Abstract
Purpose
Antibiotic de-escalation is promoted to limit prolonged exposure to broad-spectrum antibiotics, but proof that it prevents the emergence of resistance is lacking. We evaluated determinants of antibiotic de-escalation in an attempt to assess whether the latter is associated with a lower emergence of antimicrobial resistance.
Methods
Antibiotic treatments, starting with empirical beta-lactam prescriptions, were prospectively documented during 2013 and 2014 in a tertiary intensive care unit (ICU) and categorized as continuation, de-escalation or escalation of the empirical antimicrobial treatment. Determinants of the de-escalation or escalation treatments were identified by multivariate logistic regression; the continuation category was used as the reference group. Using systematically collected diagnostic and surveillance cultures, we estimated the cumulative incidence of antimicrobial resistance following de-escalation or continuation of therapy, with adjustment for ICU discharge and death as competing risks.
Results
Of 478 anti-pseudomonal antibiotic prescriptions, 42 (9 %) were classified as escalation of the antimicrobial treatment and 121 (25 %) were classified as de-escalation, mainly through replacement of the originally prescribed antibiotics with those having a narrower spectrum. In multivariate analysis, de-escalation was associated with the identification of etiologic pathogens (p < 0.001). The duration of the antibiotic course in the ICU in de-escalated versus continued prescriptions was 8 (range 6–10) versus 5 (range 4–7) days, respectively (p < 0.001). Mortality did not differ between patients in the de-escalation and continuation categories. The cumulative incidence estimates of the emergence of resistance to the initial beta-lactam antibiotic on day 14 were 30.6 and 23.5 % for de-escalation and continuation, respectively (p = 0.22). For the selection of multi-drug resistant pathogens, these values were 23.5 (de-escalation) and 18.6 % (continuation) respectively (p = 0.35).
Conclusion
The emergence of antibiotic-resistant bacteria after exposure to anti-pseudomonal beta-lactam antibiotics was not lower following de-escalation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selection of the appropriate antimicrobial therapy for critically ill patients is challenging in the context of the increasing prevalence of antimicrobial resistance. International and local guidelines advocate the use of broad-spectrum antibiotics in severe healthcare-associated infections for maximal empirical coverage, coupled with antibiotic de-escalation to reduce overall exposure to broad-spectrum antibiotics and its detrimental ecological effects [1, 2]. De-escalation may be achieved through replacing broad-spectrum antibiotics by narrow-spectrum drugs, through stopping components of an antibiotic combination, or by early withdrawal of antibiotics in the absence of infection [1, 3–8]. The widely promoted strategy of de-escalation is backed up by only a few studies which used heterogeneous definitions of de-escalation and provided equivocal results [6, 7]. The survival benefit related to de-escalation which was reported in some observational trials [9–11] could not be confirmed in other studies [12, 13], nor in a recent multicenter randomized trial [14], although none showed increased mortality associated with de-escalation. Furthermore, there is a lack of microbiological data in support of the presumption that de-escalation limits the emergence of multi-drug resistant (MDR) pathogens [7].
In the observational study reported here, we describe treatment changes (de-escalation and escalation) following empirical beta-lactam antibiotic prescription in intensive care unit (ICU) patients and identify determinants of the different treatment patterns. We subsequently relate these patterns to patient outcome, focusing on the effect of de-escalation of anti-pseudomonal beta-lactam antibiotics on the emergence of antibiotic resistance.
