Abstract
Purpose
Post-resuscitation guidelines recommend a multimodal algorithm for outcome prediction after cardiac arrest (CA). We aimed at evaluating the prevalence of indeterminate prognosis after application of this algorithm and providing a strategy for improving prognostication in this population.
Methods
We examined a prospective cohort of comatose CA patients (n = 485) in whom the ERC/ESICM algorithm was applied. In patients with an indeterminate outcome, prognostication was investigated using standardized EEG classification (benign, malignant, highly malignant) and serum neuron-specific enolase (NSE). Neurological recovery at 3 months was dichotomized as good (Cerebral Performance Categories [CPC] 1–2) vs. poor (CPC 3–5).
Results
Using the ERC/ESICM algorithm, 155 (32%) patients were prognosticated with poor outcome; all died at 3 months. Among the remaining 330 (68%) patients with an indeterminate outcome, the majority (212/330; 64%) showed good recovery. In this patient subgroup, absence of a highly malignant EEG by day 3 had 99.5 [97.4–99.9] % sensitivity for good recovery, which was superior to NSE < 33 μg/L (84.9 [79.3–89.4] % when used alone; 84.4 [78.8–89] % when combined with EEG, both p < 0.001). Highly malignant EEG had equal specificity (99.5 [97.4–99.9] %) but higher sensitivity than NSE for poor recovery. Further analysis of the discriminative power of outcome predictors revealed limited value of NSE over EEG.
Conclusions
In the majority of comatose CA patients, the outcome remains indeterminate after application of ERC/ESICM prognostication algorithm. Standardized EEG background analysis enables accurate prediction of both good and poor recovery, thereby greatly reducing uncertainty about coma prognostication in this patient population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In more than two-thirds of comatose cardiac arrest patients, the prognosis remains indeterminate after application of the ERC/ESICM prognostication algorithm. In these patients, standardized EEG analysis allows accurate prediction of good and poor recovery, thereby reducing early prognostic uncertainty. |
Introduction
Hypoxic-ischemic brain injury following cardiac arrest (CA) is a leading cause of intensive care unit (ICU) admission and acute neurological damage [1,2,3]. In comatose resuscitated CA patients, the majority of deaths are due to neurological causes [4], mostly because of withdrawal of life-sustaining therapies (WLST) following the prognostication of poor neurological outcome [5,6,7]. An accurate prognostication is therefore essential to avoid an inappropriate WLST in these patients. The 2015 guidelines on post-resuscitation care co-issued by the European Resuscitation Council (ERC) and European Society of Intensive Care Medicine (ESICM) provide a stepwise multimodal approach to support clinicians in the prediction of neurological outcome starting from 72 h after CA [8, 9]. This approach includes predictors based on clinical examination, electrophysiology, biomarkers and neuroimaging. Its main objective is to identify patients in whom, based on the concordance of several prognostic tools, poor neurological outcome is highly likely, so that the risk of a falsely pessimistic prediction is minimized [10,11,12]. However, the prognosis remains indeterminate in the remaining patients [13]. The ERC/ESICM guidelines recommend a strategy of “observe and re-evaluate” in patients with an indeterminate outcome; however, they do not provide specific prognostic guidance in this population. Because part of these patients are expected to achieve good neurological recovery, accurate prediction of outcome is particularly important [14, 15] and helps guiding the intensity of care, e.g. in conditions of delayed awakening [16,17,18,19,20] or multiple organ dysfunction [21,22,23]. New strategies are desirable to reduce prognostic uncertainty in those not identified as likely poor outcome by the ERC/ESICM algorithm, especially regarding the prediction of good neurological recovery.
This is the first study that aimed at quantifying the rate of patients remaining with an initial indeterminate outcome after applying the ERC/ESICM prognostication algorithm. We hypothesized a high rate of indeterminate outcome and further examined whether specific electroencephalogram (EEG) patterns, based on a standardized analysis, and serum neuron-specific enolase (NSE) levels, can be used to reduce prognostic uncertainty in this patient population.
