Abstract
Introduction
Local infiltration analgesia (LIA) is a popular method for decreasing post-operative pain after total knee arthroplasty (TKA). The goal of this meta-analysis is to compare the effect of LIA with placebo on the intensity of post-operative pain and the consumption of opioids.
Methods
A search was performed in the PubMed/MEDLINE, Cochrane, EMBASE and TRIP databases. All (quasi)-randomized controlled trials (RCTs) were included. LIA consists of intra-operative infiltration with at least one analgesic component. Data were pooled using Cochrane software.
Results
Seven placebo-controlled RCTs were included, involving 405 TKAs. On the first post-operative day, LIA provides an average decrease in VAS scores at rest of 12.3 % compared to placebo. Six RCTs studied opioid consumption in patients following TKA. There was a decrease in opioid consumption of 14.8 % compared to placebo 24 h after surgery. This suggests a reduced pain perception due to LIA. On the second post-operative day, the effect on both outcome measures was diminished and no longer significant. Heterogeneity between the studies was 71 % for pain and 39 % for opioid consumption (p = 0.002 and p = 0.0005). No major complications were reported with the use of LIA.
Conclusion
LIA might be able to decrease pain and the use of opioids on the first post-operative day following TKA. However, due to the high level of heterogeneity between the studies, no firm conclusions can be drawn.
Level of evidence
Meta-analysis, Level II.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last few years, several studies have been conducted on local infiltration analgesia (LIA) after total knee arthroplasty (TKA) [3–6, 10–12, 26, 27]. LIA constitutes an additional form of analgesia, in which an analgesic is administered locally into the surgical wound. The injection usually contains a mixture of an anaesthetic drug and a NSAID, to which epinephrine or a corticosteroid can be added [23]. LIA is easy to use, relatively cheap, and many authors conclude that it reduces pain and opioid consumption [1, 18]. Considering the local administration, fewer side effects of medication are expected [28]. In other surgical procedures, it is also a known form of analgesia. A review on the effects of LIA in lumbar spine surgery reported varying results [19]. However, the optimal technique of performing LIA is not yet known. As a result, there is a variation in the mixture of analgesics administered and the anatomical location at which the mixture is infiltrated.
Local infiltration analgesia often consists of a single-shot intra-articular injection. However, there are studies, which perform LIA by an intra-articular catheter as a prolonged means of administration of analgesics. This meta-analysis focuses on the effect of a single-shot injection of analgesics. Published studies to date show varying results on the analgesic effect of LIA. This meta-analysis aims to provide the highest evidence for the efficacy of LIA compared to placebo on post-operative pain and the consumption of opioids. To our knowledge, this is the first meta-analysis on the effect of LIA after TKA.
Materials and methods
Inclusion was limited to the following patient population: men and women over 18 years, with an American Society of Anesthesiologists (ASA) classification I–III, undergoing primary unilateral or bilateral TKA associated with primary or secondary osteoarthritis.
TKA with and without patellar resurfacing are both included. No distinctions are made between different approaches or surgical techniques. Both cemented and uncemented prostheses are included. Revisions and hemi-knee prostheses are excluded, to keep the intervention as uniform as possible.
In this meta-analysis, LIA is compared to placebo. Studies in which LIA is compared with another type of analgesia (e.g. femoral block or spinal anaesthesia) are excluded.
Studies in which LIA was administered to the peri-articular tissue and/or intra-articular space were included, whereas studies in which LIA was infiltrated only in the subcutaneous tissue, as well as studies in which a catheter was used for peri- and post-operative infiltration were excluded.
Currently, there is no uniform and optimal way of performing LIA, so no distinctions are made between the types of anaesthetics or analgesics that are used for performing LIA. However, the mixture should at least contain one analgesic component, such as ropivacaine, bupivacaine or morphine. We did not make distinctions in concentration, volume or combinations of drugs that are used.
To increase the credibility for this meta-analysis, the search of literature was limited only to (quasi)-randomized controlled trials (RCT) (Level I or II evidence). Quasi-randomization is a method of allocating participants to a treatment group which are not strictly random, e.g. date of birth, hospital record number or alteration. No restrictions are made concerning the duration of follow-up.
Outcome measures
Primary outcome measure of this study is post-operative pain 24 and 48 h after surgery both at rest and during rehabilitation, using a 0–100 mm VAS (visual analogue scale). When a 10 point VAS score was used, the results were converted to a 100 point VAS score. When a NRS score was used, it was converted to a VAS score.
