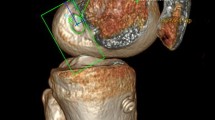
Abstract
Purpose
To evaluate the feedback from post-operative three-dimensional computed tomography (3D-CT) on femoral tunnel placement in the learning process, to obtain an anatomic anterior cruciate ligament (ACL) reconstruction.
Methods
A series of 60 consecutive patients undergoing primary ACL reconstruction using autologous hamstrings single-bundle outside-in technique were prospectively included in the study. ACL reconstructions were performed by the same trainee-surgeon during his learning phase of anatomic ACL femoral tunnel placement. A CT scan with dedicated tunnel study was performed in all patients within 48 h after surgery. The data obtained from the CT scan were processed into a three-dimensional surface model, and a true medial view of the lateral femoral condyle was used for the femoral tunnel placement analysis. Two independent examiners analysed the tunnel placements. The centre of femoral tunnel was measured using a quadrant method as described by Bernard and Hertel. The coordinates measured were compared with anatomic coordinates values described in the literature [deep-to-shallow distance (X-axis) 28.5%; high-to-low distance (Y-axis) 35.2%]. Tunnel placement was evaluated in terms of accuracy and precision. After each ACL reconstruction, results were shown to the surgeon to receive an instant feedback in order to achieve accurate correction and improve tunnel placement for the next surgery. Complications and arthroscopic time were also recorded.
Results
Results were divided into three consecutive series (1, 2, 3) of 20 patients each. A trend to placing femoral tunnel slightly shallow in deep-to-shallow distance and slightly high in high-to-low distance was observed in the first and the second series. A progressive improvement in tunnel position was recorded from the first to second series and from the second to the third series. Both accuracy (+52.4%) and precision (+55.7%) increased from the first to the third series (p < 0.001). Arthroscopic time decreased from a mean of 105 min in the first series to 57 min in the third series (p < 0.001). After 50 ACL reconstructions, a satisfactory anatomic femoral tunnel was reached.
Conclusion
Feedback from post-operative 3D-CT is effective in the learning process to improve accuracy and precision of femoral tunnel placement in order to obtain anatomic ACL reconstruction and helps to reduce also arthroscopic time and learning curve. For clinical relevance, trainee-surgeons should use feedback from post-operative 3DCT to learn anatomic ACL femoral tunnel placement and apply it appropriately.
Level of evidence
Consecutive case series, Level IV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The restoration of native anatomy is one of the fundamental principles of orthopaedics. Surgeons have learned that this principle is of crucial importance to any reconstructive procedure [29]. Tunnel placement is one of the most important factors in anterior cruciate ligament (ACL) reconstruction [16, 18, 21, 30, 36]. Recent studies affirm that anatomic ACL reconstruction is better than isometric reconstruction in restoring knee function [22, 36]. In particular, these studies state that anatomic femoral placement is crucial to replicate normal anatomy and restore native knee kinematics and it may help to decrease the incidence of joint degeneration after ACL reconstruction [1, 8]. Other studies explain that the femoral tunnel placement is more complicated and more challenging than the tibial one [15, 27].
The use of arthroscopic and radiological landmarks can help surgeons to be more precise during their personal learning phase [29]. Several intra-operative aids are available to help femoral tunnel placement. Anatomic aids as femoral ACL footprint or femoral bony landmarks (lateral intercondylar ridge-residents ridge, lateral bifurcate ridge) undergo inter-individual variability [4, 7, 31, 32] or are influenced by injury-surgery delay [4]. Instrumental aids are represented by arthroscopic ruler, intra-operative fluoroscopy or ACL computer-assisted surgery. Arthroscopic ruler also requires femoral bony landmarks, but the technique described is accurate and precise [3]. Intra-operative fluoroscopy or ACL computer-assisted surgery is still under investigation, and further studies should be performed [4, 13, 20].