Methods
The study was conducted at the 14-bed medical ICU and the 22-bed surgical ICU (SICU) of Ghent University Hospital (1056 beds). From 1 January 2013 to 31 December 2014, we prospectively registered all infections requiring antibiotics with the aid of the software application COSARA (Computer-based Surveillance and Alerting of infections, Antimicrobial Resistance and Antibiotic consumption in the ICU), developed in collaboration with the Department of Information Technology of Ghent University [15, 16]. COSARA facilitates the build-up of an extensive data warehouse by allowing linkage between automatically collected clinical and biochemical variables, antimicrobial prescription data, microbiology results and clinical diagnoses of infection. During the study period, no strict empirical antibiotic protocol was used, and all empirical choices and subsequent changes were at the liberty of the senior ICU-physician, working together in close collaboration with microbiologists and conferring three times weekly. As described previously [17], empirical antibiotic choices are essentially guided by systematically collected surveillance cultures (SC). Piperacillin–tazobactam, ceftazidime, and meropenem were administered as a continuous infusion, and non-anti-pseudomonal beta-lactam antibiotics and non-beta-lactam antibiotics were administered intermittently. Standard dosing regimens are provided in Electronic Supplemental Material (ESM) Table 1.
From the COSARA data warehouse, we retrospectively analyzed all beta-lactam antibiotic courses of at least a 48-h duration that were prescribed as first-line treatment of an infection. Only episodes in the ICU of at least a 96-h duration were included as antibiotic changes were unlikely to occur in shorter episodes. Antibiotic changes were classified as de-escalation or escalation depending on whether the changes represented a move up or down, respectively, a predefined ranking system of agents according to increasing order of Gram-negative antimicrobial activity (ESM Table 1). Roughly outlined, this ranking system was: step 1: “beta-lactam antibiotics without anti-pseudomonal activity or fluoroquinolones advocated as empirical treatment for severe community-acquired infection”; step 2: “non-carbapenem beta-lactam antibiotics with anti-pseudomonal activity or fluoroquinolones targeted at Pseudomonas”; step 3: “carbapenems”; step 4: “carbapenems in combination with a second antibiotic with Gram-negative coverage”. We did not evaluate changes in Gram-positive coverage (such as adding or withholding glycopeptides or linezolid). The ranking system was modified according to the focus of infection and the consequent need for anaerobic coverage (for example, as required in complicated intra-abdominal infections). Levofloxacin was classified as a step 1 antibiotic despite the anti-pseudomonal activity as it is a recommended treatment choice for severe community-acquired infections in national guidelines [18].
We registered patient demographics, co-morbidities, focus and severity of the infection, and daily sequential organ failure assessment (SOFA) scores. Microbiology results from 10 days prior to ICU admission until 10 days following ICU discharge were taken into consideration, comprising SC and additional cultures upon clinical suspicion of infection. SC consisted of oral, nasal, and rectal swabs upon admission, followed by once-weekly nasal samples and twice-weekly oral and rectal samples in all patients, as well as twice weekly sputum in the non-intubated patient or endotracheal aspirate in the ventilated patient. Cultured pathogens were classified as etiologic if these were considered to represent the causal pathogen of the infection and as colonizing in other cases. In case of microbiologically documented infection, antibiotic treatment was considered to be appropriate if all etiologic pathogens were covered.
For the outcome analysis, patients were included once, and the first beta-lactam prescription was considered. The following outcome parameters were recorded: ICU mortality, in-hospital mortality, subsequent infections requiring antibiotic therapy, and total antibiotic consumption in the ICU, defined as the total number of days that a patient received an antibiotic during his/her stay in the ICU. In case of combination therapy, the total antibiotic consumption equaled the sum of the number of days of the individual components of the treatment. Antibiotic-free days were noted in the subgroup of patients with a length of stay (LOS) in the ICU of at least 14 days. In addition, emergence of pathogens resistant to the initial beta-lactam antibiotic and emergence of MDR pathogens was registered. Pathogens isolated in any culture from day 2 following the start of the antibiotic treatment under study and not present before that time were defined as having emerged after treatment. The following pathogens were categorized as MDR: methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococcus, Stenotrophomonas maltophilia, Achromobacter spp., MDR Enterobacteriaceae, MDR Pseudomonas aeruginosa, and MDR Acinetobacter spp. modified from the publication of Magiorakos et al., in accordance with the MDR definition employed by the multicenter research project R-GNOSIS, work package 6 (ESM Table 2) [19, 20]. In addition, we included Enterobacteriaceae resistant for both 3rd generation cephalosporins and piperacillin-tazobactam and Clostridium difficile.