Methods
Study design
A single-center prospective observational cohort of comatose CA patients admitted to the Department of Intensive Care Medicine, Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne, Switzerland, was analyzed. All consecutive comatose adult patients resuscitated from CA who were admitted to our medical-surgical ICU from December 2009 to March 2019, for whom data were available for N20 response on somatosensory-evoked potentials (SSEP), complete pupillary and corneal reflexes evaluation, electroencephalogram (EEG) and neuron-specific enolase (NSE) determination, were included in the analysis. Patients in whom brain death occurred within 24 h were excluded from the registry. Approval was obtained from the Ethical Research Committee of the University of Lausanne (CER_VD 23.05), and waiver of consent was allowed since all examinations were part of standard patient care. Reporting of the study conforms to the STARD statement for the report of observational prognostic studies.
Patient management
All patients were managed according to a standardized protocol of post-resuscitation care based on international guidelines [8]. Targeted temperature management was applied to all patients (target temperature either 33 °C or 36 °C) and maintained for 24 h by means of surface cooling devices. A standardized sedation–analgesia protocol was applied (midazolam 0.1–0, 15 mg/kg/h and/or propofol iv. 2–4 mg/kg/h, plus fentanyl 1–1.5 μg/kg/h). Neuromuscular blockade was provided in case of shivering (rocuronium i.v. bolus, 0.6 mg/kg). After the first 24 h, sedatives were weaned when normothermia was achieved, unless any medical indication for maintaining sedation existed (e.g. respiratory and/or cardio-circulatory failure).
Prognostication algorithm
A multimodal approach was applied in all patients for prognostication purposes, according to a written protocol. Clinical examination—including Glasgow Coma Scale (GCS), pupillary and corneal reflexes evaluation—was performed daily by a certified neurologist, between 48 and 72 h from ICU admission. SSEP examination was performed at 24–48 h, without continuous sedation or under sedation weaning, and interpreted for presence or bilateral absence of median nerve N20 response by certified neurophysiologists. Video electroencephalography (video EEG, Viasys Neurocare, Madison, WI using a standard 10–20 electrode system) was performed continuously or twice, at 12–36 and 36–72 h, and examined by certified neurophysiologists for its background (continuity and reactivity, scored according to current American Clinical Neurophysiology Society terminology [24], prospectively since 2013, and retrospectively for the previous years, both blinded to outcome), and the presence of epileptiform patterns. Serum was sampled at ≈24 and ≈48 h for blood NSE concentrations, by means of an automated immunofluorescent assay (Thermo Scientific Brahms NSE Kryptor Immunoassay). The presence or absence of clinically evident myoclonus during the first 72 h was also prospectively recorded.
Decisions of withdrawal of life-sustaining therapy
Decision of WLST was considered when multimodal neurologic assessment was indicative of a poor outcome. This was deemed as highly likely when at least two of the following were present upon return to normothermia and off sedation (72 h after CA): absent brainstem reflexes (both pupillary and corneal), bilaterally absent N20 cortical response to SSEP, peak serum NSE levels > 75 μg/L, EEG with unreactive background [10, 25], and/or abundant epileptiform discharges. The presence or absence of these criteria was assessed in a multidisciplinary evaluation performed by intensivists and neurologists.
Data collection and processing
Demographic data and clinical variables were prospectively recorded for each patient according to the Utstein style [26]. For each patient, data about time from CA to awakening, neurological outcome and duration of sedation were collected. We calculated the time to awakening as the time from CA to the first evidence of a motor score of 6 on the Glasgow Coma Scale, as previously outlined [18].
Early prognostic categorization
For the purpose of this analysis, patients were categorized according to the ERC/ESICM 2015 guidelines stepwise approach [13], into two main subgroups: poor predicted outcome and indeterminate outcome.
For prediction of poor neurological outcome according to the ERC/ESICM guidelines, first-line criteria were a bilaterally absent N20 SSEP wave (unilateral cortical SSEP were categorized as present) and/or absence of both pupillary and corneal reflexes. Second-line criteria included at least two of the following: status myoclonus ≤ 48 h after ROSC, peak serum NSE levels > 75 μg/L, unreactive EEG with burst suppression and/or status epilepticus after rewarming, diffuse anoxic injury on brain CT/MRI. The NSE threshold of 75 μg/L was selected according to previous data from our group as the value with the highest specificity for poor outcome, and is the threshold clinically considered for robust poor outcome prediction in our center [10]. Brain imaging (CT and MRI) was not performed routinely for prognostication purposes.
