstrings autografts [9, 15].
Since revision surgery is a relatively rare incident, some studies are hampered by a low number of revision cases—possibly affecting reliability of the results [9, 10, 15]. Typically registry studies, including large prospective cohorts of patients, have the largest number of revisions and are therefore more likely to provide knowledge on the effect of graft size on risk of revision. Such studies, like the ones by Snaebjörnson et al. and Spraggs et al. have both found a clear effect of increasing graft size—providing support for the choice to size up the small graft diameter where encountered [16, 17].
The current study has made use of a prospective cohort of patients from the Norwegian Knee Ligament Registry (NKLR) to investigate the factors influencing the risk of undergoing revision surgery. The primary aims were (1) to describe the baseline distribution of graft diameter related to height, weight and body mass index in a large cohort of patients reconstructed using hamstrings autograft, and (2) to investigate the effect of graft size and body mass index (BMI) on the relative risk of undergoing revision surgery.
Materials and methods
Data were requested and received depersonalized from the NKLR. Only primary ACL reconstructions with hamstring autografts were considered and patients having other ligament injuries in addition to a ruptured ACL were not included. The following variables were considered: date of primary surgery, potential date of revision, time from injury to surgery, age at surgery, sex, graft size, height and weight at the time of the primary reconstruction, activity at the time when the primary injury occurred and fixation method for the femur and the tibia. The NKLR was established in 2004, however, information on graft size, height and weight for each patient was included in the NKLR registration form in 2010. All patients in the NKLR gave a written consent before the primary ACL reconstruction and the NKLR has been approved by the Norwegian Data Inspectorate. No new information was collected for the purpose of the current study. Therefore, no further ethical approval was needed.
Statistical analyses
The data was processed in IBM SPSS Statistics 23. Graft size was reported in 0.5-mm intervals and was therefore classified as a categorical variable. BMI was calculated and divided into five groups based on the World Health Organization’s international classification, weight in kilograms divided by the square of the height in meters (kg/m2). The univariate Kaplan–Meier survival analysis (with log-rank test) and the Cox proportional hazard (PH) regression model were applied to compare risk of revision between groups of patients. Schoenfelds residuals were used to check for assumptions and the Cox PH regression model was found suitable [18]. Variables known to be possible confounders based on previous studies were included in the Cox PH regression model [19, 20]. The hazard rate ratio (HR) with 95% confidence interval (CIs) was used as an estimate of relative risk of revision. Mutual adjustment for gender, age (in 5-year categories), activity at the time when the primary injury occurred and fixation method of the graft in the femur and in the tibia was performed in the adjusted Cox model.
Results
Demographic data
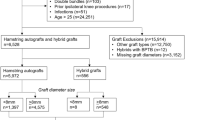
A total of 7837 patients were recorded in the NKLR undergoing primary ACL reconstructions with hamstring autografts between 2010 and 2015. Four thousand and twenty-nine of these patients (51%) had all three values of graft size, height and weight registered and were therefore included in the study. The mean follow-up time was 2.5 years (SD 1.3). During this period, 150 patients had undergone revision surgery. Mean age at time of surgery was 29.1 (SD 10.7), and the mean time from injury to surgery was 22 months (SD 44). Further, the mean age at surgery for patients that underwent revision during follow-up was 22.7 years (SD 7.6). Detailed demographic data, thereunder activity at injury and fixational method can be found in Table 1.
Graft size and BMI
Graft diameter ranged from 5.5 to 11.0 mm, with a median of 8.0 mm. Those that had undergone revision surgery also had a median graft size of 8.0 mm (range 6.5–10.0). The most commonly reported graft size was 8.0, found in 43% (N = 1726) of patients (Table 2). BMI, reported according to WHO standards, ranged from 15 to 57, with a median of 25 in the population. A total of 46% of patients were overweight (BMI 25 or above) at the time of surgery. Further, 23% of all patients were obese (BMI 30 or above) at the time of surgery. The distribution of BMI in the study population can be found in Table 2.
