Abstract
Purpose
The purpose of the present study is to compare newer designs of various symmetric and asymmetric tibial components and measure tibial bone coverage using the rotational safe zone defined by two commonly utilized anatomic rotational landmarks.
Methods
Computed tomography scans (CT scans) of one hundred consecutive patients scheduled for total knee arthroplasty were obtained pre-operatively. A virtual proximal tibial cut was performed and two commonly used rotational axes were added for each image: the medio-lateral axis (ML-axis) and the medial 1/3 tibial tubercle axis (med-1/3-axis). Different symmetric and asymmetric implant designs were then superimposed in various rotational positions for best cancellous and cortical coverage. The images were imported to a public domain imaging software, and cancellous and cortical bone coverage was computed for each image, with each implant design in various rotational positions.
Results
One single implant type could not be identified that provided the best cortical and cancellous coverage of the tibia, irrespective of using the med-1/3-axis or the ML-axis for rotational alignment. However, it could be confirmed that the best bone coverage was dependent on the selected rotational landmark. Furthermore, improved bone coverage was observed when tibial implant positions were optimized between the two rotational axes.
Conclusions
Tibial coverage is similar for symmetric and asymmetric designs, but depends on the rotational landmark for which the implant is designed. The surgeon has the option to improve tibial coverage by optimizing placement between the two anatomic rotational alignment landmarks, the medial 1/3 and the ML-axis. Surgeons should be careful assessing intraoperative rotational tibial placement using the described anatomic rotational landmarks to optimize tibial bony coverage without compromising patella tracking.
Level of Evidence
III.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tibial coverage of modern total knee designs is an important variable to ensure long-term survivorship and function of total knee replacement [6]. Improved tibial coverage decreases transferred forces to bony surfaces, specifically if the tibial cut is not perpendicular to tibial mechanical axis. In vivo measurements have shown increased high mechanical peak forces with incremental tibial varus positioning of the tibial baseplate [17]. The medial and lateral tibial plateau have different anteroposterior (AP) and mediolateral (ML) dimensions [11, 22], and optimizing tibial bone coverage with symmetric implants may result in internal rotation of the implant [20], which may affect patellar kinematics [16] and clinical outcomes [21]. Component overhang, on the other side, may lead to increased pain and poorer functional outcome, specifically in small women [4, 12]. Proponents of asymmetric implants designs argue that an asymmetric design provides better tibial coverage with less overhang and is easier to place, but avoids internal rotation of the tibial component [8, 26].
The geometry of modern tibial implants is controversially discussed in the literature. Stulberg et al. found equal tibial coverage between symmetric, asymmetric, and anatomic designs [26]. Clary et al. [6] compared four tibial implant designs (two symmetric and two asymmetric) to cover the resected tibia plateau and found that a symmetric tibial implant design had better overall coverage of the tibial plateau compared to the asymmetric designs. Dai et al. [8], on the other hand, compared six contemporary tibial implant designs and found that asymmetric designs resulted in higher tibial coverage, including improved tibial cortical support when proper rotational alignment was enforced. The controversy of Dai et al. and Clary et al. lies on the selected rotational tibial landmark. Dai et al. [8] used “Akagi’s axis,” which is defined as the line connecting the medial 1/3 of the tibial tubercle to the center of the posterior cruciate ligament [2], whereas Clary et al. [6] placed the tibial component along an axis defined as the line connecting the medial 1/3 of the tibial tubercle and the tibial origin, which is the midpoint between the centers of the medial and lateral tibial plateaus.
Lawrie et al. [18] defined a safe zone of tibial component alignment using two rotational landmarks: the medial–lateral axis (ML-axis) as described by Cobb et al. [7] and the medial third of the tibial tubercle (med-1/3-axis) as described by Uehara et al. [27].
The purpose of the present study is to compare symmetric and asymmetric tibial components using the safe zone of tibial rotation defined by Lawrie et al. [18] and quantify tibial cancellous and cortical coverage for each design for the ML-axis, the med 1/3 axis, and a position between the both lines.
The first hypothesis was that one implant geometry would yield superior coverage using the ML-axis; the second hypothesis was that a different geometry would provide better coverage using the medial 1/3 tibial axis. The third hypothesis was that aligning different implant geometries between the med-1/3-axis and the ML-axis would not increase tibial bone coverage.