The Ghent University Hospital Ethics Committee approved the study (registration number B670201524161) and waived informed consent. Only patients aged 16 years or older were included.
Statistics
Categorical variables were expressed as frequencies (percentages), continuous variables were described as median values with the interquartile range (IQR; 25–75th percentile). Continuation of the antibiotic treatment was defined as the standard to which de-escalation and escalation were compared. Differences in categorical variables were calculated using Pearson Chi-square test or Fisher’s exact test as appropriate. The Mann–Whitney U test was used to compare continuous variables. A multivariate logistic regression model was used to identify factors associated with de-escalation and escalation. All variables with a p value of 0.15 or lower and considered to be clinically important were entered into the model. The Hosmer–Lemeshow test was used to evaluate goodness-of-fit. Statistical significance was defined as p < 0.05. As systematic SC are no longer performed in patients discharged from ICU or in patients who die during their stay in the ICU, and hence the non-informative censoring assumption is likely to be violated, a competing risk analysis was performed when estimating the cumulative incidence of the emergence of antibiotic resistance [21–23]. Cumulative incidence functions (CIFs) of de-escalation and continuation were compared using a modified Chi-square test, with statistical significance defined as p < 0.05 [24]. All statistical analyses were performed with SPSS® software (SPSS, version 23; IBM Corp., Armonk, NY), and the R 3.2.2 software package [25]. The competing risk analysis was performed using the “cuminc” routine available in the “cmprsk” package developed by Gray [26].
Results
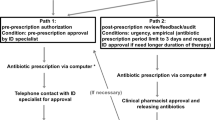
In total, we included 782 prescriptions of beta-lactam antibiotics for 615 patients in our analysis. Changes that could not be categorized as de-escalation or escalation [n = 50 (6.4 %)] were omitted from the analysis. Of the remaining prescriptions (n = 732), 254 (35 %) had no anti-pseudomonal activity [amoxicillin–clavulanate, n = 178 (24 %); cefuroxime, n = 53 (7 %), ceftriaxone n = 23 (3 %)]. Piperacillin–tazobactam was the most frequently used anti-pseudomonal antibiotic [n = 343 (47 %)], followed by meropenem [n = 111 (15 %)] and ceftazidime [n = 24 (3 %)]. Treatment changes are detailed in Fig. 1a, b. Anti-pseudomonal beta-lactam antibiotics were de-escalated in 25 % of the treatments and escalated in 9 %; subsequent changes occurred in 26 % of treatments; de-escalation was maintained in 81 % of the treatment courses. Initial beta-lactam therapy was continued during the entire treatment course in 66 % of treatments; 67 % of continued treatments for microbiologically documented infections could have been de-escalated based on susceptibility data of the etiologic pathogen.
Determinants of de-escalation and escalation
To identify the determinants of de-escalation and escalation we included prescriptions with anti-pseudomonal activity only (n = 478). The median time interval to antibiotic change was 3 days (IQR for de-escalation and escalation was 3–5 and 2–7 days, respectively). De-escalation was achieved by narrowing the Gram-negative spectrum in 111 treatments, by reducing the number of antimicrobials in three treatments, and by a combination of both in seven treatments. In 63 % of de-escalations the empirical beta-lactam antibiotic was changed to another beta-lactam antiobiotic. Levofloxacin was the most frequently prescribed non-beta-lactam antibiotic in the case of de-escalation (21 %) (Tables 1, 2).
Factors associated with de-escalation or escalation are detailed in Table 2. In the multivariate analysis, de-escalation was significantly associated with the identification of etiologic pathogens (p < 0.001), and escalation of therapy was significantly associated with severe sepsis or septic shock at presentation (p = 0.03), worsening SOFA score (p = 0.008), the presence of additional (non-etiologic) isolates resistant to the initial antibiotic (p = 0.01), admission to the SICU (p = 0.003) and hospitalization duration prior to start of the infection (p = 0.04).
