Abstract
Purpose
This study aimed at evaluating the efficacy and safety of high-dose (> 0.2 L/kg of treated plasma per day) coupled plasma filtration-adsorption (CPFA) in treating patients with septic shock.
Methods
Multicentre, randomised, adaptive trial, performed in 12 Italian intensive care units (ICUs). Patients aged 14 or more, admitted to the ICU with septic shock, or had developed it during the stay were eligible. The final outcome was mortality at discharge from the last hospital at which the patient received care.
Results
Between May 2015, and October 2017, 115 patients were randomised. The first interim analysis revealed a number of early deaths, prompting an unplanned analysis. Last hospital mortality was non-significantly higher in the CPFA (55.6%) than in the control group (46.2%, p = 0.35). The 90-day survival curves diverged in favour of the controls early after randomisation and remained separated afterwards (p = 0.100). An unplanned analysis showed higher mortality in CPFA compared to controls among patients without severe renal failure (p = 0.025); a dose–response relationship was observed between treated plasma volume and mortality (p = 0.010).
Conclusion
The COMPACT-2 trial was stopped due to the possible harmful effect of CPFA in patients with septic shock. The harmful effect, if present, was particularly marked in the early phase of septic shock. Patients not requiring renal replacement therapy seemed most exposed to the possible harm, with evidence of a dose–response effect. Until the mechanisms behind these results are fully understood, the use of CPFA for the treatment of patients with septic shock is not recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The clear lack of benefit, combined with the high costs and the invasiveness of the technique, do not support the use of coupled plasma filtration-adsorption (CPFA) in treating patients with septic shock. |
This study reveals the potentially harmful effects of extracorporeal depurative techniques aimed at removing inflammatory mediators in patients with septic shock and stressed the need for rigorous studies to assess the efficacy and safety of these procedures. |
Introduction
Septic shock is a complex, life-threatening clinical condition characterised by infection-induced circulatory, cellular, and metabolic abnormalities [1]. The most accredited hypothesis refers to an acute dysregulation between pro- and anti-inflammatory mediators. Accordingly, different extracorporeal depurative techniques have been developed to remove inflammatory substances from the bloodstream, thus restoring immune homeostasis. The efficacy of these techniques is still controversial [2].
Coupled plasma filtration and adsorption (CPFA) is one such technology. It uses a cartridge containing a synthetic resin designed to aspecifically adsorb several mediators from plasma. A haemofiltration step additionally purifies small molecules not removed by adsorption [3]. The encouraging results of pre-clinical and clinical studies [4,5,6,7,8] led GiViTI, the Italian intensive care unit (ICU) network, to organise a randomised clinical trial (entitled COMPACT) in 2007 to evaluate the efficacy and safety of CPFA in patients with septic shock. The study was interrupted for futility at the planned interim analysis. Hospital mortality was similar between the control (44/93, 47.3%) and experimental groups (41/91, 45.1%, p = 0.76) [9]. However, a high number of protocol violations was observed in terms of a low volume of plasma treated with CPFA due to the technique’s complexity. A pre-planned subgroup analysis showed that patients receiving a CPFA dose of > 0.18 L/kg/day in the first 3–5 days had lower mortality than controls (odds ratio 0.36, 95%CI 0.13–0.99) [9]. This result generated the hypothesis that CPFA might be effective when treating high volumes of plasma. In 2010, a new machine allowing regional anticoagulation with citrate became available for CPFA, making it easier to reach a much higher treated plasma volume compared to the previous system based on systemic anticoagulation with heparin [10, 11]. Several ICUs started to use citrate-based CPFA in their clinical practice, despite the lack of satisfactory clinical evidence. Hence, GiViTI launched the second COMPACT study (COMPACT-2) in 2015, with the aim to evaluate the efficacy and safety of high-dose CPFA in the treatment of patients with septic shock.