Secondary outcome measures were opioid consumption at 24 and 48 h after surgery and complications as well as side effects. For opioid consumption, only studies in which opioids were administered by a PCA pump were included. So, patients have a direct influence on the amount of administered medication, and no external factors could restrict the speed of medication delivery.
Search strategy
The following electronic databases were searched: PubMed/MEDLINE, Cochrane database of randomized trials, EMBASE, TRIP database and Google scholar (period to April 2012). The references of retrieved publications were also manually checked to add studies potentially meeting the inclusion criteria, missed by the electronic search. The following PubMed/MEDLINE search was performed:
-
1.
(((“Anesthesia, local” [mesh] OR “Anesthetics, local” [Mesh] OR “Analgesia” [Mesh] OR “injections, Intra-Articular” [Mesh] OR anesthe*[tiab])) AND (“Arthroplasty, Replacement, Knee” [Mesh] OR “Knee Prosthesis” [Mesh]) AND Randomized Controlled Trial [publication Type])
-
2.
Local infiltration analgesia knee
-
3.
“Arthroplasty, Replacement, Knee” [mesh] AND “injections, Intra-articular” [mesh]
Methods of the review
Trial selection was done by reviewing title and abstract to identify potentially relevant articles for our review. The full manuscript was retrieved when the abstract was potentially relevant. All articles written in English, German or Dutch are included in this study. All identified trials were independently assessed according to the MOOSE guidelines for inclusion using the above-mentioned criteria [25]. The articles were not blinded for affiliation, author and source.
From the included studies, data were extracted for meta-analysis. In case of doubt, the second author was consulted. Disagreements were resolved by a third author. In case of missing or unclear data, authors were contacted by email for additional information.
The relevant data were pooled using Cochrane software (Review Manager (RevMan) version 5.1. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2011). The results of comparable studies were pooled using the fixed effects model or random effects model. In the presence of heterogeneity, a random effects model weights the studies more equally than a fixed effects model.
Statistical analysis
Continuous outcome measures were reported as weighted mean difference (WMD) with a 95 % confidence interval. Significance exists if p < 0.05. Heterogeneity between the different studies was expressed as I 2-index.
Results
Included studies
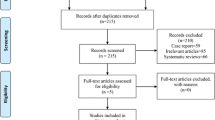
The initial search resulted in 292 articles. After screening of the titles and abstracts, 72 articles met the inclusion criteria. The selection of relevant trials is shown in Fig. 1.
Seven articles were included for meta-analysis [9, 16–18, 20, 22, 30]. The characteristics of the seven trials are summarized in Table 1. The studies were published between 1997 and 2011. Four studies originated from the United States, two from Asia and one from Brazil. A total of 406 TKAs in 374 patients were included in this review. The majority of patients were female (67 %). One study investigated the effect of LIA by placement of a bilateral TKA [22].
Practice of local infiltration analgesia
A lot of variation was present in the LIA group concerning the mixture of infiltrated local analgesia, concentration and volume. In three trials, a solution with ropivacaine was used; in three trials, bupivacaine was used and one study used morphine only. This analgesics were combined with epinephrine (n = 3), ketorolac (n = 2) or morphine (n = 2). The total infiltrated volume ranged from 20 to 152 mL (Table 1).
Pain scores
All seven included RCTs reported pain scores at rest on the first post-operative day (see Fig. 1). Of these seven RCTs, four studies investigated the pain scores at rest on the second post-operative day [9, 16, 18, 22]. In this case, the pain scores closest to 48 h were examined (see Fig. 2). Only two studies investigated pain score during activity [9, 16].
Post-operative VAS scores at rest after 24 h were in favour of LIA [WMD −5.93 (95 % CI −11.62, −0.25)] (Fig. 2). On the first post-operative day, LIA shows an average pain reduction of 6 points on a 100-points VAS scale, which corresponds to an average decrease of 12.3 %. Heterogeneity between the studies was 71 %.
After the second post-operative day, the decrease in VAS score at rest was not significant anymore (n.s.) (Fig. 3).
During activity, no positive effect of LIA could be demonstrated compared to placebo on post-operative VAS scores after 24 h (n.s.) (Fig. 4) and 48 h [WMD −2.42 (−3.72, 8.56)].
Opioid use
Six studies assessed opioid use during the first 24 h after surgery [8, 14, 15, 20, 22, 30], and only two studies also assessed opioid use on the second post-operative day [15, 30]. In four studies, morphine was used [8, 14, 22, 30]; in the study of Han et al. [15], tramadol was used and Krenzel et al. [20] used fentanyl for PCA.