The use of post-operative three-dimensional computed tomography (3D-CT) feedback in anatomic antero-medial ACL reconstruction has been evaluated in a recent study [13].
To the best of our knowledge, no studies explore the effects of feedback from post-operative 3D-CT in the learning phase of femoral tunnel placement in anatomic outside-in ACL reconstruction.
The purpose of the current study was to evaluate the effects of feedback from post-operative 3D-CT on improvement of accuracy and precision of femoral tunnel placement [A] and reduction of learning curve [B] and arthroscopic time [C] in the learning process of anatomic outside-in ACL reconstruction.
The hypothesis was that the post-operative 3D-CT would help to place the femoral tunnel into the anatomic centre and would reduce learning curve and arthroscopic time.
Materials and methods
A consecutive case series in vivo study was conducted to evaluate the feedback from post-operative 3D-CT in the learning curve of anatomic femoral tunnel placement in ACL reconstruction. A trainee-surgeon (FM) in the transitional phase of switching his approach of drilling the femoral tunnel from an isometric technique—transtibial (TT)—to an anatomic technique—outside-in (OUT-IN)—has been assisted by a post-operative 3D-CT feedback.
Study design
All patients, undergoing primary ACL reconstruction performed by the same trainee-surgeon (FM), were evaluated by a CT scan with dedicated tunnel study within 48 h after surgery. The data obtained from the CT scan were processed into a 3D surface model, and a true medial view of the lateral femoral condyle was used for the femoral tunnel placement analysis. Two independent examiners (LS, MtI) analysed the tunnel placements. The centre of femoral tunnel was measured using a quadrant method as described by Bernard and Hertel [2]. The coordinates measured were compared with anatomic coordinates values described by Piefer et al. [25]. Tunnel placement was evaluated in terms of accuracy and reproducibility. After each ACL reconstruction, results were shown to the surgeon to receive an instant feedback in order to achieve accurate correction and improve tunnel placement for the next surgery (Fig. 1).
Patient selection
Between January 2014 and March 2016, a total of 60 consecutive patients with primary isolated ACL tears, screened for an arthroscopically assisted reconstruction, were included in the study. Inclusion criteria were as follows: primary isolated ACL tears, ACL chronic injury (≥3 weeks following injury), skeletal maturity (≥18 years) and 3D-CT follow-up guaranteed. A flow chart describing patient selection in shown in Fig. 2. For a better analysis of trainee-surgeon’s progression, patients were divided into three consecutive series (1, 2, 3) of 20 patients each. The series covered the same time laps in order to avoid any bias on the speed of learning curve. Patient characteristics (age, gender, BMI and injury/surgery delay) are reported in Table 1.
Surgical technique
All ACL reconstructions were performed using the same surgical technique: antero-medial (AM) and antero-lateral (AL) arthroscopic portals, OUT-IN technique, single bundle and duplicated autologous hamstrings.
After joint debridement, the aimer of the tibial drill guide (Arthrex® Inc, Naples, FL, USA) set on 55° was introduced through AM portal and the tip of this guide placed into the ideal centre of ACL tibial insertion. Extra-articularly, the guide sleeve was placed 1 cm above the insertion of the pes anserinus and 1.5 cm medial to the tibial tubercle. After the guide-wire was inserted, a tibial tunnel was created using a cannulated drill bit matching the graft diameter [28].