Materials and methods
Trial design
This was a multicentre, randomised, controlled, adaptive trial performed in 12 Italian ICUs. The adaptive design envisaged two study gates with interim analyses: the first to assess the feasibility of high-dose CPFA using citrate-based anticoagulation (after the first 50 treated patients) and the second to evaluate its ability to accelerate recovery from septic shock (after the first 166 randomised patients). Only if the interim analyses were positive would the study continue until achieving the target size (350 patients) to evaluate hospital mortality.
The Ethics Committees of all the participating centres approved the protocol (accessible at https://giviti.marionegri.it/attachments/Projects/COMPACT-2/COMPACT-2-English_protocol_PDFA.pdf). The study was performed following the principles of Good Clinical Practice and the 1964 Helsinki Declaration and its later amendments.
Patients and treatments
Eligible patients were aged 14 or more, admitted to the ICU with septic shock, or had developed septic shock during ICU stay. Septic shock was defined according to the modified 2012 Surviving Sepsis Campaign criteria [12] (Supplementary material). Exclusion criteria were pregnancy, presence of relative or absolute contraindications to extracorporeal depurative techniques, admission from another ICU where the patient had spent more than 24 h, estimated life expectancy of less than 90 days based on clinical judgement, and absence of informed consent.
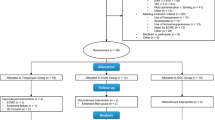
Eligible patients were randomly assigned in a 1:1 ratio to have CPFA added to the standard care or not. We used a blocked randomisation schedule (randomly permuting blocks of four and six), stratified by centre and the presence of septic shock at admission. Investigators collected clinical information (Supplementary material) through an electronic case report form developed by GiViTI [13], which also randomly allocated the patients. To guarantee concealment, the allocation was revealed only after registration of the baseline information.
Patients randomised to the experimental arm were to be treated according to the ICU’s current practice, with the addition of high-dose CPFA (more than 0.2 L/kg of treated plasma per day). The treatment was to be initiated as early as possible, but no later than 12 h after diagnosis of septic shock. It was to last at least 10 h per day and could be followed by continuous renal replacement therapy (RRT) in renal failure patients. Treatment was to be discontinued after three consecutive days if the patient was no longer in shock. The use of high-flow continuous venovenous haemofiltration was not permitted for either study arm.
The clinical follow-up started on randomisation and ended at the discharge from the ICU. During ICU stay, the daily SOFA score (Sequential Organ Failure Assessment) [14] and other parameters to assess the various organ function were recorded. The vital status was registered at ICU discharge, hospital discharge and 90 days after randomisation.
At the first study gate, the primary endpoint was the percentage of patients reaching the plasma treatment target, with a value below 90% as the stopping rule. At the second study gate, the primary endpoint was the number of shock-free days in the first 15 days after randomisation, with a difference of fewer than 2.5 days (in favour of CPFA) as the stopping rule. A sample of 166 patients (83 per arm) was needed to have 80% power to detect said difference (from 8.2 seen in the COMPACT-study controls to a hypothesised 10.7 in CPFA, with a SD of 5.7), with a type-one error of 5%, using a two-tailed t test.
The final primary endpoint was last hospital mortality, which means that in patients transferred between hospitals, mortality was assessed at discharge from the last hospital at which they had received care [9]. The protocol required a total of 350 patients to have 80% power to detect improvement achieved in the subgroup analysis of the COMPACT study: from 47 to 32% hospital mortality, with a two-tailed, type-I error of 5%.
The secondary endpoints were 90-day mortality and ICU-free days during the first 30 days after randomisation. A subgroup analysis of the primary endpoint was planned for patients starting CPFA within or later than 6 h of randomisation. The interim analysis at the second study gate could call for early termination either for efficacy, according to the Haybittle–Peto criterion for multiple comparisons, or futility, in the case of < 10% conditional power of achieving a positive result at the end of the study, based on the primary hypothesis used for the final sample size calculation.
