Abstract
Purpose
To evaluate the effect of the local infiltration of analgesics for pain after total knee arthroplasty in patients treated with femoral and sciatic peripheral nerve blocks. The secondary objective was to detect differences in analgesic consumption as well as blood loss after local infiltration of analgesics.
Methods
Prospective randomized double-blinded study in patients who underwent a TKA for knee osteoarthritis under spinal anesthesia and treated with femoral and sciatic nerve blocks. This study compared 50 patients treated with local infiltration with ropivacaine, epinephrine, ketorolac and clonidine and 50 patients treated with a placebo with the same technique. The visual analogic score was registered postoperatively at 2, 6, 12, 24, 36, 48 and 72 h after surgery. Analgesic consumption was also registered. Both groups of patients were treated with the same surgical and rehabilitation protocols.
Results
A significant difference of one point was found in the visual analogic pain scores 12 h after surgery (0.6 ± 1.5 vs. 1.7 ± 2.3). There were no significant differences in the visual analogic pain scores evaluated at any other time between 2 and 72 h after surgery. No significant differences were found in the required doses of tramadol or morphine in the postoperative period. Postoperative hemoglobin and blood loss were also similar in both groups.
Conclusion
Adding local infiltration of analgesics to peripheral nerve blocks after TKA surgery only provides minimal benefit for pain control. This benefit may be considered as non-clinically relevant. Moreover, the need for additional analgesics was the same in both groups. Therefore, the use of local infiltration of analgesics treatment in TKA surgery cannot be recommended if peripheral nerve blocks are used.
Level of evidence
I.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pain after total knee arthroplasty (TKA) is very frequent and is sometimes severe. Failure to control pain adequately postoperatively impedes physical therapy and may cause anxiety, suboptimal range of motion (ROM), longer hospital stays, an increased incidence of thromboembolic disease and worse postoperative functional outcomes [6, 10, 27].
The classical treatment of postoperative analgesia was achieved with intravenous opioids, but this treatment caused frequent negative side effects like confusion, pruritus, constipation, nausea and vomiting or respiratory depression [27]. For decades, the use of epidural infusions with local anesthetics provided analgesia without the side effects of opioids [2], but other complications like urinary retention, hypotension and spinal hematoma were seen. Over the last decade, the use of peripheral nerve blocks (PNB) [1, 15, 33] and local infiltration of analgesics (LIA) [9, 14, 17, 22, 28, 30] techniques have been more and more frequently used after TKA surgery because of their proven benefit in postoperative analgesia. Their use encourages better collaboration in the rehabilitation process, decreases hospitalization time and makes for better functional TKA outcomes.
Several studies have compared the use of epidural anesthesia with LIA [13], and some of them have shown the greater efficacy of LIA [32]. Other studies have compared the use of PNB with LIA and shown that both techniques are useful in controlling postoperative pain in TKA [1, 33]. However, to our knowledge, no study has assessed the additional benefits of LIA in controlling pain and improving functional outcomes after TKA in patients treated postoperatively with femoral and sciatic PNB.
The main objective of this study was to evaluate pain levels in two groups of patients after TKA and femoral and sciatic PNB: one group with LIA and the other without LIA. The hypothesis was that adding LIA to PNB might lead to better pain control after TKA. The secondary objective was to detect differences in analgesic consumption and blood loss after LIA.
Materials and methods
Prospective, randomized, double-blinded study was carried out that included all patients who underwent a TKA from September 2013 to June 2014. The inclusion criteria were: age between 40 and 85 years and diagnose of knee osteoarthritis. Other diagnoses were not included. The exclusion criteria are listed in Table 1. Those patients who chose to participate provided informed consent.