Patients who did not fulfill either of the above criteria were categorized as having an indeterminate outcome.
EEG and NSE
To investigate if neurological prognosis could be further improved in patients with indeterminate outcome, we assessed the prognostic accuracy of EEG and serum NSE, as follows:
EEG EEG findings were categorized according to the presence or absence of background reactivity (activity more than or equal to 10 µV and reproducible change in amplitude or frequency upon stimulation, excluding stimulus-induced rhythmic, periodic or ictal discharges [27] and muscle artifacts), spontaneous discontinuous, burst-suppressed, or suppressed pattern (background interrupted by diffusely suppressed periods of at least 10% of the recordings), abundant epileptiform activity (repetitive, periodic or rhythmic spikes, sharp waves, spike waves or rhythmic waves). This categorization was in line with the American Critical Neurophysiology Society (ACNS) terminology [24]. Using the EEG performed at day 2, the pattern was classified by experienced neurophysiologists, blinded for patient outcome, as benign, malignant or highly malignant according to the classification proposed by Westhall et al. [25]. Importantly, these criteria for defining a malignant and highly malignant EEG were different from those of the ERC/ESICM guidelines, where the only EEG-based predictor of poor outcome is a “non-reactive burst-suppressed or epileptiform pattern”, with no standardized definition. Benign EEG was defined as the absence of all of the above-mentioned features.
NSE Serum NSE levels < 33 μg/L (using the peak level at 24–48 h) were chosen as threshold for good outcome prediction, according to previous data from our group [10, 28]. For the analysis of poor outcome prediction, the threshold of > 75 μg/L previously used in the ERC/ESICM algorithm was maintained, but the combination of NSE with standardized, ACNS-based EEG analysis was investigated.
Outcome at 3 months
Neurological outcome was prospectively assessed as part of the registry at 3 months using the Cerebral Performance Category (CPC) scale through a semi-structured phone interview with the patient, caregiver or physician, and dichotomized as good (CPC 1–2) vs. poor outcome (CPC 3–5). Mortality at ICU discharge and cause of death were also collected. Both EEG/NSE and outcome data were not blinded to the investigators.
Statistical analysis
Categorical data are reported as number of events (percentage) and continuous data as median [interquartile range]. Categorical data were compared using the Chi-squared test. Normality of continuous data distribution was assessed with the Shapiro–Wilk test. Comparisons between two-paired continuous and categorical variables were performed using the Wilcoxon–Mann–Whitney U test. Non-parametric multiple comparisons were performed using the Kruskal–Wallis test. All analyses were performed applying a bilateral hypothesis and results with p ≤ 0.05 were considered significant. The study cohort represents a convenience sample. Sensitivity, specificity, FPR, positive predictive value (PPV) and negative predictive value (NPV), with binomial 95% confidence interval when appropriate, are reported for prognostication models. The best predictor of good and poor neurological outcome was identified as the one with the highest sensitivity and specificity for good and poor outcome prediction, respectively. McNemar test was performed for comparison of sensitivities and specificities of two binary diagnostic tests. Net reclassification index (NRI) and integrated discrimination improvement (IDI) were used to assess the improved discrimination power of the outcome predictors. Positive values of standardized NRI (½ NRI) and IDI indicate better discriminative performance of the alternative model compared to the “standard” model. Multiple imputations for incomplete multivariate data by Gibbs sampling were performed to account for missing data. Statistical analysis was performed with Software R Open Source 3.5.1.
Results
Patients
A total of 581 comatose post-CA patients admitted to our ICU during the study period were considered for inclusion. All patients had full recording of demographic data. Of them, 96 were excluded from analysis, because of incomplete data about SSEPs and/or brainstem reflexes (n = 54), EEG and/or NSE (n = 33), and outcome (n = 9).
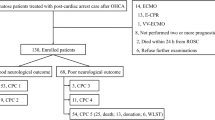
The baseline characteristics of the 485 patients included in the main analysis are summarized in Table 1. The study flow chart with main prognostication results is presented in Fig. 1.