Statistical analyses
Hospital mortality was analysed using Fisher’s exact test. The effect size was expressed in terms of absolute risk difference with its 95% confidence interval (95%CI).
At the external data and safety monitoring committee (EDSMC) request, we performed an unplanned analysis on 3-day mortality, using Fisher’s exact test. Mortality within 90 days of randomisation was assessed using Kaplan–Meier curves with any difference investigated through the logrank and Peto tests. The number of shock-free days in the first 15 days after randomisation was compared using the Wilcoxon rank-sum test.
We also performed unplanned analyses. The first to exclude any centre effect on the results, using the Breslow-Day test for homogeneity of the odds ratios. This analysis was restricted to the four units that recruited at least ten patients each, the remaining patients being merged into a fifth group. A second analysis evaluated the possible presence of a differential effect in patients with or without severe renal failure at randomisation. This is because CPFA exposes patients without renal failure to an extracorporeal treatment that doubles a preserved kidney function, which could be detrimental. We assessed this hypothesis through logistic regression on hospital mortality, the study’s primary outcome, testing the interaction between the presence of severe renal failure and treatment arm. The 90-day Kaplan–Meier survival curves were plotted, stratifying by the presence of severe renal failure at randomisation and using logrank and Peto tests to assess the difference between the curves within strata. Finally, since this analysis revealed that CPFA could have a harmful effect in patients without severe renal failure, we performed a confirmatory analysis by assessing the presence of a dose–response relationship between treated plasma volume and mortality, according to the presence of severe renal failure. We developed a Cox model on patients randomised to CPFA, taking volume as a time-varying covariate and forcing it to interact with the presence of severe renal failure. These analyses were all performed according to the intention-to-treat approach.
Since the analyses suggested that CPFA could have a harmful effect, we asked a team of independent physicians to review the medical records of recruited patients to reappraise the eligibility criteria and highlight any details that might help reconstruct the possible cause of death of deceased patients. Different sensitivity analyses were performed as a result of this review.
Results
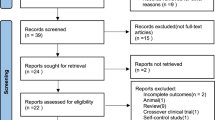
At the first study gate, 81% of patients (95%CI: 69–95%) successfully reached the target volume of treated plasma, which is compatible with a 90% success rate and demonstrates the technique’s feasibility using regional anticoagulation with citrate. This analysis was performed on 113 patients. However, the analysis revealed that some patients died very early in the experimental arm. Such finding prompted the EDSMC to request an unplanned interim analysis, further assessing 3-day mortality. These analyses (reported in the Supplementary material) did raise concerns about the possible harmful effect of CPFA in eligible patients. For this reason, the COMPACT-2 study was prematurely terminated on 23/10/2017.
Between the performance of these analyses and the termination of the study, two additional patients were randomised, giving a total of 115 for the final analyses presented here (Fig. 1). In different centres, randomisation blocks were not closed, and, by chance, this resulted in an imbalance between arms (63 patients in the CPFA, 52 in the control arm). Table 1 shows the patients characteristics (further details in Supplementary material). No serious adverse events were reported.
The mean time to start CPFA after septic shock onset was 8.7 h (SD 3.3); 16 (26.2%) patients started within 6 h, while two patients died before starting any treatment. Three control group patients were treated with CPFA in violation of the protocol; two died at 6 and 8 days post-randomisation, the third was discharged alive from the hospital 65 days after randomisation.
The analysis planned for the second study gate (originally scheduled at 166 enrolled patients) revealed a difference of 2.2 shock-free days in the first 15 days after randomisation in favour of the control group (8.2, versus 6 in CPFA, p = 0.016), well within the stopping rule at that stage for lack of activity.