Risk of revision related to graft size, BMI and demographic data
When analyzing the relative risk of revision related to graft diameter, graft size was collapsed into five groups: ≤ 7 mm, 7.5 mm, 8.0 mm, 8.5 mm, ≥ 9 mm. The most frequent graft diameter (8 mm) was the comparator for further analyses. In the same manner, BMI was collapsed into five groups: < 18.5, 18.5–24.9, 25.0–29.9, 30.0–34.9, ≥ 35 and the largest group (18.5–24.9) was used as the comparator. The results from the Cox PH regression model is presented in Table 3. The graft size of 8.5 was found to have the lowest risk of revision throughout the follow-up period (Fig. 1) but this effect was neither significant in the unadjusted (HR 0.58, 95% CI 0.31–1.10) nor in the adjusted (HR 0.60, 95% CI 0.32–1.36) model. When looking at the risk of revision related to patient BMI (Fig. 2), the relative risk (HR) of revision was significant lower in patients with a BMI of 25–29 (HR = 0.66 95% CI 0.46–0.95) in the unadjusted model. This effect was, however, no longer evident in the adjusted model [0.88 (95% CI 0.60–1.29)].
Similar results were found regarding risk of revision and graft diameter when replacing the BMI variable in the Cox PH model with height and weight as separate variables (results not shown). Sub-analyses using the smallest graft size as comparator did not change the results, nor did using the lowest BMI group as comparator (results not shown) and also when conducting the Cox analysis using fewer subgroups of graft size (creating larger patient groups with more events).
Discussion
The most important finding of the present study, investigating a cohort of 4029 ACL injured patients reconstructed with a hamstrings autograft, was that neither the graft size nor patient BMI did affect the risk of undergoing revision surgery. Contrary to former studies, use of smaller graft diameters (< 8 mm) was not found to give a higher risk of revision—and use of larger graft diameters (> 8 mm) was not found to give a lower risk of revision surgery. With regard to patient BMI, a relative large group of overweight and obese patient was seen. To the best of our knowledge, this study is the largest to date where the impact of graft size and BMI on risk of revision surgery has been investigated.
A case–control study using data from the Kaiser Permanente Registry examined the effect of graft size on risk of revision by matching 124 revised with 367 non-revised patients [17] based on age, gender, BMI and type of fixation. Graft diameter was found to be 7.9 mm in the case group and 8.1 mm in the control group, and when analyzing effect of 0.5 mm increments from 6.0 to 10.0 mm, the lower graft diameters were proportionally more frequent in the revised patients—and the higher graft diameters were more frequent in non-revised patients. The likelihood of undergoing revision was 0.82 times lower for every 0.5 mm increment in graft size throughout the population. Another study, from the Swedish National Knee Ligament Registry, did similar case–control matching—taking gender, age and fixation method into account [16]. In the group of 560 revisions, the mean graft size was 8.0 mm compared to 8.1 mm in the control group. When analyzing the effect of 0.5 mm increments in graft size from 7.0 to 10.0 mm, the likelihood of a patient requiring revision surgery was similar to the study from Kaiser Permanente. When investigating the relationship between graft size and patient-reported outcomes (KOOS and EQ-5D), no relation could, however, be found. In contrast to the above studies—where a matching algorithm was used—the whole cohort of patients surgically treated with hamstrings tendon reconstruction was included in the current work. Adjustments were made to account for patient gender, age, sex, activity at the level of injury, BMI (also including height and weight separately), and femoral/tibial fixation method. Since the two above studies came to a very similar conclusion where the current study could not find any effect, one could speculate whether differences in applied methodology could be a reason for such differences. The current applied Cox proportional hazard regression model is, however, well-established and conventionally used for registry data [18, 21]. Where the current study applied the most frequent graft size (graft diameter 8 mm) as a comparator for the analyses, the other studies have rather used the group with the smallest graft size diameter as the comparator. In the current analyses, to verify that this did not change outcomes, both approaches were applied, without any evident change in results.