On the first post-operative day, the LIA group used less opioids compared to placebo group [WMD −6.20 (95 % CI −9.71, −2.69)] (Fig. 5). There is a difference in opioid use of 14.8 % between the two groups.
After 48 h, the difference in opioid use no longer exists (n.s.) (Fig. 6).
Complications
No major complications or side effects were reported in the studies. No study reported an increased infection rate in the LIA group.
Discussion
The most important finding of the present study was that the beneficial effect on pain perception only lasts for a short period after surgery. Based on 7 RCTs enrolled in this meta-analysis, including 405 total knee prostheses, we conclude that LIA has a beneficial effect on pain perception at rest compared to placebo up till 24 h after surgery. LIA also seems to lower the consumption of opioids on the first post-operative day.
On the second post-operative day, this beneficial effect was not observed anymore. Two studies that assessed pain scores during mobilization were unable to observe a difference between LIA and placebo [15, 30].
The results of this meta-analysis should be viewed in the light of the limitations of the high heterogeneity defined by the I2 index. Therefore, despite the positive overall effect, we cannot conclude with certainty that LIA in practice actually lowers post-operative pain and opioid consumption. The high level of heterogeneity can be explained by the large diversity in the use of LIA. There was a lot of variation in the composition and dosage of medicinal components. This may indicate that the clinical homogeneity is lower than assumed.
Despite the beneficial effect of LIA, it is questionable to what extent a decrease of 6 points on a 100-point VAS pain scale is clinically relevant. And even more important is the lack of improvement in pain scores during mobilization, as this is the most important clinical outcome.
There is no widely accepted definition of LIA, this resulted in different mixtures of infiltrated analgesics used in the studies: ropivacaine, bupivacaine, morphine and ketorolac are frequently used analgesics, separately or combined. All these different compositions make it difficult to identify the “active” component of LIA. Bianconi et al. [7] and Kerr and Kohan [18] describe a cocktail consisting of ropivacaine, ketorolac and epinephrine. It seems that more and more authors follow this combination, although it is still unclear which component is effective. Similar uncertainties arise with regard to the infiltrated volume, the concentration of the analgesics and the location of infiltration
There are also other potentially effective forms of analgesia after knee surgery. A study by Kristensen et al. [21] reported that LIA and femoral nerve block are similar in the management of post-operative pain after ACL reconstruction. Future research is needed to determine what is most effective. The short duration of the analgesic effect of LIA can largely be explained by the pharmacological duration of action of the infiltrated analgesics [9]. Epinephrine could potentially enhance the other locally applied analgesics because it causes local vasoconstriction and thus a delay in the clearance of these drugs; therefore, it is regularly added to the mixture [1]. This vasoconstriction could also contribute to a decreased wound leakage and haematoma formation.
To improve the reliability of this study, we searched most of the available databases to discover all relevant studies that compare LIA with placebo.
Local infiltration analgesia can be a part of “fast-track arthroplasty surgery”. This method of treatment aims to shorten the hospital stay by optimizing the individual components of health care during the pre- and post-operative processes [17]. Hospital stay can be reduced from 3–5 to 1–2 days [18]. “Fast-track surgery” focuses on fast mobilization. One of the main goals is to reduce post-operative pain in the first days after surgery. If patients experience less pain, they are able to mobilize more quickly, which is an important contribution to the rehabilitation process [16, 24].
Patients are also often hindered in their mobilization by nausea. This is most frequently observed on the first post-operative day [29]. The lower need for opioids on the first post-operative day by the use of LIA could possibly reduce side effects such as nausea, which in turn facilitates early rehabilitation. It is important to look at the entire process of post-operative care to achieve less pain, better results, faster mobilization, shorter hospital stay and higher satisfaction rate amongst patients. Post-operative analgesia is a very important factor, but other aspects such as education, physiotherapy and other pain medications also contribute to a fast recovery.
Andersen et al. [2] investigated the effect of different concentrations of ropivacaine for LIA. No difference in analgesic effect was found. In another study by Anderson, the additional effect of subcutaneous infiltration of the wound area, in addition to the intra-articular infiltration, was examined [3]. Subcutaneous infiltration seemed to have no additional effect when combined with intra-articular infiltration. Although these two studies were not able to demonstrate the influence of volume and location of the infiltration, more research is needed on the different aspects of LIA.
