Abstract
Objective
To determine the effectiveness of blood component therapy in the correction of trauma-induced coagulopathy during hemorrhage.
Background
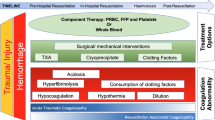
Severe hemorrhage remains a leading cause of mortality in trauma. Damage control resuscitation strategies target trauma-induced coagulopathy (TIC) with the early delivery of high-dose blood components such as fresh frozen plasma (FFP) and platelet transfusions. However, the ability of these products to correct TIC during hemorrhage and resuscitation is unknown.
Methods
This was an international prospective cohort study of bleeding trauma patients at three major trauma centers. A blood sample was drawn immediately on arrival and after 4, 8 and 12 packed red blood cell (PRBC) transfusions. FFP, platelet and cryoprecipitate use was recorded during these intervals. Samples were analyzed for functional coagulation and procoagulant factor levels.
Results
One hundred six patients who received at least four PRBC units were included. Thirty-four patients (32 %) required a massive transfusion. On admission 40 % of patients were coagulopathic (ROTEM CA5 ≤ 35 mm). This increased to 58 % after four PRBCs and 81 % after eight PRBCs. On average all functional coagulation parameters and procoagulant factor concentrations deteriorated during hemorrhage. There was no clear benefit to high-dose FFP therapy in any parameter. Only combined high-dose FFP, cryoprecipitate and platelet therapy with a high total fibrinogen load appeared to produce a consistent improvement in coagulation.
Conclusions
Damage control resuscitation with standard doses of blood components did not consistently correct trauma-induced coagulopathy during hemorrhage. There is an important opportunity to improve TIC management during damage control resuscitation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe hemorrhage remains a leading cause of mortality in trauma despite improvements in our understanding of trauma-induced coagulopathy (TIC) and the adoption of the principles of damage control resuscitation [1–3]. These strategies are thought to improve outcome by promoting hemostasis through the early correction of coagulopathy [4]. Blood component therapy is the mainstay of hemostatic resuscitation, with high-dose plasma and platelet transfusions recommended alongside red cell transfusions during acute hemorrhage. However the efficacy of these blood components, and damage control resuscitation overall, to correct TIC during bleeding is unknown.
Most studies supporting the use of high-dose blood components in trauma hemorrhage have retrospectively associated their use with survival, with the assumption that this is due to improved hemostasis during resuscitation [5, 6]. However, observed survival benefits may be attributable to other factors, such as differential rates of bleeding and delayed provision of products, avoidance of crystalloid dilution or other unknown potential biases [7–10]. In this same study population, our group has shown that physiological and hemostatic markers are not maintained during hemorrhage and damage control resuscitation [11, 12]. In a previous pilot study we also examined the ability of plasma to correct functional coagulation tests but could not identify an association showing that high-dose plasma conferred a hemostatic benefit during bleeding [7]. This study was small and therefore limited to the examination of one blood component only. To date there have been no further prospective studies of the efficacy of type, dose or combinations of blood component therapies in correcting trauma-induced coagulopathy during hemorrhage and damage control resuscitation.
The overall objective of this study was therefore to characterize the effect of damage control resuscitation and blood component therapy in correcting TIC during the acute phase of trauma hemorrhage. The specific aims were to characterize the incidence and severity of TIC during the acute phase of damage control resuscitation, to describe the effect of individual blood components on TIC and to examine the effect of increasing doses of FFP and fibrinogen on the correction of TIC. We conducted a prospective cohort study of bleeding trauma patients presenting directly to three major trauma centers.
Methods
Study design
Adult trauma patients who met the local criteria for trauma team activation were eligible for enrollment in the prospective activation of coagulation and inflammation in trauma (ACIT) observational study. ACIT is being conducted at trauma centers that are members of the International Trauma Research Network (INTRN). Subjects were enrolled between January 2009 and April 2013. At this time, there were three active study sites with data-sharing protocols, and recruitment was limited to times when research personnel were present.
The ACIT protocol samples blood immediately on arrival of the patient in the emergency department (ED). Patients who continue to bleed have further samples drawn after 4, 8 and 12 PRBC units have been administered during the acute bleeding phase.
Patient selection
All adult trauma patients requiring trauma team activation were eligible for inclusion. Criteria for trauma team activation were similar across all three sites and included abnormal physiology (Glasgow Coma Scale score <14, respiratory rate <10 or <29, systolic BP <90), anatomical injury (chest trauma with any altered physiology, suspected pelvic fracture, suspected open or depressed skull fracture, amputation proximal to the wrist or ankle, penetrating trauma), high-energy mechanism of injury (person hit by a train, person ejected from a vehicle or fatality in the same vehicle as the occupant, a person trapped under a vehicle, fall from >2 m, explosions, industrial accidents).