Materials and methods
After approval from the Institutional Review Board at Brigham and Women’s Hospital (BWH, Protocol 2016P001082), a retrospective analysis of a cohort of 100 CT was computed. A total of 123 patients who had pre-op CT scans due to the custom type of TKR and were consecutively scheduled for TKR were screened for eligibility. All CT scans were taken with the same standardized protocol. The extremity was parallel to the long axis of the table with the patella facing the ceiling. The first fifty males and first fifty females were selected. Valgus or varus knees of more than 10 degrees were excluded, since the axial planes obtained from the CT scan would not perfectly display the perpendicular position to the anatomic tibial axis. Only CT scans of varus deformed knees were selected for the same reason. By excluding severely deformed knees, the display of the proximal tibia was standardized. 23 patients (13 males and 10 females) were excluded with 2 valgus deformities above 15 degrees, 17 patients with irregular subchondral sclerosis and cystic changes, 2 patients with bony defects after anterior cruciate ligament reconstructions, and 2 after hardware removal. This is because greyscale image processing and the subsequent differentiation of cortical and cancellous bone are detrimentally impacted by such bony changes.
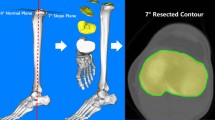
Using Centricity (GE Healthcare, Chicago, IL, USA), the above-mentioned axial images were obtained 3–5 mm below the deepest medial point on the central tibial plateau in the transverse tibial plane and superimposed with a second transverse plane 2 cm below to image to superimpose the medial 1/3 of the tibial tubercle as one anatomic landmark on one image.
Two symmetric designs, Sym1 and Sym2 (ATTUNE® and PFC SIGMA® Depuy Orthopedics, Warsaw IN, USA), and two asymmetric designs, Asym1 and Asym2 (Smith&Nephew Journey®, Smith and Nephew Inc., Memphis TN, USA; Zimmer Persona®, ZimmerBiomet, Warsaw, IN, USA), were chosen (Fig. 1). Virtual templates of various tibial implants of various sizes were created from each manufacturer’s user guide. Rules for sizing were based on common surgical practice: any tibial cortical overhang of more than 2 mm was avoided. After selecting the correct best fitting implant for each image, the images superimposed on the CT scans were then imported into ImageJ (Public Domain, National Institutes of Health NIH, Bethesda, Maryland) to compute coverage for each implant in square millimeters for the cancellous and cortical coverage. A relatively simple 2-D software in the public domain was chosen, so that any surgeon could use this technique to study bony coverage.
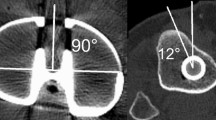
To identify the ML-axis and the med-1/3-axis, first the anatomical landmarks of the tibia had to be determined. This was done according to the techniques as described by Lawrie et al. [18] using ImageJ. For identifying the ML-axis, the tibial plateau center (TPC), the medial plateau center (MPC), and the lateral plateau center (LPC) were defined. To define the MPC, 15 points were placed along the cortical edge of the medial tibia, and the center of the circle best fitting these points was calculated as the MPC. Likewise, for defining the LPC, 15 points were placed on the edge of the lateral compartment of the tibia, and the center of the circle best fitting these points was calculated as the LPC. From these two points, the TPC was found by drawing a line through the MPC and LPC, extending this line to the medial and lateral edges of the tibia and defining the midpoint of this line as the TPC. The ML-axis as one rotational alignment was then simply a line starting at the TPC and perpendicular to the line connecting the MPC and LPC (Fig. 2a, b).
For identifying the med-1/3-axis as the other rotational alignment, first the medial third of the tibial tubercle and the center of the tibia in the second plane was selected. A line was drawn connecting the most lateral and the most medial aspects of the tibial tubercle, divided by three and the medial one-third selected. The center of the tibia in the second plane was determined as the center of the circle best fitting 15 points placed on the cortex of the tibia (Fig. 2c, d).
Each image was converted from an RGB (Red Green Blue) image to an 8-bit greyscale image, so that each pixel’s intensity would be one of 28 = 256 greyscale values. The cortical bone area of each image was chosen by selecting a threshold interval of greyscale values, i.e., by separating pixels which fall within a desired range of intensity values from those which do not. A pixel with an intensity 0 would be black, a pixel value of 256 would be white, and everything in between would be a shade of gray [21]. To avoid miscalculations, the images were cleared of anything that did not represent cortical bone, and a greyscale threshold value of average 63 (50–90) was selected to differentiate between cortical and cancellous bone. Greyscale units were used rather than Hounsfield units. Quantification of accuracy of measurements is determined by visually selecting a threshold of greyscale values that discriminated cortical from cancellous bone. This provided an individual pixel distribution for each individual tibia, reflecting cortical and cancellous bone. Due to the visual determination, there may be deviations between the absolute values obtained and the true values of cortical and cancellous bone. However, absolute values would be determined by intra- and inter-rater reliability.