Randomization was done with a software program, and the list was known by the Pharmacy Department of our institution for the preparation of the liquid to infiltrate, but remained blinded to the surgeon and postoperative evaluators until the end of the study. Patients were randomized into group A (study group) or group B (control group). In group A, the periarticular injections included 246.25 mg of ropivacaine 5 mg/mL (49.25 mL), 0.5 mg of epinephrine 1 mg/mL (0.5 mL), 30 mg of ketorolac 30 mg/mL (1 mL) and 0.08 mg of clonidine 0.1 mg/mL (0.8 mL), and 48.45 mL of saline was added to the medications to make a total of 100 mL. In group B, the periarticular injections included 100 mL of normal saline, which was injected in the same way as for the study group.
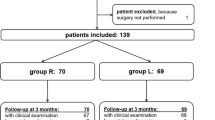
The hospital pharmacy prepared and labeled the medication according to the randomization schedule (which had peel-off labels that were removed sequentially, one by one, as each subject was enrolled) and kept the documentation. The Pharmacy Department delivered the injections to the operating room unmarked so that the surgeons were blinded to group assignment. The surgeons, patients, nurses and physical therapists remained blinded to the assigned group throughout the study. Blinding remained unbroken for all patients. Of the 101 patients enrolled, one patient was excluded from the analysis because he was exitus at 5 months postoperatively (not related to TKA). The final analysis included 100 patients (50 in each group) (Fig. 1).
The sample analyzed consisted of 100 patients (75 women and 25 men) with 72.1 years (SD 6.9) in average. There were 55 right knees and 45 left knees. The mean body mass index (BMI) was 30.6 (SD 4.9). The American Society of Anesthesiology (ASA) score was 1 in 2 % of the cases, 2 in 69 % and 3 in 29 %.
The main associated pathologies requiring chronic medication were hypertension (82 %), dyslipidemia (43 %), diabetes mellitus (29 %) and depression (18 %).
Procedures
All surgeries were done under spinal anesthesia with 10–15 mg of bupivacaine. Intraoperative conscious sedation was not restricted by the protocol.
A medial parapatellar approach was used for all patients. All implants were posterior-stabilized and cemented and included patellar resurfacing. Electrocautery was used for hemostasis. The tourniquet was used in all cases and released after wound closure. A deep drain was placed for drainage and kept in the knee for 24 h. A dose of 10 mg/kg of tranexamic acid was administered intravenously to the patients 15 min before tourniquet release to decrease postoperative bleeding. Postoperative cryotherapy was used in all cases.
In all patients, the surgeon infiltrated the 100 mL of liquid prepared by the hospital pharmacy in the soft tissues around the joint with a syringe and a 0.8 mm gauge needle. The injections were given after component cemented implantation as follows: behind the knee into posterior soft tissues (30 mL), into the medial (25 mL) and lateral (25 mL) gutters (including the femoral periosteum) and into subcutaneous tissue (20 mL) [11]. After joint infiltration, the surgeon proceeded to the suture by planes.
Postoperatively, the anesthesiologist proceeded to PNB guided by ultrasound (femoral and sciatic blocks with a single dose of 40 mL of bupivacaine 0.25 % + adrenaline, making for a total of 100 mg of bupivacaine administered). The PNB were always done at least 1 h after the intra-articular infiltration of the study group to minimize the possibility of toxic doses of anesthetic drugs in plasma.
The parenteral protocol of postoperative analgesia was as follows: paracetamol 1 g/8 h iv. and dexketoprofen 50 mg/8 h iv. If the patient had a pain <3 on the VAS, tramadol 1 mg/kg iv every 6 h was used. If the VAS remained >3 at 30 min after a tramadol dose, a dose of morphine 0.01 mg/kg s.c. was administered.
Early ambulation and enoxaparin 40 mg/24 h s.c. for 4 weeks were the standard prophylaxis against deep venous thrombosis. Cefazoline for 24 h was used as the antibiotic prophylaxis.
During the first 3 days after surgery, a nurse asked for and registered the patients VAS scores from 0 (indicating no pain) to 10 (indicating extreme pain) at 2, 6, 12, 24, 36, 48 and 72 h postoperatively. The use of rescue analgesia (tramadol and morphine) was also registered.