The current overall mean age was 29.9 years at surgery, but when adjusting relative to those that had undergone revision (N = 150), the mean age was a much lower 22.7. The studies by Snaebjörnsson et al. and Spragg et al. reported a median of 17.6 and a mean of 21.7 respectively. Although the current model did adjust for age when investigating for effect of graft diameter and BMI, age differences between populations are important to report since younger age at time of surgery is a well-known risk of failure after ACL surgery [19, 22, 23]. In a joint study from all Scandinavian ACL registries, a decrease in risk for revision surgery was seen with increasing age (in 5 year intervals) from 20 years and above—as compared to those less than 20 years in age at the time of surgery [20]. One would believe that younger knees would have an advantage in healing and rehabilitation after surgery—but this effect is probably counterweighted by the tendency to continue risk-seeking behavior after their initial ACL reconstruction. The young ACL-reconstructed patient will more likely return to pivoting sports than those who are older at the time of reconstruction [6, 24, 25].
The current relatively high incidence of adipose and obese patients has rarely before been reported in ACL injured patients. BMI is limited as a measure for overweight, since both fat and muscle tissue can contribute to a high score. Athletic patients can therefore appear overweight due to a substantial muscle mass [26] The high incidence (23%) of obese patients, however, indicates that the current finding might be linked to the general trend of increasing weight in the Western world [27]. An additional factor, probably affecting the preoperative BMI, is the time from injury to surgery. For most patients, an ACL tear leads to a sudden and dramatic reduction in activity as compared to the preinjury level. Time to surgery, a current mean of 22 months, comes with a risk that inactivity can negatively affect patients’ weight. Registry-based studies reflect the population as a whole—therefore timing of surgery as well as body composition might differ from studies that report on selected populations of athletes. Underlying differences in study populations are therefore important to consider when assessing generalizability of and comparability between studies. In the current work, height and weight were also included as separate factors in the Cox regression model to account for limitations in BMI. Results did, however, not change from only using BMI.
Although commonly reported, BMI has rarely been investigated as a primary risk factor for inferior outcomes after ACL reconstruction. A systematic review evaluating both risk of worse outcomes and postoperative complications could not find any clear relation to the patient baseline BMI [28]. Another review reported that patients with increased BMI at baseline had a lower level of activity after reconstruction—but no increase in risk of undergoing revision surgery [29]. Pietrosimone et al. recently investigated whether BMI above or under 25 was related to inferior outcomes at mean 30 months after ACL reconstruction [30]. Only a weak, and clinically non-relevant, correlation to IKDC subjective scores was seen in those with a BMI above 25. In a report from the NKLR, the effect of BMI above and below 25 was also investigated [20]. In that study, a higher risk of undergoing revision surgery was found in patients with a BMI of less than 25. The authors interpreted these findings to be related to a confounding higher level of activity rather than the relative BMI itself. The current data had a trend towards lower risk of revision in patients with a BMI of 25–29, perhaps due to a similar effect. The results did, however, disappear in the adjusted model.
There are, inevitably, some limitations in the current work. Data collected from a registry are volunteered from patients and surgeons and error in registrations might therefore occur. Further, the current study is somewhat conservative when only using revision to define failure after primary surgery. It is well-known that revision surgery is not performed in all patients that experience failure after ACL reconstruction. A study by Crawford et al., including findings from clinical examination in addition to revision surgery, displayed how the actual failure rate can be up to double of what is accounted for by only including revision surgery as a measure for failure [5]. Another limitation, inherent in the Norwegian registry, is that graft diameter is reported as the thickest part of the construct. The graft tunnel diameter, as a proxy for graft diameter, is commonly used in other studies—so this issue is not only related to the current work [17]. Biomechanically, the thinnest part of the graft has the lowest load to failure and should perhaps rather have been registered. Using data from a registry also has a certain limitation in the available data. Therefore, factors that have previously been shown to affect outcomes—surgeon experience, graft tunnel placement, rehabilitation protocol, timing of return to sport—has not been controlled for. Strengths of the current study include a rare and relative high number of patients (N = 150) undergoing revision surgery. Adjusting for age, gender, level of activity at time of injury, BMI (and height and weight) and fixation method in tibia/femur did account for some potential confounders and strengthens the current results. Finally, reporting data from a community-based registry might increase the generalizability of results for the common surgeon as compared to case series of more homogenous patients from single-center high-volume surgeons.
The findings from the current study do not align with other studies displaying that a bigger hamstrings autograft diameter reduces the risk of revision surgery as compared to smaller graft sizes. The current results does therefore not support the choice of upsizing the smaller graft diameters when encountered. The authors do, however, feel that more studies should reproduce these findings before general recommendations on accepting smaller graft size is given.