The size of the virtual implant template was selected for best medial–lateral and anterior–posterior fit, but no overhang of more than 2 mm was accepted. These templates were superimposed to the CT scans for alignment using Keynote 2003–2015 (Apple Inc. Cupertino, CA, USA). Each virtual implant template was then aligned along the ML-axis, along the medial-1/3-axis, and in between to find the best cortical and cancellous coverage. The resulting overlay images were then exported into ImageJ by measuring the following six coverage parameters: total, medial, and lateral tibial cortical coverage, and total, medial, and lateral tibial cancellous coverage. The steps were repeated for each of the four designs. Figure 1 shows the resulting images created in Keynote before each image was exported into ImageJ for calculation.
Statistical analysis
Descriptive statistics were presented for each region of interest and implant type, overall and by sex. A linear mixed effects model (to account for repeated measurements on the same knee) was used to assess the association between tibial coverage in percentage and implant type. The comparison between implant types was assessed using Tukey’s adjustment for multiple comparisons. A two-sided sample size was assumed with an α (Type I error rate) of 5% and statistical power of 80%. A sample size of 100 would allow for the detection of differences in tibial coverage between implant types of 0.4 standard deviations.
To assess inter-reader and intra-reader agreement, the Shrout-Fleiss intraclass correlation coefficient (ICC) [23] was used. Three different observers measured 10% of all exams and repeated the measurements after 1 h. The ICC ranges from − 1 to 1, with values closer to zero indicating weaker reliability. Guidelines suggest that ICCs less than 0.40 indicate poor reliability, 0.40 to 0.59 fair, 0.60 to 0.74 good, and 0.75 to 1 excellent [5].
Results
Mean patient age was 66.1 years (53–80) for males and 65 years (49–93) for females. Using the ML-axis, Sym1 provided the best bone coverage of the total tibia (87.5 ± 3.1%), while Asym1 provided the best bone coverage of the medial tibia (87.5 ± 2.6%). Asym1 provided the greatest cortical coverage overall (52.9 ± 12.7%) and for the medial cortical tibia (58.7 ± 15.1%) (Tables 1, 2; Fig. 3).
Using the med-1/3-axis, Asym2 provided not only the best bone coverage of the entire tibia (83.5 ± 4.4%), but also of the medial tibia (81.0 ± 4.8%). The Asym2 provided the best cortical coverage of the entire tibia (50.7 ± 14.4%) and the medial tibia (51.2 ± 17.9%) (Fig. 4). Coverage of the lateral tibia is displayed in Fig. 5.
Optimizing the coverage between the two lines, an additional improvement of tibial coverage was observed; the cortical coverage of the implants did increase for each implant: by 12.4% for Sym1, by 21.9% for Sym2, by 7.9% for Asym1 and by 13.0% for Asym2. Aligning the implants between both lines for best cancellous coverage led only to marginal improvements: Sym1 improved by 0.4%, Sym2 by 0.7%, Asym1 by 0.3% and Asym2 by 1.9% (Figs. 6, 7).
The intra-rater reliability was high, ranging from a median of 0.957 (Sym1) to 0.989 (Sym2). The inter-rater reliability was good to excellent, with median ICCs ranging from 0.754 (Sym2) to 0.951 (Sym1).
Discussion
The most important finding of the present study is the fact that one implant (Asym2) has the best fit for the medial 1/3-axis but the worst for the ML-axis. The second most important finding is the fact that implant placement between the medial 1/3 axis and the ML-axis enhances tibial coverage for all implants studied.
It is clear that accurate tibial component rotation improves knee kinematics, decreases patella complications, and improves functional outcomes [3, 21]. One in vitro study demonstrated a significant reduction of retropatellar pressure by rotating the tibial component from 3° internal to 3° external rotation [25], suggesting this might decrease anterior knee pain. It is unclear which method to determine tibial rotation is best; the total number of methods speak for themselves: Akagi et al., Dao Trong et al., Incavo et al., Lawrie et al., Lützner et al., Silva et al., Uehara et al. [2, 9, 15, 18, 19, 24, 27]. The safe zone defined by Lawrie et al. [18] was used for this research to allow the surgeon to rotate the various designs within the two landmarks to optimize tibial implant coverage. The disadvantage of this selected method is the fact that the surgeon cannot rely solely on the medial 1/3 of the tibial tubercle.