Study flowchart with early outcome prognostication following the application of ERC/ESICM algorithm. Study flowchart showing patient early (by day 3) outcome classifications, dichotomized as indeterminate vs. poor outcome, after the application of the ERC/ESICM prognostication algorithm, with subsequent patient outcome trajectories, including awakening, and 3-month Cerebral Performance Categories (CPC; 1–2 = good vs. 3–5 = poor neurological recovery). BSR brainstem reflexes (pupillary and corneal), BS burst suppression, CA cardiac arrest, CT computerized tomography, EEG electroencephalography, ICU intensive care unit, MRI magnetic resonance imaging, NSE neuron-specific enolase, SE status epilepticus, SSEP N20 somatosensory-evoked potentials
Accuracy of the ERC/ESICM prognostication algorithm
A minority of patients (155/485; 32%) were identified as having a poor outcome by the ERC/ESICM prognostication algorithm; of these, 129 (83%) were detected by first-line prognostic tests, including 67 patients who had bilateral absence of both pupillary/corneal reflexes and SSEP and 62 patients who had bilaterally absent SSEP and unilaterally or bilaterally present pupillary/corneal reflexes. The remaining 26 patients (17%) were identified by second-line predictors; of these, 11 (42.3%) had diffuse hypoxic/ischemic injury on brain CT/MRI, 15 (58%) had status myoclonus, 16 (61.5%) had non-reactive burst suppression or status epilepticus on EEG and 14 (54%) had NSE > 75 µg/L. Two poor outcome predictors were present in 22 patients (85%) and three in four patients (15%). All patients identified as having a poor outcome according to the ERC/ESICM algorithm died without awakening, except one patient who awoke and subsequently died from extracerebral causes (Fig. 1).
Prevalence of indeterminate outcome based on ERC/ESICM prognostication algorithm
Based on the current European ERC/ESICM prognostication algorithm, more than two-thirds of patients (330/485; 68%) were predicted to have an indeterminate outcome. Among them, 261 (79%) awoke in the ICU (of which 69 [26%] after day 5), and the majority had good neurological outcome at 3 months (Fig. 1). No difference in the rate of good outcome was observed between patients who awoke before or after day 5 (160/192 [83%] vs. 52/69 [75%] respectively, p = 0.15). No correlation was observed between the duration of sedation and the time to awakening after sedation stop (Spearman’s correlation coefficient 0.0007, p = 0.99). Sixty-nine (21%) patients never awoke; the majority (52; 75%) died in the ICU. A single ERC/ESICM second-line poor outcome predictor was found in 33/330 (10%) of patients with indeterminate outcome, the most common being status myoclonus (10/33; 30%) followed by hypoxic/ischemic injury on CT/MRI (9/33; 27%), non-reactive burst suppression or status epilepticus on EEG (8/33; 24%), NSE > 75 (6/33; 18%).
Three-month prognosis in the early indeterminate outcome population based on EEG and NSE findings
Figure 2 shows EEG and NSE findings in patients with an initial indeterminate outcome. The rate of good recovery was higher in patients with a benign EEG pattern than in those with a malignant or highly malignant EEG (75% vs. 39% vs. 11%, p < 0.0001), and with NSE < 33 μg/L (72.5% vs. 40% when NSE was between 33 and 75 μg/L vs. 16.7% when NSE was > 75 μg/L, p < 0.0001). The prevalence of the different combination patterns of EEG and NSE and the associated prevalence of good outcome are reported in the Electronic Supplementary Material (ESM_Figure 1).
EEG and NSE findings in patients prognosticated as indeterminate outcome by day 3, with subsequent proportions of good recovery (cerebral performance categories 1 or 2) at 3 months. EEG background at day 2 was classified according to [25], as benign, malignant or highly malignant. Serum NSE was classified into three categories according to peak levels at 24–48 h. The figure illustrates the percentage of patients for each EEG and NSE category and the corresponding rate of good neurological outcome (CPC 1–2) at 3 months
Prediction of good and poor outcome at 3 months in the early indeterminate outcome population
Prediction of good outcome
As shown in Table 2, in patients with an initially indeterminate outcome the absence of a highly malignant pattern on the EEG performed at day 2 had the highest sensitivity (99.5%; 95% confidence interval 97.4–99.9%) for predicting a good neurological recovery at 3 months. Among the 211 patients who were correctly identified as having good recovery, 51 (24.1%) awoke after day 5. The last patient awoke on day 29.