Last hospital mortality (the primary outcome of the study) was not significantly higher in the CPFA (55.6%) compared to the control group (46.2%, p = 0.35). The conditional power needed to demonstrate that last hospital mortality in the CPFA arm was lower than in controls under the protocol hypothesis was 13%, slightly greater than 10% envisaged in the protocol for early termination due to futility. However, the threshold provided in the protocol was to be calculated at a later stage (166 patients). Three-day mortality, whose assessment was requested by EDSMC, was significantly different between the two groups (30.2% in CPFA, 13.5% in controls, p = 0.044, Table 2).
The 90-day survival curves (Fig. 2) diverged in favour of the controls early after randomisation and remained separated afterwards (logrank test, p = 0.0996; Peto test, p = 0.0418; the latter attributes a higher weight to early events). The controls spent more days outside the ICU during the first 30 days after randomisation (9.3 vs. 6.6 in CPFA, p = 0.026).
There was no difference in the a priori determined subgroup analysis: hospital mortality was comparable between patients starting CPFA within 6 h of septic shock onset (8/16, 50%) and those starting later (25/45, 55.6%). The OR did not differ between centres (Breslow-Day test, 0.7513, p = 0.945), excluding a possible “centre effect”.
The survival analysis showed higher mortality in CPFA than controls, but the difference was statistically significant only among patients without severe renal failure (Fig. 3). The interaction term between the randomisation arm and the presence of severe renal failure was not significant (p = 0.177).
The Cox model performed on patients randomised to CPFA, revealed that the volume of CPFA, considered as time-varying covariate, was positively and significantly associated with mortality (p = 0.010). Conversely, both the presence of severe renal failure and its interaction with the volume of CPFA were not statistically significant (Supplementary material). Since the latter two variables were forced into the model, we have the hazard ratio of CPFA volume in the presence and absence of severe renal failure, respectively, 4.17 (0.47–36.92) and 3.55 (95%CI 1.35–9.29).
The review of the clinical records revealed that 8 cases (7.2% of the 111 patients for whom this assessment was possible) had inappropriate antibiotic therapy. Eligibility was questioned in 31 patients (10 patients were considered terminally ill at the time of randomisation, 13 were diagnosed as not having septic shock, 8 had septic shock onset over twelve hours before randomisation). The sensitivity analysis performed by excluding patients considered non-eligible for the study yielded very similar results to the main analysis (Supplementary material).
Discussion
The COMPACT-2 study was designed to test the hypothesis—emerging from the previous trial—that high-dose CPFA reduces hospital mortality in patients with septic shock. Instead, the study was terminated earlier on suspicion that CPFA might have caused excess mortality, particularly in the first phase of treatment. The primary study analysis showed a non-significant but clinically relevant absolute increase in overall hospital and 90-day mortality in the experimental arm. Patients in the control group spent significantly less (almost 3) days outside the ICU in the first 30 days after randomisation, which corroborates the hypothesis that CPFA provides no benefit in this context. An unplanned analysis showed more than twofold 3-day mortality in the CPFA compared to the control group, further suggesting that CPFA could be harmful. Although these results cannot be considered conclusive, they were sufficiently suggestive to terminate the study prematurely to avoid exposing patients to unjustified risk.
It is always painful to stop a randomised trial for futility or, worse, for potential harm. What is the explanation for such unexpected findings? First, CPFA may cause harm to patients with septic shock. In the first COMPACT study, there were no hints of such effect, but a considerable percentage of patients randomised to CPFA (48.4%) did not reach a substantial treated plasma volume. It can be speculated that the high rate of protocol violations prevented the true harmful effect of CPFA from being observed. The new technique of performing the treatment with regional anticoagulation with citrate, enabling most patients to reach the target treated plasma volume, appears to have allowed the effect to be seen. The host response against infectious pathogens is modulated through pro- and anti-inflammatory responses [15]. The amount and timing of release of different mediators, their relatively short half-lives, their limited range of action, their considerable redundancy and pleiomorphisms and the underexpression or overexpression of their receptors make the process extremely complex [16, 17]. The strategy beyond CPFA, as well as most of the other extracorporeal depurative techniques, of simultaneously removing several inflammatory mediators may do more harm than good.