ACL main bulk and additional soft tissue were removed from the medial wall of the lateral femoral condyle. Femoral tunnel was realized using the outside-in FlipCutter® RetroConstruction™ technique (Arthrex®). The aimer of the femoral RetroConstruction™ drill guide (Arthrex®) set on 110° was introduced through AL portal [6]. The arthroscopic calibrated femoral aimer of RetroConstruction™ drill guide (Arthrex®) was the unique intra-operative reference permitted to place the tunnel. As described by Wilson et al. [33], the guide was used to identify and mark the centre of the ACL femoral footprint. Two measurements were made: the “deep distance” and the “height distance” of the ACL femoral footprint centre. The distance between the deep and shallow articular cartilage (deep-to-shallow distance) was obtained using the ruler marked on the long axis of the guide (Fig. 3a). The “deep distance” was the midpoint-line of the deep-to-shallow distance measured from the shallow articular cartilage (Fig. 3b); instead the “height distance” was 2 mm plus the radius of the graft measured from the low articular cartilage (Fig. 3c). The centre of the femoral RetroConstruction™ drill guide (Arthrex®) was positioned in the centre of the ACL femoral footprint identified, with an extra-articular visual angle of 45° relative to the frontal and sagittal axes of the femur [6]. Extra-articularly, the guide sleeve was placed proximal and anterior to the lateral epicondyle to avoid injury to lateral soft tissue structures and to maximize tunnel length. The FlipCutter® (Arthrex®), matching the graft diameter, was than advanced as anterograde drilling until it reached the tip of the femoral aimer. After blade flipping, the retrograde reamer was pulled back until femoral socket was created at 25 mm of depth. Autologus hamstring graft was fixed using ACL TightRope® RT (Arthrex®), an adjustable loop cortical fixation device, on femoral side, and Bio-Intrafix™ (DePuy-Mitek©, Raynham, MA, USA), a bioabsorbable interference screw, on tibial side, with 40-N tension applied to graft using the ACL Tie Tensioner (DePuy-Mitek©) at 30° of knee flexion [5].
Radiological evaluation
All patients underwent dedicate CT scan using Siemens SOMATOM® Sensation 16 CT scanner (Siemens AG©, Munich, Germany) on fully extended knee, within 48 h after surgery. The images were obtained at 120 kV and 450 mA, with a slice thickness of 1.25 mm and a pitch of 0.5 mm/rev. The data from the CT scans were exported to an image analysis software syngo ® VRT (Siemens AG©, Munich, Germany) and processed into 3D surface model. The models were co-registered with properly scaled male or female base models, which had been pre-aligned to an anatomic coordinate system based on the femoral head and tibial malleoli centre as recommended by the International Society of Biomechanics [9, 34].
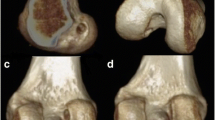
In order to evaluate the femoral tunnel placement, the 3D surface model was oriented in a true lateral view and the medial condyle was progressively digitally subtracted until the roof of the inter-condyle notch was reached.
The analysis was performed using a true medial view of the lateral femoral condyle. The centre of the femoral tunnel was measured using a 10 × 10 grid as described by Bernard and Hertel quadrant method [2]. The parallel and the perpendicular to the Blumensaat’s line positions were used to determine femoral tunnel coordinates. Parallel to the Blumensaat’s line position was calculated as percentage of the total sagittal diameter of the lateral condyle measured along Blumensaat’s line [deep-to-shallow distance (X-axis)]. Perpendicular to the Blumensaat’s line position was calculated as percentage of the maximum intercondylar notch height [high-to-low distance (Y-axis)] (Fig. 4). The coordinates measured were compared with anatomic coordinates values described by Piefer et al. [deep-to-shallow distance (X-axis), normal value = 28.5%; high-to-low distance (Y-axis), normal value = 35.2%] [25] (Fig. 5).
All measurements were performed by two other independent and blinded surgeons (LS, MtI) not participating in the surgical procedures. To examine the reproducibility of this method, we randomly selected ten knees from the 3D-CT surface models and repeated all measurements three times by three other independent surgeons (MI, RC, CC), not participating in the surgical procedure, who were blinded to the results reported by the other observers (LS, MtI). The intra-observer and inter-observer reliabilities were analysed both for the deep-to-shallow distance and for the high-to-low distance and assessed by determining the intraclass/interclass correlation coefficient (ICCs).