Outcome after de-escalation of therapy
Both de-escalation and escalation were associated with a longer antibiotic course [8 (IQR 6–10) (de-escalation) vs. 11 (IQR 8–19) (escalation) vs. 5 (IQR 4–7) (continuation) days; p < 0.001] and a higher total antibiotic consumption while in the ICU [12 (7–22) (de-escalation) vs. 24 (13–39) (escalation) vs. 7 (4–15) (continuation) days; p < 0.001]. As compared to the LOS in the ICU of patients who continued on the original therapy [continuation: 8 (IQR 5–15) days], that of patients in the de-escalation and escalation categories was significantly longer [11 (6–19) days, p = 0.001 and 17 (10–23) days, p < 0.001, respectively]. The number of antibiotic-free days on day 14 was significantly lower for patients in the de-escalation and escalation categories [1 (0–3) (de-escalation), p = 0.04 vs. 0 (0–1) (escalation), p < 0.001 vs. 2 (0–6) (continuation) days]. A subsequent infection in the ICU was more frequent following escalation of treatment than following continuation (55.3 vs. 33 %, respectively; p = 0.008). Neither ICU mortality nor hospital mortality differed between the three categories (Table 3).
Pathogens with in vitro resistance to the initial anti-pseudomonal beta-lactam antibiotic emerged in 32.6 % of patients, and MDR pathogens emerged in 28.8 % of patients; these values did not differ significantly when the initial beta-lactam therapy was continued, de-escalated, or escalated. The cumulative incidence estimate (CIE) of emergence of pathogens resistant to the initial beta-lactam on day 14 was 23.5 % when the initial beta-lactam was continued and 30.6 % when therapy was de-escalated (p = 0.22). The CIE of emergence of MDR pathogens on day 14 was 18.6 and 23.5 % for continuation and de-escalation of therapy, respectively (p = 0.35). Both CIF curves are displayed in Fig. 2a, b. Equally, subgroup analyses on microbiologically confirmed infections (ESM Fig. 2c, d) or on only those including antibiotic courses of >5 days (ESM Fig. 2e, f) found no differences in the CIFs of antibiotic resistance when de-escalation was compared to continuation.
a Cumulative incidence function (after adjustment for ICU discharge and death as competing risk events) of emergence of pathogens resistant to the initial anti-pseudomonal betalactam antibiotic. b Cumulative incidence function (after adjustment for ICU discharge and death as competing risk events) of emergence of MDR pathogens
Discussion
To date no data have been published which confirm a beneficial effect of de-escalation on MDR emergence [7]. Previous studies that were not designed to investigate this subject were unable to demonstrate an impact of de-escalation on the selection of resistance [12, 14, 27]. Our analysis of routinely collected diagnostic and surveillance cultures is the first study to address this topic systematically. Our data show no impact of the de-escalation of empirical anti-pseudomonal beta-lactam therapy on the emergence of resistance to antibiotics.
De-escalation of anti-pseudomonal beta-lactam antibiotics was performed in one-quarter of the prescriptions, which is low in comparison with the rates reported in previous studies, ranging from 30 to 60 % [7, 9–13, 27, 28]. However, comparison between studies is hampered by the lack of a universal definition for de-escalation. Our definition of de-escalation was strict and limited to Gram-negative coverage only. Gonzalez et al. reported a de-escalation rate of 51 %, with >90 % achieved by a reduction in the number of antimicrobials [12]. In contrast, the majority of de-escalations in our study resulted from substitution of the initial antibiotic by an antibiotic with a more limited spectrum [111/121 (92 %)].