A blood analysis (hemogram, including red cell count and hemoglobin concentration) was requested at 24 and 72 h postoperatively. Three physical therapists measured the passive ROM of the knee joint at 4 days, 2 weeks and 6 months after surgery with the patient in a supine position with a manual 30-cm plastic goniometer 0° to 360° per 1°. The rehabilitation protocol included beginning passive motion of the knee and standing-up 24 h after surgery.
The clinical and functional status of the patient was evaluated with the Knee Society Score (KSS) [4] preoperatively and 6 months after surgery.
The study received approval from the IRB (Parc de Salut Mar 2012/4987).
Statistical analysis
All data collected for this study were entered into an Excel database (Microsoft Office 2003, Redmond, WA) and analyzed using the SPSS 18.0 (SPSS Inc., Chicago, IL) statistical program. A descriptive analysis of the sample was done using rates for categorical variables and the mean and standard deviations for continuous variables. Demographic and clinical characteristics were examined across the two treatment groups to test the randomization. To compare differences between the two treatment groups, either a Chi-square or a Fisher exact test was used for the analysis of categorical variables and the Student’s t test was used for continuous variables. Statistical significance was set at p < 0.05.
Based on a power analysis, a sample size of 48 patients in each group was estimated to detect a 1.5-point difference in the visual analogic scale (VAS) score with a standard deviation of 2.0 points, a p value of 0.05, and a power of 80 % or higher.
Results
The main preoperative hemoglobin was 13.8 g/dL (SD 1.2). The main preoperative ROM was 108.2 (SD 10.9) degrees. The main preoperative KSS was 40.5 (SD 13.4) in the Knee Score and 52.0 (SD 13.9) in the Functional Score.
No significant differences were found between both groups in terms of baseline demographics (age, gender, BMI, ASA score), side of surgery or in preoperative assessment of pain, range of motion (ROM) or KSS.
After analyzing the postoperative VAS pain scores, a significant difference of 1 point was found at 12 h after surgery, being lower in the study group (p = 0.008). There were no significant differences in VAS pain scores evaluated at 2, 6, 24, 36, 48 or 72 h after surgery (Fig. 2). Upon analyzing the VAS scores in the categories of good pain control (VAS ≤ 3) or bad pain control (VAS > 3), no significant differences between the groups in any of the times of evaluation after surgery were observed.
No significant differences between groups were found in the cumulative required doses of tramadol in the postoperative period (Table 2). The number of patients requiring morphine as a rescue medication was also similar in the control group and the study group (23 vs. 18 on the first day, 14 vs. 9 on the second day and 1 vs. 4 on the third day).
The postoperative hemoglobin evaluated at 1 day (mean 11.1 g/dL SD 1.0) and 3 days (mean 10.1 g/dL, SD 1.2) after surgery showed no differences between both groups. The hemoglobin drop (difference between postoperative and preoperative values) was also similar in both groups (n.s.). The blood loss measured in the drainage (mean 190 mL, SD 146 mL) was also similar in both groups. Only three patients (two in the control group and one in the study group) needed an allogenic blood transfusion (n.s.).
No patient fall was registered during the postoperative period in the control or in the study groups. The mean ROM was similar between both groups on the fourth day (group A 75.9° vs. group B 75.0°, n.s.), second week (97.3° vs. 98.7°) and sixth month (109.0° vs. 107.6°) postoperatively.
The postoperative KSS increased very significantly with respect to the preoperative values in both groups but was similar in the study group (Knee 89.9 (SD 12.2) Function 83.1 (SD 18.8)) and in the control group (Knee 91.1 (SD 9.3) Function 82.4 (SD 15.1)).
There were no complications that we could attribute to the injected medications. In the study group, there was a case of stiffness requiring arthroscopic arthrolysis and two acute infections that were successfully treated after debridement, an insert exchange and antibiotics. In the control group, there was a pulmonary embolism, two cases of stiffness requiring arthroscopic arthrolysis and one exitus caused by a brain lymphoma 5 months after surgery (this patient was excluded in the final analysis).