Outcome measures
The differences in positions between anatomic ACL origin centre (AC) and post-operative femoral tunnel centre (TC) for each distance were recorded for each patient. Tunnel placement was evaluated in terms of accuracy and precision as described by Luites and colleagues [16]. The accuracy (the agreement between the positions) was calculated using the mean of all the individual absolute differences (Euclidean distance). The Euclidean distance (ΔXY) from the anatomic ACL origin centre (AC) to the post-operative femoral tunnel centre (TC) was measured with the following formula ΔXY = √(X AC − X TC)2 + (Y AC − Y TC)2 [16]. The precision (the reproducibility of the procedure) was defined by means of the standard deviation (SD) of the absolute differences [16]. Complications and arthroscopic time were also recorded.
According to Sadoghi et al. [26], femoral tunnel was rated as “anatomic” if it was placed inside anatomic coordinates values with a range of 1.0 units of the grid.
Feedback
After each ACL reconstruction, results recorded from a single patient were shown to the surgeon in order to assess the previous tunnel performed, to achieve the accurate correction and improve tunnel placement in the following surgery. As a result, the surgeon obtained a feedback every time.
Ethical standards
All patients accepted the proposed treatment and follow-up after an adequate information and written consent. The study and follow-up, respecting the criteria of the Declaration of Helsinki, have been approved by Institutional Review Board of Azienda Ospedaliera-Universitaria Careggi—Department of Surgery and Translational Medicine. The IRB number was DCMT2012/DIC/STUDI.SPER/2012.009.ORT.
Statistical analysis
Statistical analysis was performed using SPSS® statistics software (IBM®, Armonk, New York, USA). The demographic data of the study were examined using repeated-measures ANOVA (age, BMI, injury/surgery delay) and Fisher’s exact Chi-squared test (gender). The outcome measures between the three series were compared using repeated-measures ANOVA (accuracy, arthroscopic time, complication) and Levene’s test equality of variance (precision). A p level of 0.05 was considered significant. When statistically significant effects were found, pair-wise comparisons were performed using the Bonferroni post hoc test to determine the exact location of the group difference. A repeated one-way analysis of variance (ANOVA) was performed to calculate the intra-observer reliability of the data recorded for the three measurements. The inter-observer reliability was calculated using an unrepeated two-way ANOVA of the averages of the three measurements obtained by each of the three observers. A time period of 4 weeks elapsed between test and retest measurements. Sample sizes were based on predicted power to detect a difference of 5% between series with an alpha 0.05 and 80% power. A difference of 5% was considered clinically relevant. Considering the means and the standard deviations (SD) of the absolute differences, minimal sample size of 16 subjects per series was calculated from our pilot study of five patients per series.
Results
Patient characteristics
No significant differences (n.s.) were recorded between the three series in age, gender, BMI and injury/surgery delay, and values are shown in Table 1.
Femoral tunnel placement
A trend to placing femoral tunnel slightly shallow in deep-to-shallow distance and slightly high in high-to-low distance was observed in the first and the second series. Femoral tunnel placements, divided in corresponding series, are all presented in Fig. 6.
Great reliability of the measurement method used in the 3D-CT surface model was obtained. The intraclass/interclass correlation coefficient (ICCs) values were 0.986/0.987 and 0.992/0.962 for the deep-to-shallow and high-to-low distance, respectively.
Accuracy and precision of femoral tunnel placement
Both accuracy (+52.4%) and precision (+55.7%) increased from the first to the third series.
Reverse trend was observed for all the individual absolute differences and the SD of the absolute differences. The continuous improvement of ACL femoral tunnel placement is presented in Fig. 7. A significant difference between the three series was observed in accuracy (<0.001) and precision (<0.001) (Table 2). In particular, pair-wise comparisons revealed no significant difference (n.s.) between first and second series among accuracy and precision, whereas accuracy and precision in third series were significantly higher than first and second series (0.001 and <0.001, respectively) (Table 3).