One implant (Asym2) showed the best coverage in the med-1/3-axis alignment. It is recommended to place this implant within 5° of an axis created by the medial 1/3 of the tibial tubercle and the posterior cruciate ligament (PCL) attachment point [1]. This explains why this asymmetric design has inferior bone coverage for the ML-axis. Whether better cortical coverage is important or clinical relevant cannot be answered with this study.
The study’s first hypothesis could not be completely answered, since no single implant provides the best coverage for either observed anatomical landmark. However, the second hypothesis, that the selection of rotational landmarks influences best bone coverage, can be confirmed. The third hypothesis, that cortical coverage can be optimized if the implant is placed between the medial-1/3-axis and the ML-axis can be confirmed: cortical coverage was increased between 7.9% (Asym1), Sym1 by 12.4%, Asym2 by 13.0%. and 21.9% (Sym2). Tibial coverage is optimized by trying to find best tibial coverage between the two landmark lines.
The results obtained for tibial bone coverage, ranging from 79.8 to 83.5%, comply with the results of Clary et al. [6], who reported total tibia coverage of four different designs in 14,791 total knee arthroplasty patients. Three of the implant designs used by Clary et al., i.e., were assessed in the present study, and similar coverage values were observed, ranging from 80.2 to 83.8%. Their results showed that one symmetric tibial implant had better overall coverage of the tibial plateau as compared to the asymmetric designs, while asymmetric designs tend to create more internal rotation. Other authors also found no major differences between symmetric and asymmetric designs [26]. Only 2 mm overhang was accepted, similarly to Clary et al., which may explain why the observed coverage was slightly low compared to Wernecke et al. [28]. Overhang of more than 2 mm may lead to excessive medio-collateral ligament (MCL) loading and should be avoided [12].
Westrich et al. [29] observed tibial coverage of three historic tibial implant designs ranging from 80.6 to 84.7% and concluded that the shape of the tibial component and the number of sizes are more important to improve tibial coverage then asymmetrical or symmetrical designs. Dai et al. [8] compared six tibial designs and found one asymmetric design that had the best total tibial coverage among 6 designs (92–87%). However, Dai et al. used an implant which was specifically designed using a slightly modified med-1/3-axis: a line connecting the center of the posterior cruciate ligament to the medial third of the tibial tubercle. They did not discuss the high anatomic variability of the tibial tubercle relative to the tibial center and the ML-axis.
This study has several limitations. The resection level was below the osteophytes to avoid any interference with cortical measures using the image software, which may be below the normal surgical resection. This suggests that the geometries were not identical to what surgeons find intra-operatively. However, a resection level below the osteophytes was necessary to calculate the true cortical coverage, since the software depends on a greyscale to differentiate between cancellous and cortical bone. Another limitation of this study is that only four different implant designs were used. However, these four implants represent current modern symmetric and asymmetric designs. A relatively simple public domain software was also used, rather than a sophisticated commercial software [6, 8]. This may, however, be an advantage, in that it allows surgeons to research certain questions that are relevant to their practices without relying on commercial entities.
The accuracy of this study’s method cannot be determined based on the “true” value of coverage, as these readings were based on in vivo studies and thus there is no gold standard. The scaling error of the CT images was controlled in relation to the used implants. Every image had a known calibrating line with a known length and pixel amount. The image size was calibrated using the set scale function in ImageJ. The average scale of all 100 used images was 2.8349 pixels/mm with a standard deviation of 0.00146 pixels/mm. Therefore, 95% of measurements fell within 0.003 pixels/mm, with a calculated standard error of 0.000146. Since the greyscale threshold was set for each image prior to superimposing and calculating tibial coverage, the observed error did not impact the computed results, which is reflected in the high intra- and inter-observer reliability of the results.
The strength of the present study is the fact that all patients were scheduled for total knee replacement compared to other studies who selected non-arthritic patients. A further strength of the present study is that the coverage of different implants was not only considered for one particular axis, but for the two axes i.e., the med-1/3 and the ML-Axis and allowed for rotation within the safe zone. Cancellous and cortical coverage were considered separately given the fact that cortical bone is ten times denser then cancellous bone. Surprisingly, differences of up to 15% were found, which could influence long-term outcomes and tibial loosening.