A second hypothesis is that CPFA may have caused harm to a subset of patients. Although unplanned, the subgroup analysis based on the presence of renal failure is suggestive in this respect. We have no evidence of a differential effect of CPFA with and without severe renal failure (the interaction between the treatment and renal failure was not statistically significant), but at least we demonstrated its detrimental effect in the latter subgroup. It is tempting to think that it could be harmful to subject a patient with septic shock but good renal function to an RRT (even independently of the presence of the CPFA cartridge). Doubling the depurative function could remove important substances at an excessive rate. The prime suspect is antibiotics, as any delay in receiving appropriate antibiotic therapy in severe sepsis or septic shock patients is associated with excess mortality [18,19,20]. Although the qualitative review of the medical records found that the correct therapy was given to 92.8% of patients, we cannot rule out that increasing antibiotic clearance by adding the effect of at least 10 h’ RRT to a well-functioning kidney could have caused treatment underdosing. Moreover, we calculated that CPFA removes 50% more antibiotics than does standard continuous RRT (Supplementary material), increasing the possibility of undertreatment. In this respect, a significant dose–response effect of treated plasma on mortality was demonstrated in patients without severe renal failure. This is particularly worrisome, as it reinforces the idea that the harmful effect was not the result of confounding.
Finally, the review of the clinical records revealed that several patients were randomised despite being ineligible. The sensitivity analysis performed by excluding these patients yielded very similar results to the main analysis, thus rejecting the hypothesis that CPFA was contraindicated in these cases.
Study limitations
Our study has weaknesses. As most trials prematurely stopped, the number of patients is lower than expected and does not allow to address all the questions raised by the interim analysis. This limitation is particularly disappointing when the results are contrary to what was anticipated, as in this case. In such circumstances, it would be essential to investigate the possible reasons for the findings with enough data. Contrariwise, when the interim analysis shows potential harm to patients, it is an ethical imperative to stop the trial. We performed some unplanned analyses to try to explain the results. However, by their nature, unplanned analyses are to be considered with caution and can at best confirm that a hypothesis is worth investigating. According to the literature [21,22,23], we did not consider appropriate to adjust for multiple comparisons. The unplanned analyses were indeed driven by specific hypotheses, and a type II error (which would have led to dismiss the hypothesis as worth investigating) would have had worst consequence than a type I error.
Finally, the lack of cytokine measurement on recruited patients, due to budget constraints, has limited our hypothesis investigation capacity and represents another study weaknesses.
Conclusions
The COMPACT-2 trial was prematurely stopped due to the possible harmful effect of CPFA in patients with septic shock. Although not conclusive, the performed analyses suggest the following: (1) the harmful effect, if present, is particularly marked in the early phase of treatment or of the septic shock condition; (2) patients not requiring RRT seem most exposed to possible excess CPFA-related mortality; (3) there is evidence of a dose–response effect, which could be related to the CPFA cartridge, the RRT, or both.
Until full understanding of the mechanisms behind the negative results has been further investigated, it is not recommended to use CPFA for the treatment of patients with septic shock.
Availability of data and material
All data generated or analysed during this study are included in this published article and its supplementary information files.
Code availability
Not applicable.