Microbiological documentation of the infection has been identified as a prerequisite for de-escalation in many studies [10, 11, 13, 14, 27–29]. Although 27 % of the de-escalations in our study were for the treatment of culture-negative infections, multivariate analysis of the determinants of de-escalation found that identification of the etiologic pathogen was the single factor promoting de-escalation. However, a high number (67 %) of continued treatments for microbiologically documented infections were not de-escalated despite this being microbiologically possible, indicating that other, unresolved barriers for de-escalation may exist [30]. In contrast with prior observations we did not find an association between de-escalation and clinical improvement or less severity of the infection [9, 10, 29, 31]. Interestingly, factors associated with escalation were more complex. Escalation was significantly associated with a higher clinical severity upon presentation and unfavorable evolution under treatment, an observation which was also reported by Garnacho-Montero et al. [9]. Additionally, the presence of resistant colonizing pathogens triggered the physician to escalate therapy. As the presence of resistant colonizing pathogens did not inhibit de-escalation, we suspect that during the treatment course SC are mainly used to alter the treatment in the case of severe and sustained infections. Escalation of therapy was also associated with the ICU department (surgical/medical) regardless of focus of infection, suggesting that the decision to alter the therapy may be related to more subjective characteristics or attitudes of the physician [30].
An unexpected finding of our analysis was that the treatment duration was significantly longer in the de-escalated population (p < 0,001) [13]. To account for potential bias, we repeated this analysis in different subgroups of patients (i.e., with the antibiotic course completed in the ICU, with etiologic pathogens identified) and calculated antibiotic-free days in the subgroup with a LOS in the ICU of ≥14 days after initiation of the infection—obtained the same result. One possible explanation is that de-escalation under the form of early antibiotic discontinuation may be hidden in the subgroup of patients who continued treatment. However, as the results are identical in different subgroups, we assume that this last reasoning cannot fully explain our observation. Alternatively, prolonged antibiotic treatment may be an unwanted side-effect of de-escalation. Although we have no firm explanation, it is tempting to propose a few potential explanations. The first is that physicians may not take the first days of empirical therapy into account when determining the full treatment duration. A second plausible explanation is the subjective perception that extending a course of a narrow-spectrum antibiotic for a few days may have fewer harmful ecological consequences than extending that of a broad-spectrum drug. The total antibiotic consumption in the ICU was also significantly higher in our de-escalated patients, but these results were mainly determined by the initial infectious episode. In contrast to our findings, Leone et al. observed an increased number of superinfections in patients following de-escalation, leading to a significantly higher total antibiotic consumption [14]. Clearly, de-escalation may itself provoke subsequent attitudes or behavior, an aspect of this study which deserves further attention.
Cumulative incidence functions were analyzed both for the emergence of pathogens resistant to the initial anti-pseudomonal beta-lactam antibiotic and for emergence of MDR pathogens, adjusting for ICU discharge and death as competing risks for the selection of resistance, and did not differ significantly between patients in the de-escalation and continuation categories. The increased emergence (although not reaching significance) of resistance in patients in the de-escalation category, as compared to those who continued the therapy, disappeared altogether when the analysis was restricted to microbiologically confirmed infections, as well as in the subgroup of antibiotic courses of >5 days; as such this increased resistance might be due to the higher number of short antibiotic exposures in the continuation group. The observation that pathogens resistant to the prescribed beta-lactam antibiotic were isolated after a median time interval of 5 days of treatment suggests that there is a widespread reservoir of resistance which rapidly results in detectable colonization even after short treatment courses. Our results find support in the study of Armand-Lefèvre et al. [32] who describe an odds ratio of 5.9 for colonization with imipenem-resistant Gram-negative bacilli in the intestinal flora of ICU patients after 1–3 days of exposure to imipenem. These findings suggest that a reduction of the number of exposures to broad-spectrum antibiotics may be a better approach to limit the emergence of resistance. An alternative hypothesis for the rapid selection of resistance is derived from simulation studies that demonstrate a lower probability to achieve adequate pharmacokinetic/pharmacodynamic targets for more narrow-spectrum agents [33].