As compared with EEG, NSE < 33 µg/L had a lower sensitivity for good outcome prediction, either when used alone (84.9% [79.3–89.4%]) or when in combination with EEG (84.4% [78.8–89%], both p < 0.001; Table 2).
When considering a non-highly malignant EEG as “standard” test, the addition of NSE < 33 µg/L resulted only in a modest improvement of the discriminative power (½ NRI = 0.25 [0.17–0.37], IDI = 0.08 [0.05–0.11]).
Prediction of poor outcome
As shown in Table 3, the presence of a highly malignant EEG pattern on day 2 was very specific (99.5 [97.4–99.9] %) for poor prognosis in patients with initial indeterminate outcome. Peak serum NSE > 75 μg/L had equal specificity, but lower sensitivity than highly malignant EEG (4.2% vs. 8.5%; p = 0.19). When compared to a highly malignant EEG, an NSE > 75 µg/L did not result in better discriminative power (½ NRI = − 0.08 [− 0.13 to − 0.33], IDI = − 0.03 [− 0.06 to 0.01]).
Six patients had a 3-month poor recovery despite the absence of a highly malignant EEG (two benign, four malignant): all had an NSE > 75 μg/L, this combination providing a 100 [98.2–100] % specificity for poor recovery. Additional data on NSE are given in ESM_Table 1 (sensitivity analysis of NSE thresholds) and ESM_Figure 2 (area under the ROC curve for NSE as poor outcome predictor). Multiple imputations for incomplete multivariate data by Gibbs sampling (mean results from 1000 different simulations for missing data allocation) confirmed comparable results as in the true main analysis (ESM_Appendix).
Discussion
This is the first large prospective study to examine the rate of indeterminate outcome following the application of 2015 ERC/ESICM guidelines, and to explore optimal prognostic strategies for better prediction of recovery in this setting. Our study showed that in more than two-thirds of patients who are comatose after resuscitation from CA the outcome remains indeterminate after application of the ERC/ESICM prognostication algorithm. This constitutes a substantial challenge in clinical practice. Decreasing the rate of prognostic uncertainty in these patients is of utmost importance, because the majority of them achieve a good neurological recovery at 3 months. Relying onto an accurate prediction, especially for good recovery, may increase clinician’s confidence in providing longer observation time and avoiding inappropriate WLST.
Our study identified EEG, classified using the ACNS terminology [25] as the most powerful prognostic tool in the group of patients with an initial indeterminate outcome. Indeed, the analysis of NRI and IDI showed that the overall added discriminative power of NSE above that of EEG was low. The absence of a highly malignant pattern on the EEG performed at day 2 yielded a sensitivity of 99.5% for good outcome prediction, and accurately predicted a favorable recovery in 66% of patients. Importantly, almost 25% of these patients had a delayed awakening, underlining the importance of an early good outcome prediction. Identifying a recovery potential when prognosis appears indeterminate is helpful in choosing when aggressive support of organ function may be indicated [21,22,23]. Although self-fulfilling prophecy may still be an issue when prognosticating a poor outcome, the prediction of good recovery remains on the contrary unaffected by it; achieving a high sensitivity (and low false negatives) is important since, ideally, no patients destined to a good recovery should be missed. Indeed, using absence of a highly malignant EEG as a criterion, only one patient with good outcome in our cohort would have been missed. A potential drawback of using this approach is the number of false positives, i.e. patients who have a poor outcome despite the absence of a highly malignant EEG, and in whom intensive care would be nonetheless maintained. In this regard, in a limited subset of patients (six in our cohort), the combination of NSE > 75 μg/L with a malignant EEG predicted poor outcome with 100% specificity, and could thus provide a useful “second-line” prognosticator to avoid unnecessary continuation of care.
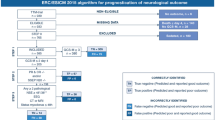
In summary, based on the findings of our study, we suggest the application of a standardized EEG analysis as a complement to the current ERC/ESICM algorithm to reduce prognostic uncertainty in patients with an indeterminate outcome, as outlined in Fig. 3. This proposal for a new prognostication algorithm should be used with caution due to the remaining uncertainties on patient prognosis, however, we believe it may be of potential help for the clinical practice.