References
Singer M, Deutschman CS, Seymour CW et al (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315:801–810. https://doi.org/10.1001/jama.2016.0287
Rhodes A, Evans LE, Alhazzani W et al (2017) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 45:486–552. https://doi.org/10.1097/CCM.0000000000002255
Franchi M, Giacalone M, Traupe I et al (2016) Coupled plasma filtration adsorption improves hemodynamics in septic shock. J Crit Care 33:100–105. https://doi.org/10.1016/j.jcrc.2016.02.005
Tetta C, Gianotti L, Cavaillon JM et al (2000) Coupled plasma filtration-adsorption in a rabbit model of endotoxic shock. Crit Care Med 28:1526–1533. https://doi.org/10.1097/00003246-200005000-00045
Formica M, Olivieri C, Livigni S et al (2003) Hemodynamic response to coupled plasmafiltration-adsorption in human septic shock. Intensive Care Med 29:703–708. https://doi.org/10.1007/s00134-003-1724-0
Ronco C, Brendolan A, Lonnemann G et al (2002) A pilot study of coupled plasma filtration with adsorption in septic shock. Crit Care Med 30:1250–1255. https://doi.org/10.1097/00003246-200206000-00015
Hassan J, Cader RA, Kong NC et al (2013) Coupled Plasma Filtration Adsorption (CPFA) plus Continuous Veno-Venous Haemofiltration (CVVH) versus CVVH alone as an adjunctive therapy in the treatment of sepsis. EXCLI J 12:681–692
Hu D, Sun S, Zhu B et al (2012) Effects of coupled plasma filtration adsorption on septic patients with multiple organ dysfunction syndrome. Ren Fail 34:834–839. https://doi.org/10.3109/0886022X.2012.684553
Livigni S, Bertolini G, Rossi C et al (2014) Efficacy of coupled plasma filtration adsorption (CPFA) in patients with septic shock: a multicenter randomised controlled clinical trial. BMJ Open 4:e003536. https://doi.org/10.1136/bmjopen-2013-003536
Mariano F, Tetta C, Stella M et al (2004) Regional citrate anticoagulation in critically ill patients treated with plasma filtration and adsorption. Blood Purif 22:313–319. https://doi.org/10.1159/000078788
Zhang Z, Hongying N (2012) Efficacy and safety of regional citrate anticoagulation in critically ill patients undergoing continuous renal replacement therapy. Intensive Care Med 38:20–28. https://doi.org/10.1007/s00134-011-2438-3
Dellinger RP, Levy MM, Rhodes A et al (2013) Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock, 2012. Intensive Care Med 39:165–228. https://doi.org/10.1007/s00134-012-2769-8
Finazzi S, Paci G, Antiga L et al (2020) PROSAFE: a European endeavor to improve quality of critical care medicine in seven countries. Minerva Anestesiol. https://doi.org/10.23736/S0375-9393.20.14112-9
Vincent JL, de Mendonca A, Cantraine F et al (1998) Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 26:1793–1800
Tamayo E, Fernandez A, Almansa R et al (2011) Pro- and anti-inflammatory responses are regulated simultaneously from the first moments of septic shock. Eur Cytokine Netw 22:82–87. https://doi.org/10.1684/ecn.2011.0281
Kellum JA, Kong L, Fink MP et al (2007) Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med 167:1655–1663. https://doi.org/10.1001/archinte.167.15.1655
Angus DC, van der Poll T (2013) Severe sepsis and septic shock. N Engl J Med 369:840–851. https://doi.org/10.1056/NEJMra1208623
Andersson M, Östholm-Balkhed Å, Fredrikson M et al (2019) Delay of appropriate antibiotic treatment is associated with high mortality in patients with community-onset sepsis in a Swedish setting. Eur J Clin Microbiol Infect Dis 38:1223–1234. https://doi.org/10.1007/s10096-019-03529-8
Seymour CW, Kahn JM, Martin-Gill C et al (2017) Delays from first medical contact to antibiotic administration for sepsis. Crit Care Med 45:759. https://doi.org/10.1097/CCM.0000000000002264
Weiss SL, Fitzgerald JC, Balamuth F et al (2014) Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med 42:2409–2417. https://doi.org/10.1097/CCM.0000000000000509