Learning curve effect
After 50 ACL reconstructions, femoral tunnels were placed inside anatomic coordinates values with a range of 1.0 units of the grid and, according to Sadoghi et al. [26], a satisfactory anatomic femoral tunnel placement was reached.
Arthroscopic time
Arthroscopic time decreased from a mean of 105 min in the first series to 57 min in the third series. A significant difference was recorded comparing arthroscopic time (<0.001) between the three series (Table 2). Pair-wise comparisons showed a significant difference between first, second and third series (<0.001) (Table 3).
Discussion
The most important findings of the current study are progressive improvement of femoral tunnel placement to anatomic ACL centre [A], reduction of arthroscopic time [B] and learning curve [C] as effects of feedback from post-operative 3D-CT.
Regarding surgical technique, a ruler marked on femoral tunnel drill guide is the unique intra-operative aid used by surgeon in this study. According to Wilson et al. [33], arthroscopic ruler helps the surgeon to identify and mark ACL footprint better. Anatomic aids as femoral bony landmarks (lateral intercondylar ridge-residents ridge, lateral bifurcate ridge) or femoral ACL residual often undergo inter-individual [7, 31, 32] or time variability [4]. Instrumental aids as intra-operative fluoroscopy and computer-assisted surgery use methods not jet validated; reference studies are poor with opposite results [4, 13, 20].
Focusing on radiological evaluation, the quadrant method described by Bernard and Hertel [2] is used to obtain standardized results on ACL tunnel placements analysis. Quadrant method can be realized on traditional radiograph as well as on CT scans [2, 3, 12, 14, 19, 23, 35]. Bernard and Hertel grid can be created on 3D-CT reconstructions to evaluate tunnel placements after ACL reconstruction [18, 24]. Tunnel coordinates obtained are compared to anatomic ACL coordinates described by a systematic review of ACL femoral footprint anatomy performed by Piefer et al. [25]. These “ideal” coordinates result from 7 cadaveric studies and 1 radiological in vivo study that evaluate ACL femoral footprint anatomy using Bernard and Hertel grid. In a recent study, Parkar et al. [23] suggest the CT as “gold standard” for evaluation of tunnel placements after ACL reconstruction. In that study, traditional radiographs, MRI and CT were compared for measurements of graft tunnel placements using Bernard and Hertel grid. Authors define CT consistently reliable in tunnel measurements and 3D-CT the only image modality and image type able to depict both deep-to-shallow and high-to-low directions [23]. According to Heckel et al. [10], knee CT radiation dose can be reduced by adjusting the technical parameter of CT machine to the same level as three views of knee traditional radiographs. In that way, post-operative 3D-CT advantages can be extended from clinical studies to routine clinical practices [23].
About learning curve, an anatomic femoral placement was reached after about 50 ACL reconstructions using post-operative 3D-CT feedback in the current study. Hohmann et al. [11] described that approximately 100 ACL reconstructions were required to complete learning curve performing repeated surgery without radiological feedback. That study describes learning process of single-bundle transtibial technique, a type of ACL reconstruction easier than single-bundle outside-in technique. In a retrospective review, Luthringer et al. [17] explained that a number of cases between 32 and 64 were needed to complete learning curve of femoral tunnel placement in single-bundle antero-medial ACL reconstruction. Literature is poor of studies that explain clearly the number of ACL reconstructions required to complete learning curve.
The current study has several limitations.
Firstly, a control study group without radiological feedback was not included. Femoral tunnel placement improvements can be the result of feedback from post-operative 3D-CT as well as repeated surgery. The current study can be compared to a similar study explained by Inderhaug et al. [13] that evaluates femoral tunnel placements performed using antero-medial technique without radiological feedback and antero-medial technique with post-operative 3D-CT feedback. Authors underline the importance of both radiological feedback and repeated surgery to improve femoral tunnel placements in learning process.