Studies are only included when there was a single, direct intra- or peri-articular infiltration of a local anaesthetic during surgery. Many studies on the effect of LIA use a catheter. After the local infiltration during surgery, a catheter is left behind with the tip located within the joint space. Through this catheter, an additional bolus of analgesics can be administered in the first hours after surgery, varying from 6 to 28 h after surgery. This aims to prolong the analgesic effect. To make the intervention as uniform as possible, it was decided to exclude these studies for meta-analysis. However, the effect of LIA in combination with a catheter is very interesting for future research. When it can achieve pain relief in the first 24 post-operative hours by one bolus injection, it might be possible to prolong the analgesic effect by giving additional boluses through a catheter.
The effect of LIA may also be influenced by the type of anaesthesia administered at surgery. For example, when a spinal block is positioned properly, this often gives an analgesic effect for hours after surgery. This can already reduce the pain score by several points, leaving only a minimal margin for the locally applied analgesics. The study by Han et al. [15] used a combination of epidural and spinal anaesthesia; epidural anaesthesia is along-acting form of analgesia, and this can explain why they did not find a beneficial effect on pain scores when using LIA. When multiple bolus injections are administered, the effect of the spinal anaesthesia will be reduced so that the effect of LIA will be more detectable.
To our knowledge, this study is the first meta-analysis on LIA after TKA. Despite the limitations of this study, this is currently the highest possible level of evidence. The high degree of heterogeneity was inevitable because the optimal way of performing LIA is not known.
Therefore, more research is needed on the separate components of LIA, to find the optimal analgesic cocktail. In order to draw better conclusions about the efficacy of LIA, more uniformity is needed in the use of it. In this way, there will be more homogeneity between studies and more reliable comparisons can be drawn.
Conclusion
Based on this meta-analysis, we conclude that LIA might be able to decrease the post-operative pain and the use of opioids on the first post-operative day following TKA. However, the beneficial effect is very small and is therefore not clinically relevant. There is a high level of heterogeneity between the studies, and more homogenous research is necessary. Until then we recommend, following this meta-analysis, not to use LIA on routine basis.
References
Affas F, Nygards EB, Stiller CO, Wretenberg P, Olofsson C (2011) Pain control after total knee arthroplasty: a randomized trial comparing local infiltration anesthesia and continuous femoral block. Acta Orthop 82(4):441–447
Andersen LO, Gaarn-Larsen L, Kristensen BB, Husted H, Otte KS, Kehlet H (2010) Analgesic efficacy of local anaesthetic wound administration in knee arthroplasty: volume vs concentration. Anaesthesia 65(10):984–990
Andersen LO, Husted H, Kristensen BB, Otte KS, Gaarn-Larsen L, Kehlet H (2010) Analgesic efficacy of subcutaneous local anaesthetic wound infiltration in bilateral knee arthroplasty: a randomised, placebo-controlled, double-blind trial. Acta Anaesthesiol Scand 54(5):543–548
Andersen LO, Husted H, Otte KS, Kristensen BB, Kehlet H (2008) A compression bandage improves local infiltration analgesia in total knee arthroplasty. Acta Orthop 79(6):806–811
Andersen LO, Husted H, Otte KS, Kristensen BB, Kehlet H (2009) High-volume infiltration analgesia in total knee arthroplasty: a randomized, double-blind, placebo-controlled trial. Acta Anaesthesiol Scand 52(10):1331–1335
Andersen LO, Kristensen BB, Husted H, Otte KS, Kehlet H (2008) Local anesthetics after total knee arthroplasty: intraarticular or extraarticular administration? A randomized, double-blind, placebo-controlled study. Acta Orthop 79(6):800–805
Bianconi M, Ferraro L, Traina GC, Zanoli G, Antonelli T, Guberti A et al (2003) Pharmacokinetics and efficacy of ropivacaine continuous wound instillation after joint replacement surgery. Br J Anaesth 91(6):830–835
Browne C, Copp S, Reden L, Pulido P, Colwell C Jr (2004) Bupivacaine bolus injection versus placebo for pain management following total knee arthroplasty. J Arthroplasty 19(3):377–380
Bader AM, Datta S, Flanagan H, Covino BG (1989) Comparison of bupivacaine- and ropivacaine-induced conduction blockade in the isolated rabbit vagus nerve. Anesth Analg 68(6):724–727