Perneger TV (1998) What’s wrong with Bonferroni adjustments. BMJ 316:1236–1238
Feise RJ (2002) Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol 2:8. https://doi.org/10.1186/1471-2288-2-8
Rothman KJ (1990) No adjustments are needed for multiple comparisons. Epidemiology 1:43–46
Acknowledgements
The authors thank Joanne Fleming for her help with the English editing of the text. The members of the GiViTI involved in the study are as follows: Valeria Bonato (Department of Intensive Care, Azienda Ospedaliera SS. Antonio e Biagio e Cesare Arrigo, Alessandria, Italy); Italo Calamai (Anesthesia and Intensive Care Unit AUSL Toscana Centro, San Giuseppe Hospital, Empoli, Firenze, Italy); Gilberto Fiore (Department of Anesthesia and Intensive Care, Hospital of Santa Croce di Moncalieri, Torino, Italy); Valentina Gori (Anestesia e Rianimazione, Ospedale SS. Cosma e Damiano, AUSL Toscana Centro, Pescia, Pistoia, Italy); Ugo Lefons (Anestesia Rianimazione, Ospedale di Campostaggia Alta Val d'Elsa, Poggibonsi, Siena, Italy); Sergio Livigni (SC Anestesia Rianimazione Ospedale San Giovanni Bosco, ASL Città Di Torino, Torino, Italy); Manlio Cosimo Claudio Meca (Dipartimento Grandi Traumi, Unità Operativa di Anestesia e Rianimazione, Ospedale Maurizio Bufalini di Cesena, Italy); Stefano Meinardi (SC Anestesia Rianimazione, Ospedale Maggiore di Chieri, Chieri, Torino, Italy); Giuseppe Nattino (Intensive Care Unit, Azienda Socio Sanitaria Territoriale di Lecco, Lecco, Italy); Luisa Tedeschi (Dipartimento di Anestesia e Rianimazione, Citta’ della Salute e della Scienza di Torino, Ospedale CTO, Torino, Italy); Marina Terzitta (Department of Anesthesia and Intensive Care, Morgagni-Pierantoni Hospital, Forlì, Italy); Francesco Zuccaro (Dipartimento di Anestesia e Rianimazione, Ospedale Madonna delle Grazie, Azienda Sanitaria Locale di Matera, Italy).
Funding
Bellco, the CPFA patent holder, gave an unconditional research grant for the study. They had no role in study design, data collection, data analysis, data interpretation, or writing of the report. GiViTI—Istituto di Ricerche Farmacologiche Mario Negri IRCCS—has full ownership of the data and the dissemination policy of the results. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit.
Author information
Authors and Affiliations
Consortia
Contributions
The authors substantially contributed to the paper as follows: conception and design (EG, SL, FF, MP, GB), analysis (EG, SF, CR, GB) and interpretation (all authors) of data, drafting the article (EG, GB) or critical revision (all authors). All authors approved the final version of the manuscript. EG, SF, CR, and GB had full access to all study data and take responsibility for its integrity and the accuracy of data analysis.
Corresponding author
Ethics declarations
Conflicts of interest
BV reports grants from Pfizer, Nordic Pharma, MSD Italia, Angelini, Cepheid, Becton Dickinson, Biomerieux, Gilead, outside the submitted work. CO reports personal fees from Intersurgical SpA, outside the submitted work. All other authors declare no competing interests.
Ethical approval
The COMPACT-2 study was approved by the Ethical Committee of the Coordinating Center of the project, Ospedale San Giovanni Bosco of Turin, and subsequently by all participating centers’ Ethics Committees.
Consent to participate
Written informed consent was obtained according to Italian legislation.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The members of the GiViTI are mentioned in the Acknowledgments section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Garbero, E., Livigni, S., Ferrari, F. et al. High dose coupled plasma filtration and adsorption in septic shock patients. Results of the COMPACT-2: a multicentre, adaptive, randomised clinical trial. Intensive Care Med 47, 1303–1311 (2021). https://doi.org/10.1007/s00134-021-06501-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-021-06501-3