Discussion
The main finding of this study is that adding LIA to PNB after TKA surgery provides only minimal benefit to pain control. In the present study, only the VAS 12 h after surgery was significantly reduced in the LIA group, but the difference (one point in the VAS score) can be considered as non-clinically relevant. Moreover, the need for additional analgesics was the same in both groups. As in previous reports [22], no complications related to these techniques were found in this study.
Spinal anesthesia reduces postoperative morbidity as well as postoperative pain and morphine consumption in comparison with general anesthesia [26]. It was used in all the analyzed patients.
Advances in nerve stimulation and ultrasound technologies have made the PNB more popular over the last decade by achieving good pain control in the immediate postoperative period after TKA reducing the side effects [10]. On the other hand, the use of PNB can have some inconveniences, which includes difficult ambulation, while the nerve block is in effect that can cause the patient to falls. Nevertheless, such a fall is more frequent if a continuous catheter has been used instead of single-shot techniques [19]. The most frequently used PNB in TKA surgery is the femoral block with a single shot which may warrant good analgesia up to 24 h [12]. The use of the sciatic block with the femoral block seems to improve pain control in the posterior aspect of the knee [7] and is also associated with increased patient satisfaction [12]. This combination was used in this study. With the rehabilitation protocol used, that of not trying to stand up for the first 24 h after surgery, no patient fall was registered during the postoperative period.
The use of intraoperative LIA with a combination of medications is very useful in reducing postoperative pain and opioid consumption and provides effective pain relief while permitting early rehabilitation [16, 20, 21, 24, 29, 31]. Its use is effective for the first 6–12 h after surgery. Several combinations have been tried. One of the most successful cocktails combines ropivacaine, ketorolac and epinephrine, with or without clonidine [3, 11, 23]. This mixture should be injected in the periosteum, joint capsule and soft tissues under direct visual control before wound closure. LIA has been proposed for use in addition to PNB [16], but as far as we know, there is a lack of evidence about this combination.
As the medication included in the injection in the study group had 0.5 mg of epinephrine, a reduction effect in the bleeding was to be expected [8, 25]. Nevertheless, the postoperative hemoglobin and the drained blood were similar in both groups and there was no reduction in the transfusion requirements. The reason may be that a low rate of transfusion was observed in this study in comparison with previous studies that evaluated transfusion requirements after LIA which included epinephrine [8]. Therefore, the potential benefit in decreasing the bleeding after surgery has not been proved in this study.
This study was designed considering a multimodal analgesia approach, which is based on the use of multiple techniques and multiple pain modulators at relatively low doses, which affect different steps along the pain pathway to use lower opioid doses [5, 18]. Good pain control has been stated to be necessary for the patient to perform the usual postoperative rehabilitation [5, 10]. In this study, as both groups of patients achieved low VAS scores (no higher than 3, on average, at any time studied after surgery), the final ROM and KSS were similar in both groups. Therefore, we failed to prove a better functional result if LIA is added to PNB in controlling postoperative pain.
This study has some limitations. The first is that using PNB, a fast-track postoperative rehabilitation was not used because of the risk of patient falls. However, beginning to stand up the day following surgery is a widely accepted rehabilitation protocol. The second is that the study was powered to detect VAS differences but the study might have been underpowered to detect differences in other secondary variables like the doses of analgesics or blood loss. A third limitation is that three different anesthesiologists, four surgeons and three physical therapists were involved in the treatment of these patients, but the bias of different people involved was minimized for the randomized assignment of treatment. The last limitation is that only one cocktail of medications in LIA has been tested, but other combinations might be more effective when added to PNB. However, some studies considered the combination used the most useful one when used in LIA [11].
Conclusion
An attempt was made to add the known benefits of LIA to the benefits of PNB, but the effect on the pain perceived by patients after TKA surgery is minimal, with no effect on consumption of analgesics needed. Moreover, no differences in the blood loss were found. Therefore, the addition of treatment with LIA after TKA surgery is not recommended when PNB are used.