The clinical implications of this research are related to improving tibial bone coverage without internally rotating the tibial component. This is important to optimize patellar tracking and knee kinematics. Each day, surgeons must strike a balance between better tibial coverage and optimal tibial rotation. To do so, they must understand the different geometries of symmetric and asymmetric tibial components. Asymmetric tibia have different asymmetries, and surgeons should know how different anteroposterior lengths for medial and lateral tibial condyles impact patella tracking and knee kinematics.
Conclusion
Tibial coverage is similar for symmetric and asymmetric designs, but depends on the rotational landmark for which the implant is designed. The surgeon has the option to improve tibial coverage by optimizing placement between the two anatomic rotational alignment landmarks, the medial 1/3 and the ML-axis. Surgeons should be careful assessing intraoperative rotational tibial placement using the described anatomic rotational landmarks to optimize tibial bony coverage without compromising patella tracking.
Abbreviations
- TKR:
-
Total knee replacement
- med-1/3-axis:
-
Medial-third-axis
- ML-axis:
-
Medial-lateral-axis
- TPC:
-
Tibial plateau center
- MPC:
-
Medial plateau center
- LPC:
-
Lateral plateau center
- RGB:
-
Red Green Blue
- PCL:
-
Posterior cruciate ligament
- PTA:
-
Posterior tibial axis
- AP:
-
Anteroposterior
- ML:
-
Mediolateral
- MCL:
-
Medio-collateral ligament
- Sym1:
-
Attune
- Sym2:
-
Sigma
- Asym1:
-
Journey
- Asym2:
-
Persona
References
Akagi M, Matsusue Y, Mata T, Asada Y, Horiguchi M, Iida H, Nakamura T (1999) Effect of rotational alignment on patellar tracking in total knee arthroplasty. Clin Orthop Relat Res 366:155–163
Akagi M, Oh M, Nonaka T, Tsujimoto H, Asano T, Hamanishi C (2004) An anteroposterior axis of the tibia for total knee arthroplasty. Clin Orthop Relat Res 420:213–219
Berger RA, Crossett LS, Jacobs JJ, Rubash HE (1998) Malrotation causing patellofemoral complications after total knee arthroplasty. Clin Orthop Relat Res 356:144–153
Bonnin MP, Schmidt A, Basiglini L, Bossard N, Dantony E (2013) Mediolateral oversizing influences pain, function, and flexion after TKA. Knee Surg Sports Traumatol Arthrosc 21(10):2314–2324
Cicchetti DV (1994) Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess 6(4):284–290
Clary C, Aram L, Deffenbaugh D, Heldreth M (2014) Tibial base design and patient morphology affecting tibial coverage and rotational alignment after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 22(12):3012–3018
Cobb JP, Dixon H, Dandachli W, Iranpour F (2008) The anatomical tibial axis: reliable rotational orientation in knee replacement. J Bone Joint Surg Br 90(8):1032–1038
Dai Y, Scuderi GR, Bischoff JE, Bertin K, Tarabichi S, Rajgopal A (2014) Anatomic tibial component design can increase tibial coverage and rotational alignment accuracy: a comparison of six contemporary designs. Knee Surg Sports Traumatol Arthrosc 22(12):2911–2923
Dao Trong ML, Diezi C, Goerres G, Helmy N (2015) Improved positioning of the tibial component in unicompartmental knee arthroplasty with patient-specific cutting blocks. Knee Surg Sports Traumatol Arthrosc 23(7):1993–1998
De Valk EJ, Noorduyn JCA, Mutsaerts ELAR. (2016) How to assess femoral and tibial component rotation after total knee arthroplasty with computed tomography: a systematic review. Knee Surg Sports Traumatol Arthrosc 24(11):3517–3528
Fitz W, Bliss R, Losina E (2013) Current fit of medial and lateral unicompartmental knee arthroplasty. Acta Orthop Belg 79(2):191–196
Gudena R, Pilambaraei MA, Werle J, Shrive NG, Frank CB (2013) A safe overhang limit for unicompartmental knee arthroplasties based on medial collateral ligament strains: an in vitro study. J Arthroplasty 28(2):227–233
Hirakawa M, Miyazaki M, Ikeda S, Matsumoto Y, Kondo M, Tsumura H (2017) Evaluation of the rotational alignment of the tibial component in total knee arthroplasty: position prioritizing maximum coverage. Eur J Orthop Surg Traumatol 27(1):119–124
Hirschmann MT, Konala P, Amsler F, Iranpour F, Friederich NF, Cobb JP (2011) The position and orientation of total knee replacement components: a comparison of conventional radiographs, transverse 2D-CT slices and 3D-CT reconstruction. J Bone Joint Surg Br 93(5):629–633
Incavo SJ, Coughlin K, Pappas C, Beynnon B (2003) Anatomic rotational relationships of the proximal tibia, distal femur, and patella: implications for rotational alignment in total knee arthroplasty. J Arthroplasty 18(5):643–648