Essving P, Axelsson K, Aberg E, Spannar H, Gupta A, Lundin A (2011) Local infiltration analgesia versus intrathecal morphine for postoperative pain management after total knee arthroplasty: a randomized controlled trial. Anesth Analg 113(4):926–933
Essving P, Axelsson K, Kjellberg J, Wallgren O, Gupta A, Lundin A (2009) Reduced hospital stay, morphine consumption, and pain intensity with local infiltration analgesia after unicompartmental knee arthroplasty. Acta Orthop 80(2):213–219
Essving P, Axelsson K, Kjellberg J, Wallgren O, Gupta A, Lundin A (2010) Reduced morphine consumption and pain intensity with local infiltration analgesia (LIA) following total knee arthroplasty. Acta Orthop 81(3):354–360
Fajardo M, Collins J, Landa J, Adler E, Meere P, Di Cesare PE (2011) Effect of a perioperative intra-articular injection on pain control and early range of motion following bilateral TKA. Orthopedics 34(5):354
Garcia JB, Barbosa Neto JO, Vasconcelos JW, Ferro LS, Silva RC (2010) Analgesic efficacy of the intra-articular administration of high doses of morphine in patients undergoing total knee arthroplasty. Rev Bras Anestesiol 60(1):1–12
Han CD, Lee DH, Yang IH (2007) Intra-synovial ropivacaine and morphine for pain relief after total knee arthroplasty: a prospective, randomized, double blind study. Yonsei Med J 48(2):295–300
Huang N, Cunningham F, Laurito CE, Chen C (2001) Can we do better with postoperative pain management? Am J Surg 182(5):440–448
Kehlet H, Soballe K (2010) Fast-track hip and knee replacement—what are the issues? Acta Orthop 81(3):271–272
Kerr DR, Kohan L (2008) Local infiltration analgesia: a technique for the control of acute postoperative pain following knee and hip surgery: a case study of 325 patients. Acta Orthop 79(2):174–183
Kjærgaard M, Møiniche SandK, Olsen S (2012) Wound infiltration with local anesthetics for post-operative pain relief in lumbar spine surgery: a systematic review. Acta Anaesthesiol Scand 56:282–290
Krenzel BA, Cook C, Martin GN, Vail TP, Attarian DE, Bolognesi MP (2009) Posterior capsular injections of ropivacaine during total knee arthroplasty: a randomized, double-blind, placebo-controlled study. J Arthroplasty 24(6 Suppl):138–143
Kristensen PK, Pfeiffer-Jensen M, Storm JO, Thillemann TM (2013) Local infiltration analgesia is comparable to femoral nerve block after anterior cruciate ligament reconstruction with hamstring tendon graft: a randomised controlled trial. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-013-2399-x
Mauerhan DR, Campbell M, Miller JS, Mokris JG, Gregory A, Kiebzak GM (1997) Intra-articular morphine and/or bupivacaine in the management of pain after total knee arthroplasty. J Arthroplasty 12(5):546–552
Raeder JC (2012) Local infiltration analgesia for pain after total knee replacement surgery: a winner or just a strong runner-up? Anesth Analg 113(4):684–686
Reuben SS, Sklar J (2000) Pain management in patients who undergo outpatient arthroscopic surgery of the knee. J Bone Joint Surg Am 82-A(12):1754–1766
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283(15):2008–2012
Toftdahl K, Nikolajsen L, Haraldsted V, Madsen F, Tonnesen EK, Soballe K (2007) Comparison of peri- and intraarticular analgesia with femoral nerve block after total knee arthroplasty: a randomized clinical trial. Acta Orthop 78(2):172–179
Toftdahl K, Nikolajsen L, Soballe K, Tonnesen E (2006) Postoperative analgesia following total knee arthroplasty. Ugeskr Laeger 168(20):1991–1996
Vendittoli PA, Makinen P, Drolet P, Lavigne M, Fallaha M, Guertin MC, Varin F (2006) A multimodal analgesia protocol for total knee arthroplasty. A randomized, controlled study. J Bone Joint Surg Am 88(2):282–289
Wheeler M, Oderda GM, Ashburn MA, Lipman AG (2002) Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain 3(3):159–180
Zhang S, Wang F, Lu ZD, Li YP, Zhang L, Jin QH (2011) Effect of single-injection versus continuous local infiltration analgesia after total knee arthroplasty: a randomized, double-blind, placebo-controlled study. J Int Med Res 39(4):1369–1380
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keijsers, R., van Delft, R., van den Bekerom, M.P.J. et al. Local infiltration analgesia following total knee arthroplasty: effect on post-operative pain and opioid consumption—a meta-analysis. Knee Surg Sports Traumatol Arthrosc 23, 1956–1963 (2015). https://doi.org/10.1007/s00167-013-2788-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-013-2788-1