Standardized EEG analysis as a complement to current ERC/ESICM prognostication algorithm. The figure illustrates how a standardized EEG analysis can be used in clinical practice to improve prognostication in patients with an indeterminate outcome based on current ERC/ESICM multimodal algorithm [8, 9]. NSE neuron-specific enolase, ROSC return of spontaneous circulation
Study limitations
Some limitations of our study need to be acknowledged. First, the study was single center, therefore limiting generalizability, in part because of potential variations of prevalence of good and poor outcomes across studies. However, the results of multiple imputations analysis confirmed comparable results as in the main analysis, thereby reinforcing our message. Second, the timeframe was relatively long and, as in all prognostication studies, our results may have been affected by a self-fulfilling prophecy bias towards poor outcome. Nevertheless, the use of a pre-specified written prognostication algorithm and WLST strategy, which remained unchanged over the study timeline, confers internal validation. In addition, the standardized EEG criteria we used were not included in our WLST strategy and their analysis was performed blinded to patient outcome. Furthermore, WLST is less likely to influence the strength of predictors of good prognosis. Third, we considered brain imaging (when performed) only for the initial stratification of patients according to the second line of ERC/ESICM guidelines, as neuroimaging was not routinely performed for the purpose of coma prognostication. Indeed, CT/MRI findings are still used inconstantly and without definitive accepted quantitative variables at the early phase following CA [29]. Fourth, there is no strict consensus on the definition of awakening: we used the first GCS motor score of 6 [7, 20, 30], but other definitions have been used [16, 17, 31]. We did not account for the duration of sedation in line with previous studies [29, 30, 32, 33] and indeed we found no correlation between the duration of sedation and the time to awakening from sedation stop. Fifth, we applied ERC/ESICM guidelines and subsequent EEG and NSE analysis retrospectively, which is not as optimal as a true prospective application of an algorithm during patient care. Finally, outcome was available at 3 months, however, CPC score may continue to evolve beyond this timeline.
Conclusions
In the majority of comatose patients resuscitated from cardiac arrest, outcome remains indeterminate after application of ERC/ESICM algorithm. In this population, a standardized EEG background analysis enables accurate prediction of both good and poor outcome, thereby greatly reducing prognostic uncertainty.
Abbreviations
- BS:
-
Burst suppression;
- BSR:
-
Brainstem reflexes (including pupillary and corneal)
- CA:
-
Cardiac arrest
- CPC:
-
Cerebral Performance Category
- CT:
-
Brain computerized tomography
- ERC:
-
European Resuscitation Council
- ESICM:
-
European Society of Intensive Care Medicine
- EEG:
-
Electroencephalogram
- FPR:
-
False positive rate
- GCS:
-
Glasgow Coma Scale
- ICU:
-
Intensive care unit
- MRI:
-
Brain magnetic resonance imaging
- NPV:
-
Negative predictive value
- NSE:
-
Serum neuron-specific enolase
- PPV:
-
Positive predictive value
- ROC:
-
Receiving operator characteristic
- SE:
-
Status epilepticus
- SSEP:
-
Somatosensory-evoked potentials
- WLST:
-
Withdrawal of life-sustaining therapies
References
Laver S, Farrow C, Turner D, Nolan J (2004) Mode of death after admission to an intensive care unit following cardiac arrest. Intensiv Care Med 30:2126–2128. https://doi.org/10.1007/s00134-004-2425-z
Nolan JP, Ferrando P, Soar J et al (2016) Increasing survival after admission to UK critical care units following cardiopulmonary resuscitation. Crit Care Lond Engl 20:219. https://doi.org/10.1186/s13054-016-1390-6
Nobile L, Taccone FS, Szakmany T et al (2016) The impact of extracerebral organ failure on outcome of patients after cardiac arrest: an observational study from the ICON database. Crit Care. https://doi.org/10.1186/s13054-016-1528-6