Secondly, this study does not analyse the clinical effects of outliers in femoral tunnel position during learning process of anatomic femoral tunnel placement reconstruction. Many recent studies underline the importance of anatomic femoral position to obtain better clinical results. The clinical effects of different femoral tunnel placements were not object of the current study; however, they could become a topic of future and follow-up study.
Thirdly, ACL reconstructions are performed by a unique trainee-surgeon in this study. Inter-surgeon variability can influence learning process and tunnel placements.
The lack of a control study group, the absence of a clinical evaluation and the presence of a unique trainee-surgeon performing the ACL reconstructions are all limitations.
The “instant feedback” from post-operative 3D-CT provided to the trainee-surgeon is the strength of the current study. In contrast to Inderhaug et al. [13], that performed post-operative 3D-CT between 6 and 12 weeks after surgery, in the current study 3D-CT scans and related measurements were conducted within 48 h after surgery. In that way after each ACL reconstruction, results recorded from a single patient were shown to the surgeon in order to assess the previous tunnel performed, to achieve the accurate correction and improve tunnel placement in the following surgery. As a result, the surgeon obtained a true feedback every time.
For clinical relevance, as shown by the results of this study, trainee-surgeons should use feedback from post-operative 3D-CT to learn anatomic ACL femoral tunnel placement and apply it appropriately. As a matter of fact, we recommend the use of post-operative low-dose radiation 3D-CT in daily practice during the learning phase of a surgeon approaching an anatomic technique to reduce the number of surgical procedures required to perform a quick high-quality anatomic ACL reconstruction.
Conclusion
Feedback from post-operative 3D-CT is effective in the learning process to improve accuracy and precision of femoral tunnel placement in order to obtain anatomic ACL reconstruction. This study does not evaluate any clinical result, but it underlines the importance of post-operative 3D-CT feedback in helping a trainee-surgeon to reduce arthroscopic time and accelerate the learning curve.
References
Abebe ES, Utturkar GM, Taylor DC, Spritzer CE, Kim JP, Moorman CT III, Garrett WE, DeFrate LE (2011) The effects of femoral graft placement on in vivo knee kinematics after anterior cruciate ligament reconstruction. J Biomech 44(5):924–929
Bernard M, Hertel P, Hornung H, Cierpinski T (1997) Femoral insertion of the ACL. Radiographic quadrant method. Am J Knee Surg 10:14–21
Bird JH, Carmont MR, Dhillon M, Smith N, Brown C, Thompson P, Spalding T (2011) Validation of a new technique to determine midbundle femoral tunnel position in anterior cruciate ligament reconstruction using 3-dimensional computed tomography analysis. Arthroscopy 27:1259–1267
Brown CH, Spalding T, Robb C (2013) Medial portal technique for single-bundle anatomical anterior cruciate ligament (ACL) reconstruction. Int Orthop 37:253–269
Carulli C, Matassi F, Soderi S, Sirleo L, Munz G, Innocenti M (2017) Resorbable screw and sheath versus resorbable interference screw and staples for ACL reconstruction: a comparison of two tibial fixation methods. Knee Surg Sports Traumatol Arthrosc 25(4):1264–1271
Collette M, Cassard X (2011) The Tape Locking Screw technique (TLS): a new ACL reconstruction method using a short hamstring graft. Orthop Traumatol Surg Res 97:555–559
Ferretti M, Ekdahl M, Shen W, Fu FH (2007) Osseous landmarks of the femoral attachment of the anterior cruciate ligament: an anatomic study. Arthroscopy 23:1218–1225