De-escalation on the second or third day of therapy is recommended [1, 2, 5]. In our study we de-escalated therapy after a median treatment duration of 3 (IQR 3–5) days, which is in accordance with previous reports [12, 14, 27]. As physicians rely on microbiology results for their decision to de-escalate, it seems almost impossible to narrow this time-frame due to the limitations of current microbiology practices.
Our study has a number of limitations. First, it is a retrospective study, although all antibiotic-related data were recorded prospectively. Second, our study is monocentric in a setting with relatively low resistance levels, and the impact of de-escalation may be different in other ecologies. Third, our ranking system of incremental Gram-negative antimicrobial activity is only one of many possible approaches. In previous papers focusing on the subject, ranking of antimicrobials by their spectrum of activity has proven to be difficult, resulting in conflicting definitions [34, 35]. Moreover, most prior observational studies do not provide the ranking of the treatments that was used, which makes interpretation and comparison difficult [9, 12, 13, 27]. Fourth, we lack information regarding the antibiotic exposition prior to ICU admission. However, keeping in mind that in the univariate analysis de-escalated patients had significantly shorter hospitalization duration before the initiation of the beta-lactam treatment and significantly less previous antibiotic exposure in the ICU, it is unlikely that prior antimicrobial consumption was higher in the de-escalated population. Finally, it is reasonable that different de-escalation strategies are not comparable with respect to patient outcome and impact on microbial ecology.
In conclusion, in our study population, de-escalation of anti-pseudomonal beta-lactam antibiotics, as performed by the replacement of antibiotic treatment by a more narrow-spectrum agent, was mainly driven by the presence of etiologic cultures. We did not observe a beneficial effect of de-escalation on the emergence of resistance. Consequently, we conclude that de-escalation should not be considered to be a safe strategy underpinning the unlimited empirical use of broad-spectrum therapy. Our results confirm the urgent need for a uniform definition on de-escalation and for future randomized controlled trials to determine the most optimal de-escalation strategy and, by extension, the most optimal antibiotic strategy for reducing overall antibiotic exposure and antimicrobial selection pressure.
References
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent J-L, Moreno R, The Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup (2013) Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 39:165–228
Dellit TH, Owens RC, McGowan JE, Gerding DN, Weinstein RA, Burke JP, Huskins WC, Paterson DL, Fishman NO, Carpenter CF, Brennan PJ, Billeter M, Hooton TM (2007) Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 44:159–177
Kollef MH (2001) Hospital-acquired pneumonia and de-escalation of antimicrobial treatment. Crit Care Med 29(7):1473–1475
Kollef MH (2001) Optimizing antibiotic therapy in the intensive care unit setting. Crit Care 5:189–195
Niederman MS (2006) De-escalation therapy in ventilator-associated pneumonia. Curr Opin Crit Care 12:452–457
Silva BNG, Andriolo RB, Atallah AN, Salomao R (2013) De-escalation of antimicrobial treatment for adults with sepsis, severe sepsis or septic shock (review). Cochrane Database Systematic Rev 3:CD007934 doi:10.1002/14651858
Tabah A, Cotta MO, Garnacho-Montero J, Schouten J, Roberts JA, Lipman J, Tacey M, Timsit JF, Leone M, Zahar JR, De Waele J, on behalf of the Working Group for Antimicrobial Use in the ICU (2015) A systematic review of the definitions, determinants and clinical outcomes of antimicrobial de-escalation in the intensive care unit. Clin Infect Dis. doi:10.1093/cid/civ1199