Lemiale V, Dumas F, Mongardon N et al (2013) Intensive care unit mortality after cardiac arrest: the relative contribution of shock and brain injury in a large cohort. Intensiv Care Med 39:1972–1980. https://doi.org/10.1007/s00134-013-3043-4
Dragancea I, Wise MP, Al-Subaie N et al (2017) Protocol-driven neurological prognostication and withdrawal of life-sustaining therapy after cardiac arrest and targeted temperature management. Resuscitation 117:50–57. https://doi.org/10.1016/j.resuscitation.2017.05.014
Dragancea I, Rundgren M, Englund E et al (2013) The influence of induced hypothermia and delayed prognostication on the mode of death after cardiac arrest. Resuscitation 84:337–342. https://doi.org/10.1016/j.resuscitation.2012.09.015
Mulder M, Gibbs HG, Smith SW et al (2014) Awakening and withdrawal of life-sustaining treatment in cardiac arrest survivors treated with therapeutic hypothermia. Crit Care Med 42:2493–2499. https://doi.org/10.1097/CCM.0000000000000540
Nolan JP, Cariou A (2015) Post-resuscitation care: ERC–ESICM guidelines 2015. Intensiv Care Med 41:2204–2206. https://doi.org/10.1007/s00134-015-4094-5
Nolan JP, Soar J, Cariou A et al (2016) Erratum to: European Resuscitation Council and European Society of Intensive Care Medicine 2015 guidelines for post-resuscitation care. Intensiv Care Med 42:488–489. https://doi.org/10.1007/s00134-015-4158-6
Oddo M, Rossetti AO (2014) Early multimodal outcome prediction after cardiac arrest in patients treated with hypothermia. Crit Care Med 42:1340–1347. https://doi.org/10.1097/CCM.0000000000000211
Scarpino M, Lanzo G, Lolli F et al (2018) Neurophysiological and neuroradiological multimodal approach for early poor outcome prediction after cardiac arrest. Resuscitation 129:114–120. https://doi.org/10.1016/j.resuscitation.2018.04.016
Tsetsou S, Novy J, Pfeiffer C et al (2018) Multimodal outcome prognostication after cardiac arrest and targeted temperature management: analysis at 36 °C. Neurocrit Care 28:104–109. https://doi.org/10.1007/s12028-017-0393-8
Cronberg T (2019) Assessing brain injury after cardiac arrest, towards a quantitative approach. Curr Opin Crit Care 25:211–217. https://doi.org/10.1097/MCC.0000000000000611
Rossetti AO, Tovar Quiroga DF, Juan E et al (2017) Electroencephalography predicts poor and good outcomes after cardiac arrest: a two-center study. Crit Care Med 45:e674–e682. https://doi.org/10.1097/CCM.0000000000002337
Ruijter BJ, Tjepkema-Cloostermans MC, Tromp SC et al (2019) Early electroencephalography for outcome prediction of postanoxic coma: a prospective cohort study. Ann Neurol 86:203–214. https://doi.org/10.1002/ana.25518
Paul M, Bougouin W, Geri G et al (2016) Delayed awakening after cardiac arrest: prevalence and risk factors in the Parisian registry. Intensiv Care Med 42:1128–1136. https://doi.org/10.1007/s00134-016-4349-9
Gold B, Puertas L, Davis SP et al (2014) Awakening after cardiac arrest and post resuscitation hypothermia: are we pulling the plug too early? Resuscitation 85:211–214. https://doi.org/10.1016/j.resuscitation.2013.10.030
Rey A, Rossetti AO, Miroz J-P et al (2019) Late awakening in survivors of postanoxic coma: early neurophysiologic predictors and association with ICU and long-term neurologic recovery. Crit Care Med 47:85–92. https://doi.org/10.1097/CCM.0000000000003470
Eid SM, Albaeni A, Vaidya D et al (2016) Awakening following cardiac arrest: determined by the definitions used or the therapies delivered? Resuscitation 100:38–44. https://doi.org/10.1016/j.resuscitation.2015.12.017
Irisawa T, Vadeboncoeur TF, Karamooz M et al (2017) Duration of coma in out-of-hospital cardiac arrest survivors treated with targeted temperature management. Ann Emerg Med 69:36–43. https://doi.org/10.1016/j.annemergmed.2016.04.021
Sandroni C (2016) EEG for prognostication in postanoxic coma: what you predict depends on when you predict. Minerva Anestesiol 82:919–922
Geri G, Guillemet L, Dumas F et al (2015) Acute kidney injury after out-of-hospital cardiac arrest: risk factors and prognosis in a large cohort. Intensiv Care Med 41:1273–1280. https://doi.org/10.1007/s00134-015-3848-4