Conclusion
Contrary to former studies—showing an increased risk of undergoing revision surgery when a smaller graft size has been used at the primary ACL reconstruction—the current work could not demonstrate such effects. The influence of BMI was also investigated, and although a high incidence of adipose patients were identified—the BMI was not found to relate to the risk of revision surgery.
Change history
28 August 2019
Unfortunately, the author Jon Olav Drogset was incorrectly published in the original version and updated here. The original article has been corrected.
References
Chalmers PN, Mall NA, Moric M, Sherman SL, Paletta GP, Bach JCB BR (2014) Does ACL reconstruction alter natural history?: A systematic literature review of long-term outcomes. J Bone Jt Surg 96:292–300
Inderhaug E, Strand T, Fischer-Bredenbeck C, Solheim E (2013) Long-term results after reconstruction of the ACL with hamstrings autograft and transtibial femoral drilling. Knee Surg Sports Traumatol Arthrosc 21:2004–2010
Lai C, Ardern C, Feller J, Webster K (2017) Return to sport following anterior cruciate ligament reconstruction in elite athletes: a systematic review and meta-analysis. J Sci Med Sport 20:e101
Grassi A, Kim C, Marcheggiani Muccioli GM, Zaffagnini S, Amendola A (2017) what is the mid-term failure rate of revision ACL reconstruction? A systematic review. Clin Orthop Relat Res 475:2484–2499
Crawford SN, Waterman BR, Lubowitz JH (2013) Long-term failure of anterior cruciate ligament reconstruction. Arthroscopy 29:1566–1571
Webster KE, Feller JA (2016) Exploring the high reinjury rate in younger patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med 44:2827–2832
Steiner M (2017) Editorial commentary: size does matter—anterior cruciate ligament graft diameter affects biomechanical and clinical outcomes. Arthroscopy 33:1014–1015
Janssen RPA, van der Velden MJF, van den Besselaar M, Reijman M (2015) Prediction of length and diameter of hamstring tendon autografts for knee ligament surgery in Caucasians. Knee Surg Sports Traumatol Arthrosc 25:1199–1204
Magnussen RA, Lawrence JTR, West RL, Toth AP, Taylor DC, Garrett WE (2012) Graft size and patient age are predictors of early revision after anterior cruciate ligament reconstruction with hamstring autograft. Arthroscopy 28:526–531
Mariscalco MW, Flanigan DC, Mitchell J, Pedroza AD, Jones MH, Andrish JT, Parker RD, Kaeding CC, Magnussen RA (2013) The influence of hamstring autograft size on patient-reported outcomes and risk of revision after anterior cruciate ligament reconstruction: a multicenter orthopaedic outcomes network (MOON) cohort study. Arthroscopy 29:1948–1953
Atbaşi Z, Erçin E, Erdem Y, Emre T, Atilla H, Parlak A (2017) Correlation between body mass index and quadrupled hamstring tendon autograft size in ACL reconstruction. Joints 4:198–201
Hamner DL, Brown CH, Steiner ME, Hecker AT, Hayes WC (1999) Hamstring tendon grafts for reconstruction of the anterior cruciate ligament. JBJS 81:549–557
Schimoler PJ, Braun DT, Miller MC, Akhavan S (2015) Quadrupled hamstring graft strength as a function of clinical sizing. Arthroscopy 31:1091–1096
Boniello MR, Schwingler PM, Bonner JM, Robinson SP, Cotter A, Bonner KF (2015) Impact of hamstring graft diameter on tendon strength: a biomechanical study. Arthroscopy 31:1084–1090
Park SY, Oh H, Park S, Lee JH, Lee SH, Yoon KH (2013) Factors predicting hamstring tendon autograft diameters and resulting failure rates after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 21:1111–1118
Snaebjörnsson T, Hamrin Senorski E, Ayeni OR, Alentorn-Geli E, Krupic F, Norberg F, Karlsson J, Samuelsson K (2017) Graft diameter as a predictor for revision anterior cruciate ligament reconstruction and KOOS and EQ-5D values: a cohort study from the Swedish national knee ligament register based on 2240 patients. Am J Sports Med 45:2092–2097