Fu FH, van Eck CF, Tashman S, Irrgang JJ, Moreland MS (2015) Anatomic anterior cruciate ligament reconstruction: a changing paradigm. Knee Surg Sports Traumatol Arthrosc 23(3):640–648
Grood ES, Suntay WJ (1983) A joint coordinate system for the clinical description of three dimensional motions: application to the knee. J Biomech Eng 105:136–144
Henckel J, Richards R, Lozhkin K, Harris S, Rodriguez y Baena FM, Barrett AR, Cobb JP (2006) Very low-dose computed tomography for planning and outcome measurement in knee replacement for planning and outcome measurements in knee replacement. The imperial knee protocol. J Bone Joint Surg Br 88:1513–1518
Hohmann E, Bryant A, Tetsworth K (2010) Tunnel positioning in anterior cruciate ligament reconstruction: how long is the learning curve? Knee Surg Sports Traumatol Arthrosc 18:1576–1582
Horie M, Muneta T, Yamazaki J, Nakamura T, Koga H, Watanabe T, Sekiya I (2015) A modified quadrant method for describing the femoral tunnel aperture positions in ACL reconstruction using two-view plain radiographs. Knee Surg Sports Traumatol Arthrosc 23(4):981–985
Inderhaug E, Larsen A, Strand T, Waaler PA, Solheim E (2016) The effect of feedback from post-operative 3D CT on placement of femoral tunnels in single-bundle anatomic ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 24(1):154–160
Iriuchishima T, Ryu K, Suruga M, Aizawa S, Fu FH (2017) The correlation of femoral tunnel length with the height and area of the lateral wall of the femoral intercondylar notch in anatomical single-bundle ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 25(5):1632–1637
Kohn D, Busche T, Carls J (1998) Drill hole position in endoscopic anterior cruciate ligament reconstruction. Results of an advanced arthroscopy course. Knee Surg Sports Traumatol Arthrosc 6(Suppl 1):S13–S15
Luites JW, Wymenga AB, Blankevoort L, Eygendaal D, Verdonschot N (2014) Accuracy of a computer-assisted planning and placement system for anatomical femoral tunnel positioning in anterior cruciate ligament reconstruction. Int J Med Robot 10(4):438–446
Luthringer TA, Blackmore SA, Singh BC, Strauss EJ (2016) The learning curve associated with anteromedial portal drilling in ACL reconstruction. Phys Sportsmed 44(2):141–147
Markolf KL, Hame S, Hunter DM, Oakes DA, Zoric B, Gause P, Finerman GA (2002) Effects of femoral tunnel placement on knee laxity and forces in an anterior cruciate ligament graft. J Orthop Res 20:1016–1024
Matassi F, Sirleo L, Carulli C, Innocenti M (2015) Anatomical anterior cruciate ligament reconstruction: transtibial versus outside-in technique: SIGASCOT Best Paper Award Finalist 2014. Joints 3(1):6–14
Meuffels DE, Reijman M, Verhaar JA (2012) Computer-assisted surgery is not more accurate or precise than conventional arthroscopic ACL reconstruction: a prospective randomized clinical trial. J Bone Joint Surg Am 94(17):1538–1545
Moisala AS, Jarvela T, Harilainen A, Sandelin J, Kannus P, Jarvinen M (2007) The effect of graft placement on the clinical outcome of the anterior cruciate ligament reconstruction a prospective study. Knee Surg Sports Traumatol Arthrosc 15:879–887
Musahl V, Plakseychuk A, VanScyoc A, Sakaki T, Debski RE, McMahon PJ, Fu FH (2005) Varying femoral tunnels between the anatomical footprint and isometric positions: effect on kinematics of the anterior cruciate ligament reconstructed knee. Am J Sports Med 33:712–718
Parkar AP, Adriaensen ME, Fischer-Bredenbeck C, Inderhaug E, Strand T, Assmus J, Solheim E (2015) Measurements of tunnel placements after anterior cruciate ligament reconstruction—a comparison between CT, radiographs and MRI. Knee 22(6):574–579