Keshmiri A, Maderbacher G, Baier C, Zeman F, Grifka J, Springorum HR (2016) Significant influence of rotational limb alignment parameters on patellar kinematics: an in vitro study. Knee Surg Sports Traumatol Arthrosc 24(8):2407–2414
Kutzner I (2012) Influence of limb alignment on mediolateral loading in total knee replacement: in vivo measurements in five patients. J Bone Joint Surg Am 94(11):1023
Lawrie CM, Noble PC, Ismaily SK, Stal D, Incavo SJ (2011) The flexion-extension axis of the knee and its relationship to the rotational orientation of the tibial plateau. J Arthroplasty 26(6):53–58.e1
Lützner J, Krummenauer F, Günther K, Kirschner S (2010) Rotational alignment of the tibial component in total knee arthroplasty is better at the medial third of tibial tuberosity than at the medial border. BMC Musculoskelet Disord 11:57
Martin S, Saurez A, Ismaily S, Ashfaq K, Noble P, Incavo SJ (2014) Maximizing tibial coverage is detrimental to proper rotational alignment. Clin Orthop Relat Res 472(1):121–125
Nicoll D, Rowley DI (2010) Internal rotational error of the tibial component is a major cause of pain after total knee replacement. J Bone Joint Surg Br 92(9):1238–1244
Servien E, Saffarini M, Lustig S, Chomel S, Neyret P (2008) Lateral versus medial tibial plateau: morphometric analysis and adaptability with current tibial component design. Knee Surg Sports Traumatol Arthrosc 16(12):1141–1145
Shrout P, Fleiss JL (1979) Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86(2):420–428
Silva A, Pinto E, Sampaio R (2016) Rotational alignment in patient-specific instrumentation in TKA: MRI or CT? Knee Surg Sports Traumatol Arthrosc 24(11):3648–3652
Steinbrück A, Schröder C, Woiczinski M, Müller T, Müller PE, Jansson V, Fottner A (2016) Influence of tibial rotation in total knee arthroplasty on knee kinematics and retropatellar pressure: an in vitro study. Knee Surg Sports Traumatol Arthrosc 24(8):2395–2401
Stulberg SD, Goyal N (2015) Which tibial tray design achieves maximum coverage and ideal rotation: anatomic, symmetric, or asymmetric? An MRI-based study. J Arthroplasty 30(10):1839–1841
Uehara K, Kadoya Y, Kobayashi A, Ohashi H, Yamano Y (2002) Bone anatomy and rotational alignment in total knee arthroplasty. Clin Orthop Relat Res 402:196–201
Wernecke G, Harris I, Houang M, Seeto B, Chen D, MacDessi S (2012) Comparison of tibial bone coverage of 6 knee prostheses: a magnetic resonance imaging study with controlled rotation. J Orthop Surg (Hong Kong) 20(2):143–147
Westrich G, Haas S, Insall J, Frachie A (1995) Resection spe 373 cimen analysis of proximal tibial anatomy based on 100 total knee arthroplasty specimens. J Arthroplasty 10(1):47–51
Author information
Authors and Affiliations
Contributions
MM: acquisition of data, analysis and interpretation of data, drafting of the manuscript, preparing of graphs and tables, has given final approval of the version to be published, participated in the design of the study, agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. JW: revising the manuscript critically for important intellectual content, participated in the design of the study. JC: participated in the design of the study and performed the statistical analysis. JB: revising the manuscript critically for important intellectual content, discussion of background and results, analysis and interpretation of data, have given final approval of the version to be published, agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. WF: provided conception and design of the study, general supervision of the research group, analysis and interpretation of data, discussion of background and results, revising the manuscript critically for important intellectual content, final approval of the version to be published, agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Funding
There was no outside funding or grants received that assisted in this study.
Ethical approval
This study has been performed in accordance with ethical standards concerning investigations in the United States of America.
Informed consent
For this type of article informed consent is not required.
Rights and permissions
About this article
Cite this article
Meier, M., Webb, J., Collins, J.E. et al. Do modern total knee replacements improve tibial coverage?. Knee Surg Sports Traumatol Arthrosc 26, 3219–3229 (2018). https://doi.org/10.1007/s00167-018-4836-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-018-4836-3