Spragg L, Chen J, Mirzayan R, Love R, Maletis G (2016) The Effect of autologous hamstring graft diameter on the likelihood for revision of anterior cruciate ligament reconstruction. Am J Sports Med 44:1475–1481
Ranstam J, Kärrholm J, Pulkkinen P, Mäkelä K, Espehaug B, Pedersen AB, Mehnert F, Furnes O, For the NARA study group (2011) Statistical analysis of arthroplasty data. II. Guidelines. Acta Orthop 82:258–267
Gifstad T, Foss OA, Engebretsen L, Lind M, Forssblad M, Albrektsen G, Drogset JO (2014) Lower risk of revision with patellar tendon autografts compared with hamstring autografts: a registry study based on 45,998 primary ACL reconstructions in Scandinavia. Am J Sports Med 42:2319–2328
Persson A, Fjeldsgaard K, Gjertsen JE, Kjellsen AB, Engebretsen L, Hole RM, Fevang JM (2014) Increased risk of revision with hamstring tendon grafts compared with patellar tendon grafts after anterior cruciate ligament reconstruction: a study of 12,643 patients from the norwegian cruciate ligament registry, 2004–2012. Am J Sports Med 42:285–291
Ranstam J, Kärrholm J, Pulkkinen P, Mäkelä K, Espehaug B, Pedersen AB, Mehnert F, Furnes O, For the NARA study group (2011) Statistical analysis of arthroplasty data. I. Introduction and background. Acta Orthop 82:253–257
Andernord D, Björnsson H, Petzold M, Eriksson BI, Forssblad M, Karlsson J, Samuelsson K (2014) Surgical predictors of early revision surgery after anterior cruciate ligament reconstruction: results from the swedish national knee ligament register on 13,102 patients. Am J Sports Med 42:1574–1582
Kamien PM, Hydrick JM, Replogle WH, Go LT, Barrett GR (2013) Age, graft size, and tegner activity level as predictors of failure in anterior cruciate ligament reconstruction with hamstring autograft. Am J Sports Med 41:1808–1812
Brophy RH, Schmitz L, Wright RW, Dunn WR, Parker RD, Andrish JT, McCarty EC, Spindler KP (2012) Return to play and future acl injury risk after acl reconstruction in soccer athletes from the multicenter orthopaedic outcomes network (MOON) group. Am J Sports Med 40:2517–2522
Shelbourne KD, Benner RW, Gray T (2014) Return to sports and subsequent injury rates after revision anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med 42:1395–1400
Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, Allison TG, Batsis JA, Sert-Kuniyoshi FH, Lopez-Jimenez F (2008) Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes 32:959–966
Ng M, Fleming T, Robinson M et al (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384:766–781
Kluczynski MA, Bisson LJ, Marzo JM (2014) Does body mass index affect outcomes of ambulatory knee and shoulder surgery? Arthroscopy 30:856–865
de Valk EJ, Moen MH, Winters M, Bakker EWP, Tamminga R, van der Hoeven H (2013) Preoperative patient and injury factors of successful rehabilitation after anterior cruciate ligament reconstruction with single-bundle techniques. Arthroscopy 29:1879–1895
Pietrosimone B, Kuenze C, Hart JM, Thigpen C, Lepley AS, Blackburn JT, Padua DA, Grindstaff T, Davis H, Bell D (2017) Weak associations between body mass index and self-reported disability in people with unilateral anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 26:1326–1334
Funding
There has been no funding for the current work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflicts of interest to declare.
Ethical approval
Ethical approval has not been sought since the NKLR has ethical approval for its enrollment of patients, and no new data has been sought for the current work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: The author name Jon Olav Drogset was incorrectly published in the original version and updated here.
Rights and permissions
About this article
Cite this article
Inderhaug, E., Drogset, J.O., Lygre, S.H.L. et al. No effect of graft size or body mass index on risk of revision after ACL reconstruction using hamstrings autograft. Knee Surg Sports Traumatol Arthrosc 28, 707–713 (2020). https://doi.org/10.1007/s00167-019-05395-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-019-05395-5