Bougouin W, Dumas F, Marijon E et al (2017) Gender differences in early invasive strategy after cardiac arrest: Insights from the PROCAT registry. Resuscitation 114:7–13. https://doi.org/10.1016/j.resuscitation.2017.02.005
Hirsch LJ, LaRoche SM, Gaspard N et al (2013) American clinical neurophysiology society’s standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol 30:27
Westhall E, Rossetti AO, van Rootselaar A-F et al (2016) Standardized EEG interpretation accurately predicts prognosis after cardiac arrest. Neurology 86:1482–1490. https://doi.org/10.1212/WNL.0000000000002462
Perkins GD, Jacobs IG, Nadkarni VM et al (2015) Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the utstein resuscitation registry templates for out-of-hospital cardiac arrest. Resuscitation 96:328–340. https://doi.org/10.1016/j.resuscitation.2014.11.002
Hirsch LJ, Claassen J, Mayer SA, Emerson RG (2004) Stimulus-induced rhythmic, periodic, or ictal discharges (SIRPIDs): a common EEG phenomenon in the critically ill. Epilepsia 45:109–123
Rossetti AO, Rabinstein AA, Oddo M (2016) Neurological prognostication of outcome in patients in coma after cardiac arrest. Lancet Neurol 15:597–609. https://doi.org/10.1016/S1474-4422(16)00015-6
Wijman CAC, Mlynash M, Caulfield AF et al (2009) Prognostic value of brain diffusion weighted imaging after cardiac arrest. Ann Neurol 65:394–402. https://doi.org/10.1002/ana.21632
Grossestreuer AV, Abella BS, Leary M et al (2013) Time to awakening and neurologic outcome in therapeutic hypothermia-treated cardiac arrest patients. Resuscitation 84:1741–1746. https://doi.org/10.1016/j.resuscitation.2013.07.009
Howell K, Grill E, Klein A-M et al (2013) Rehabilitation outcome of anoxic-ischaemic encephalopathy survivors with prolonged disorders of consciousness. Resuscitation 84:1409–1415. https://doi.org/10.1016/j.resuscitation.2013.05.015
Lybeck A, Cronberg T, Aneman A et al (2018) Time to awakening after cardiac arrest and the association with target temperature management. Resuscitation 126:166–171. https://doi.org/10.1016/j.resuscitation.2018.01.027
Ponz I, Lopez-de-Sa E, Armada E et al (2016) Influence of the temperature on the moment of awakening in patients treated with therapeutic hypothermia after cardiac arrest. Resuscitation 103:32–36. https://doi.org/10.1016/j.resuscitation.2016.03.017
Acknowledgements
The authors thank John-Paul Miroz, RN, Jan Novy, MD PhD, Christine Stähli, RN and Laura Pezzi, RN, for their help in data collection, and EEG retrieval and analysis.
Funding
Mauro Oddo and Andrea O. Rossetti are supported by the Swiss National Science Foundation.
Author information
Authors and Affiliations
Contributions
FB and FR equally contributed to data acquisition and analysis, and drafted the manuscript; GB performed EEG analysis and classification; ADR provided expert supervision of statistical analysis; AOR supervised EEG analysis and classification, contributed to data acquisition and analysis, and critically revised the manuscript; FST revised the manuscript and provided important intellectual contribution; CS and MO equally contributed to supervise the concept and design of the study, data analysis and interpretation, and critically revised the manuscript. All authors approved and agreed on the final version of the manuscript. This article is reported according to the STARD 2015 guidelines (https://www.equator-network.org/reporting-guidelines/stard/).
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Ethics approval
Approval was obtained from the Ethical Research Committee of the University of Lausanne, and waiver of consent was allowed since all examinations were part of standard patient care.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bongiovanni, F., Romagnosi, F., Barbella, G. et al. Standardized EEG analysis to reduce the uncertainty of outcome prognostication after cardiac arrest. Intensive Care Med 46, 963–972 (2020). https://doi.org/10.1007/s00134-019-05921-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-019-05921-6