Parkar AP, Adriaensen ME, Strand T, Inderhaug E, Harlem T, Solheim E (2013) How to read post-operative radiographs and CT scans after single-bundle anterior cruciate ligament reconstruction. Skeletal Radiol 42(11):1489–1500
Piefer JW, Pflugner TR, Hwang MD, Lubowitz JH (2012) Anterior cruciate ligament femoral footprint anatomy: systematic review of the 21st century literature. Arthroscopy 28:872–881
Sadoghi P, Kröpfl A, Jansson V, Müller PE, Pietschmann MF, Fischmeister MF (2011) Impact of tibial and femoral tunnel position on clinical results after anterior cruciate ligament reconstruction. Arthroscopy 27(3):355–364
Sati M, Staubli H, Bourquin Y, Kunz M, Nolte LP (2002) Real-time computerized in situ guidance system for ACL graft placement. Comput Aided Surg 7:25–40
Seo SS, Kim CW, Kim JG, Jin SY (2013) Clinical results comparing transtibial technique and outside in technique in single bundle anterior cruciate ligament reconstruction. Knee Surg Relat Res 25:133–140
Snyder GM, Johnson DL (2011) Anatomic graft placement in ACL surgery: plain radiographs are all we need. Orthopedics 34(2):116–118
Sommer C, Friederich NF, Muller W (2000) Improperly placed anterior cruciate ligament grafts: correlation between radiological parameters and clinical results. Knee Surg Sports Traumatol Arthrosc 8:207–213
Tsukada S, Fujishiro H, Watanabe K, Nimura A, Mochizuki T, Mahakkanukrauh P, Yasuda K, Akita K (2014) Anatomic variations of the lateral intercondylar ridge: relationship to the anterior margin of the anterior cruciate ligament. Am J Sports Med 42(5):1110
Van Eck CF, Martins CAQ, Vyas SM, Celentano U, Van Dijk CN, Fu FH (2010) Femoral intercondylar notch shape and dimensions in ACL-injured patients. Knee Surg Sports Traumatol Arthrosc 18:1257–1262
Wilson AJ, Yasen SK, Nancoo T, Stannard R, Smith JO, Logan JS (2013) Anatomic all-inside anterior cruciate ligament reconstruction using the translateral technique. Arthrosc Tech 2(2):e99–e104
Wu G, Siegler S, Allard P, Kirtley C, Leardini A, Rosenbaum D, Whittle M, D’Lima DD, Cristofolini L, Witte H, Schmid O, Stokes I, Standardization and Terminology Committee of International Society of Biomechanics (2002) ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion—part I: ankle, hip, and spine. J Biomech 35(4):543–548
Youm Y-S, Cho S-D, Eo J, Lee K-J, Jung K-H, Cha J-R (2013) 3D CT analysis of femoral and tibial tunnel positions after modified transtibial single bundle ACL reconstruction with varus and internal rotation of the tibia. Knee 20:272–276
Zavras TD, Race A, Amis AA (2005) The effect of femoral attachment location on anterior cruciate ligament reconstruction: graft tension patterns and restoration of normal anterior-posterior laxity patterns. Knee Surg Sports Traumatol Arthrosc 13:92–100
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
No funding sources were received for this study.
Ethical approval
The study and follow-up, respecting the criteria of the Declaration of Helsinki, has been approved by Institutional Review Board of Azienda Ospedaliera-Universitaria Careggi—Department of Surgery and Translational Medicine. The IRB number was DCMT2012/DIC/STUDI.SPER/2012.009.ORT.
Informed consent
All patients accepted the proposed treatment and follow-up after an adequate information and written consent.
Rights and permissions
About this article
Cite this article
Sirleo, L., Innocenti, M., Innocenti, M. et al. Post-operative 3D CT feedback improves accuracy and precision in the learning curve of anatomic ACL femoral tunnel placement. Knee Surg Sports Traumatol Arthrosc 26, 468–477 (2018). https://doi.org/10.1007/s00167-017-4614-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-017-4614-7