Garnacho-Montero J, Escoresca-Ortega A, Fernandez-Delgado E (2015) Antibiotic de-escalation in the ICU: how is it best done? Curr Opin Infect Dis 28:193–198
Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, Corcia-Palomo Y, Fernandez-Delgado E, Herrera-Melero I, Ortiz-Leyba C, Marquez-Vacaro JA (2014) De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med 40:32–40
Knaak E, Cavalieri SJ, Elsasser GN, Preheim LC, Gonitzke A, Destache CJ (2013) Does antibiotic de-escalation for nosocomial pneumonia impact intensive care unit length of stay? Infect Dis Clin Pract 21(3):172–176
Giantsou E, Liratzopoulos N, Efraimidou E, Panopoulou M, Alepopoulou E, Kartali-Ktenidou S, Manolas K (2007) De-escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate. Intensive Care Med 33:1533–1540
Gonzalez L, Cravoisy A, Barraud D, Conrad M, Nace L, Lemarié J, Bollaert P-E, Gibot S (2013) Factors influencing the implementation of antibiotic de-escalation and impact of this strategy in critically ill patients. Crit Care 17:R140
Mokart D, Slehofer G, Lambert J, Sannini A, Chow-Chine L, Brun J-P, Berger P, Duran S, Faucher M, Blanche J-L, Saillard C, Vey N, Leone M (2014) De-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: results of an observational study. Intensive Care Med 40:41–49
Leone M, Bechis C, Baumstarck K, Lefrant J-Y, Albanèse J, Jaber S, Lepape A, Constantin J-M, Papazian L, Bruder N, Allaouchiche B, Bézulier K, Antonini F, Textoris J, Martin C, for the AZUREA network investigators (2014) De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial. Intensive Care Med 40:1399–1408
Steurbaut K, Colpaert K, Gadeyne B, Depuydt P, Vosters P, Danneels C, Benoit D, Decruyenaere J, De Turck F (2012) COSARA: integrated service platform for infection surveillance and antibiotic management in the ICU. J Med Syst 36:3765–3775
De Bus L, Diet G, Gadeyne B, Leroux-Roels I, Claeys G, Steurbaut K, Benoit D, De Turck F, Decruyenaere J, Depuydt P (2014) Validity analysis of a unique infection surveillance system in the intensive care unit by analysis of a data warehouse built through a workflow-integrated software application. J Hosp Infect 87:159–164
De Bus L, Saerens L, Gadeyne B, Boelens J, Claeys G, De Waele JJ, Benoit DD, Decruyenaere J, Depuydt PO (2014) Development of antibiotic treatment algorithms based on local ecology and respiratory surveillance cultures to restrict the use of broad-spectrum antimicrobial drugs in the treatment of hospital-acquired pneumonia in the intensive care unit: a retrospective analysis. Crit Care 18:R152
Saag MS. Gilbert DN CH, Eliopoulos GM, Moellering RC (2012) The Sanford guide to antimicrobial therapy. 23rd edition of the Belgian/Luxembourg version 2012–2013. Antimicrobial Therapy Inc., Sperryville
Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281
R-GNOSIS (2011) Resistance in Gram-Negative Organisms: Studying Intervention Strategies. Available at: http://www.r-gnosis.eu/. Accessed 14 March 2016
Andersen PK, Abildstrom SZ, Rosthoj S (2002) Competing risk as a multi-state model. Stat Methods Med Res 11:203–215
Pepe M, Mori M (1993) Kaplan-Meier, marginal or conditional probability curves in summarizing competing risk failure time data? Stat Med 12(8):737–751
Satagopan JM, Ben-Porat L, Berwick M, Robson M, Kutler D, Auerbach AD (2004) A note on competing risks in survival data analysis. Br J Cancer 91(4):1229–1235
Gray RJ (1998) A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141–1154
(2015) R Foundation for Statistical Computing. R: a language and environment for statistical computing (version 3.2.2). Available at: http://www.R-project.org
cmprsk-package. Available at: https://cran.r-project.org/web/packages/cmprsk/cmprsk.pdf. Accessed 14 March 2016