References
Affas F, Nygårds EB, Stiller CO, Wretenberg P, Olofsson C (2011) Pain control after total knee arthroplasty: a randomized trial comparing local infiltration anesthesia and continuous femoral block. Acta Orthop 82:441–447
Al-Zahrani T, Doais KS, Aljassir F, Alshaygy I, Albishi W, Terkawi AS (2015) Randomized clinical trial of continuous femoral nerve block combined with sciatic nerve block versus epidural analgesia for unilateral total knee arthroplasty. J Arthroplasty 30:149–154
Andersen KV, Nikolajsen L, Haraldsted V, Odgaard A, Søballe K (2013) Local infiltration analgesia for total knee arthroplasty: should ketorolac be added? Br J Anaesth 111:242–248
Ares O, Castellet E, Maculé F, León V, Montañez E, Freire A, Hinarejos P, Montserrat F, Amillo JR (2013) Translation and validation of ‘the knee society clinical rating system’ into Spanish. Knee Surg Sports Traumatol Arthrosc 21:2618–2624
Baratta JL, Gandhi K, Viscusi ER (2014) Perioperative pain management for total knee arthroplasty. J Surg Orthop Adv 23:22–36
Barrington JW, Dalury DF, Emerson RH Jr, Hawkins RJ, Joshi GP, Stulberg BN (2013) Improving patient outcomes through advanced pain management techniques in total hip and knee arthroplasty. Am J Orthop 42(10 Suppl):S1–S20
Bauer MC, Pogatzki-Zahn EM, Zahn PK (2014) Regional analgesia techniques for total knee replacement. Curr Opin Anaesthesiol 27:501–506
Bhutta MA, Ajwani SH, Shepard GJ, Ryan WG (2015) Reduced blood loss and transfusion rates: additional benefits of local infiltration anaesthesia in knee arthroplasty patients. J Arthroplasty 30:2034–2037
Browne C, Copp S, Reden L, Pulido P, Colwell C Jr (2004) Bupivacaine bolus injection versus placebo for pain management following total knee arthroplasty. J Arthroplast 19:377–380
Carli F, Clemente A, Asenjo JF, Kim DJ, Mistraletti G, Gomarasca M, Morabito A, Tanzer M (2010) Analgesia and functional outcome after total knee arthroplasty: periarticular infiltration versus continuous femoral nerve block. Br J Anaesth 105:185–195
Dalury DF, Lieberman JR, MacDonald SJ (2011) Current and innovative pain management techniques in total knee arthroplasty. J Bone Joint Surg Am 93:1938–1943
Danninger T, Opperer M, Memtsoudis SG (2014) Perioperative pain control after total knee arthroplasty: an evidence based review of the role of peripheral nerve blocks. World J Orthop 5:225–232
DeWeese FT, Akbari Z, Carline E (2001) Pain control after knee arthroplasty: intraarticular versus epidural anesthesia. Clin Orthop Relat Res 392:226–231
Essving P, Axelsson K, Kjellberg J, Wallgren O, Gupta A, Lundin A (2010) Reduced morphine consumption and pain intensity with local infiltration analgesia (LIA) following total knee arthroplasty. Acta Orthop 81:354–360
Ganapathy S (2012) Wound/intra-articular infiltration or peripheral nerve blocks for orthopedic joint surgery: efficacy and safety issues. Curr Opin Anaesthesiol 25:615–620
Gibbs DM, Green TP, Esler CN (2012) The local infiltration of analgesia following total knee replacement: a review of current literature. J Bone Joint Surg Br 94:1154–1159
Gómez-Cardero P, Rodríguez-Merchán EC (2010) Postoperative analgesia in TKA: ropivacaine continuous intraarticular infusion. Clin Orthop Relat Res 468:1242–1247
Horlocker TT (2010) Pain management in total joint arthroplasty: a historical review. Orthopedics 33(9 Suppl):14–19
Johnson RL, Kopp SL, Hebl JR, Erwin PJ, Mantilla CB (2013) Falls and major orthopaedic surgery with peripheral nerve blockade: a systematic review and meta-analysis. Br J Anaesth 110:518–528
Kazak Bengisun Z, AysuSalviz E, Darcin K, Suer H, Ates Y (2010) Intraarticular levobupivacaine or bupivacaine administration decreases pain scores and provides a better recovery after total knee arthroplasty. J Anesth 24:694–699
Kehlet H, Andersen LØ (2011) Local infiltration analgesia in joint replacement: the evidence and recommendations for clinical practice. Acta Anaesthesiol Scand 55:778–784
Keijsers R, van Delft R, van den Bekerom MP, de Vries DC, Brohet RM, Nolte PA (2015) Local infiltration analgesia following total knee arthroplasty: effect on post-operative pain and opioid consumption-a meta-analysis. Knee Surg Sports Traumatol Arthrosc 23:1956–1963
Kelley TC, Adams MJ, Mulliken BD, Dalury DF (2013) Efficacy of multimodal perioperative analgesia protocol with periarticular medication injection in total knee arthroplasty: a randomized, double-blinded study. J Arthroplasty 28:1274–1277
Krenzel BA, Cook C, Martin GN, Vail TP, Attarian DE, Bolognesi MP (2009) Posterior capsular injections of ropivacaine during total knee arthroplasty: a randomized, double-blind, placebo-controlled study. J Arthroplasty 24(6 Suppl):138–143
Leownorasate M, Ruangsillapanu N (2014) Post-op pain and blood loss in total knee arthroplasty: an RCT using periarticular injection with diclofenac-based multimodal drugs. J Med Assoc Thai 97:1332–1337
Macfarlane AJ, Prasad GA, Chan VW, Brull R (2009) Does regional anesthesia improve outcome after total knee arthroplasty? Clin Orthop Relat Res 467:2379–2402
Monllau JC, Hinarejos P, Leal J, Torres-Claramunt R, Puig-Verdié L (2015) Peri-operative management in TKA. In: Bentley G (UK) (ed) European Instructional Lectures: volume 15, 2015, 16th EFFORT Congress, Prague, pp 155–168
Ong JC, Chin PL, Fook-Chong SM, Tang A, Yang KY, Tay BK (2010) Continuous infiltration of local anaesthetic following total knee arthroplasty. J Orthop Surg (Hong Kong) 18:203–207
Reinhardt KR, Duggal S, Umunna BP, Reinhardt GA, Nam D, Alexiades M, Cornell CN (2014) Intraarticular analgesia versus epidural plus femoral nerve block after TKA: a randomized, double-blind trial. Clin Orthop Relat Res 472:1400–1408
Rosen AS, Colwell CW Jr, Pulido PA, Chaffee TL, Copp SN (2010) A randomized controlled trial of intraarticular ropivacaine for pain management immediately following total knee arthroplasty. HSS J 6:155–159
Schotanus MG, Bemelmans YF, van der Kuy PH, Jansen J, Kort NP (2015) No advantage of adrenaline in the local infiltration analgesia mixture during total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-015-3723-4
Spreng UJ, Dahl V, Hjall A, Fagerland MW, Ræder J (2010) High-volume local infiltration analgesia combined with intravenous or local ketorolac + morphine compared with epidural analgesia after total knee arthroplasty. Br J Anaesth 105:675–682
Toftdahl K, Nikolajsen L, Haraldsted V, Madsen F, Tønnesen EK, Søballe K (2007) Comparison of peri and intraarticular analgesia with femoral nerve block after total knee arthroplasty: a randomized clinical trial. Acta Orthop 78:172–179
Acknowledgments
The authors gratefully thank the assistance in the statistical analysis by Sergi Mojal and the assistance in Figure design by Dr. Vito Andriola.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest relative to the funding of the research presented.
Rights and permissions
About this article
Cite this article
Hinarejos, P., Capurro, B., Santiveri, X. et al. Local infiltration analgesia adds no clinical benefit in pain control to peripheral nerve blocks after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 24, 3299–3305 (2016). https://doi.org/10.1007/s00167-016-4187-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-016-4187-x