Morel J, Casoetto J, Jospé R, Aubert G, Terrana R, Dumont A, Molliex S, Auboyer C (2010) De-escalation as part of a global strategy of empiric antibiotherapy management. A retrospective study in a medico-surgical intensive care unit. Crit Care 14(6):R225
De Waele JJ, Ravyts M, Depuydt P, Blot SI, Decruyenaere J, Vogelaers D (2010) De-escalation after empirical meropenem treatment in the intensive care unit: fiction or reality? J Crit Care 25:641–646
Álvarez-Lerma F, Alvarez B, Luque P, Ruiz F, Dominguez-Roldan J-M, Quintana E, Sanz-Rodriguez C, The ADANN Study Group (2006) Empiric broad-spectrum antibiotic therapy of nosocomial pneumonia in the intensive care unit: a prospective observational study. Crit Care 10:R78
McCullough AR, Rathboneb J, Parekh S, Hoffmann TC, Del Mar CB (2015) Not in my backyard: a systematic review of clinicians’ knowledge and beliefs about antibiotic resistance. J Antimicrob Chemother 70:2465–2473
Joung MK, Lee JA, Moon SY, Cheong HS, Joo EJ, Ha YE, Sohn KM, Chung SM, Suh GY, Chung DR, Song JH, Peck KR (2011) Impact of de-escalation therapy on clinical outcomes for intensive care unit-acquired pneumonia. Crit Care 15(2):R79
Armand-Lefèvre L, Angebault C, Barbier F, Hamelet E, Defrance G, Ruppé E, Bronchard R, Lepeule R, Lucet JC, El Mniai A, Wolff M, Montravers P, Plésiat P, Andremonta A (2013) Emergence of imipenem-resistant gram-negative bacilli in intestinal flora of intensive care patients. Antimicrob Agents Chemother 57(3):1488–1495
Carlier M, Roberts JA, Stove V, Verstraete AG, Lipman J, De Waele JJ (2015) A simulation study reveals lack of pharmacokinetic/pharmacodynamic target attainment in de-escalated antibiotic therapy in critically ill patients. Antimicrob Agents Chemother 59(8):4689–4694
Weiss E, Zahar JR, Lesprit P, Ruppe E, Leone M, Chastre J, Lucet JC, Paugam-Burtz C, Brun-Buisson C, Timsit JF, De-escalation study Group (2015) Elaboration of a consensual definition of de-escalation allowing a ranking of beta-lactams. Clin Microbiol Infect 21(7):649.e1–649.e10
Madaras-Kelly K, Jones M, Remington R, Hill N, Huttner B, Samore M (2014) Development of an antibiotic spectrum score based on veterans affairs culture and susceptibility data for the purpose of measuring antibiotic de-escalation: a modified Delphi approach. Infect Control Hosp Epidemiol 35(9):1103–1113
Acknowledgments
This research project is funded by the IWT (Institute for the Promotion of Innovation through Science and Technology in Flanders) (project IWT–TBM COSARA–project number 060517). LDB received a Clinical Research Grant from Ghent University Hospital, Belgium (project number KW/1394/INT/001/001). JDW is a senior Clinical Investigator with the Research Foundation Flanders (FWO).
Authors' contributions
LDB and PD conceived the study, participated in its design and coordination, analyzed the data, and drafted the manuscript; WD, JC, LDB, KV, and BG performed data acquisition and analyses; WD, JC, BG, KV, JB, GC, JDW, and JD critically revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Take-home message: The results of this study do not confirm the expected favorable effect of de-escalation of anti-pseudomonal beta-lactam antibiotic treatment on the selection of antimicrobial resistance. De-escalation should therefore not be considered to be a safe strategy underpinning an unlimited empirical use of broad-spectrum combination therapy. Future research to determine the most optimal de-escalation strategy and by extension the most optimal antibiotic strategy reducing overall antibiotic exposure and antimicrobial selection pressure is essential.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
De Bus, L., Denys, W., Catteeuw, J. et al. Impact of de-escalation of beta-lactam antibiotics on the emergence of antibiotic resistance in ICU patients: a retrospective observational study. Intensive Care Med 42, 1029–1039 (2016). https://doi.org/10.1007/s00134-016-4301-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-016-4301-z