Exclusion criteria included arrival in the ED more than 2 h following injury, transfer from another hospital, pregnancy and burns greater than 5 % of the total body surface area. Patients were retrospectively excluded if they declined to give consent for the research study, were receiving anticoagulant medications (not including aspirin), had moderate or severe liver disease or a known bleeding diathesis. This study reports on the subset of ACIT patients who were bleeding and received four or more units of PRBCs during the acute hemorrhage phase.
The majority of included patients were unable to provide informed consent at the time of enrollment, and consent was therefore obtained from the trauma team leader (a physician independent of the research study) who acted as the patient’s legally authorized representative (LAR). Written consent from the patient or next of kin was obtained as soon after enrollment as appropriate. The study was reviewed and approved by the respective National Research Ethics Committees.
Major hemorrhage protocols
All hospitals adhered to the principles of damage control resuscitation—early damage control surgery, permissive hypotension, limited crystalloid or colloid administration, and targeting TIC through high-dose blood product administration. Activation of the major hemorrhage protocols at all institutions provided immediate access to emergency PRBC units. Plasma was subsequently provided with PRBC aiming for a 2:3 FFP:PRBC ratio in two centers and a 1:1 (Octaplas:PRBC) ratio in one center. In two centers, platelets and cryoprecipitate are administered with the second transfusion pack (with four further units of FFP). In the third center platelets are administered with every 5 U of PRBC from the second transfusion pack and monitored according to conventional coagulation tests. In all centers resuscitation continues with these multicomponent packs until bleeding is controlled. The estimated expected fibrinogen doses transfused in each product are PLT 0.4 g, FFP/Octaplas 0.9 g and cryoprecipitate 2 g for each pool/unit transfused [13].
Sampling technique
An initial 30 ml research blood sample was drawn along with the standard trauma laboratory tests (peripheral blood count, clotting screen and arterial blood gas) within 20 min of arrival to the ED. For those patients with acute active bleeding that required immediate transfusion as part of damage control resuscitation, further blood samples were taken after the 4th, 8th and 12th unit of PRBC had been administered.
Sample analysis
ROTEM samples were processed using a ROTEM delta instrument (TEM International GmbH, Munich, Germany) at 37 °C. The methodology and parameters of ROTEM have been described previously [14]. Two separate ROTEM assays were performed for each patient: first, EXTEM measuring tissue factor initiated clotting; second, FIBTEM with the addition of cytochalasin D, a platelet inhibitor. All pipetting steps and mixing of the reagents with samples were performed with an automated electronic pipette program. Clotting time (CT), clot amplitude at 5 min (CA5), alpha angle (α) and maximum clot firmness (MCF EXTEM/FIBTEM) were reported for each sample analyzed.
Coagulation factor and fibrinogen level analysis
Fresh blood samples were prepared to extract double-spun plasma for analysis and PT, PTT and fibrinogen levels (Clauss method) measured by the laboratory staff in the central laboratory. Frozen aliquots were thawed using a water bath at 37 °C prior to analysis using the Sysmex CS2100i automated coagulation analyzer; the prothrombin ratio (PTr) was calculated as observed PT divided by the mean control PT.
Definitions
We defined coagulopathy on the ROTEM [14] as a 5-min EXTEM clot amplitude (CA) ≤35 mm. This has been shown to identify ATC and predicts transfusion requirements [15]. We also analyzed samples with a prolonged PT ratio (>1.2), which we have previously shown to be a clinically relevant diagnostic threshold for this measure [16]. We defined minor injury as a patient who had an injury severity score (ISS) [17] ≤4. These patients from our ACIT study were used as a baseline comparator as previously described and are referred to in the results [18].
Data collection and analysis
Data were collected prospectively on patient demographics, time of injury, mechanism (blunt or penetrating), prehospital fluid administration, time of arrival in the ED, baseline vital signs and total transfusion requirements in the first 12 h of admission. The amount of FFP, PLT and CRYO was recorded after each 4 U of PRBCs transfused. Ratios of FFP:PRBC, PLT:PRBC and CRYO:PRBC were then calculated for each interval. In addition, the total dose of fibrinogen was calculated using the above estimated constituent values. Coagulation changes for each 4-U PRBC interval were calculated as: (post-interval sample value − pre-interval sample value) for each individual interval, hence adjusting for any bias due to different numbers within intervals. The 4th-unit PRBC data included patients who went on to receive 8 and 12 U of PRBC. Similarly, 8-U data include patients who received 8 in addition to those who went on to receive 12 U. Statistical analysis was performed using GraphPad PRISM v5 (GraphPad Software Inc., San Diego, CA, USA) and Microsoft Excel 2003 (Microsoft, Inc., Redmond, WA, USA). Normal-quantile plots were used to test for normal distribution. Parametric data are expressed as mean ± 95 % confidence intervals (CIs). Nonparametric data are given as median (interquartile range, IQR). A p < 0.05 was chosen to represent statistical significance throughout.
Results
A total of 810 patients were included in the ACIT study over the 52-month period. Forty-nine patients were retrospectively excluded, 28 after personal or legal representative consent was declined following enrollment and 21 for retrospective exclusion criteria. Of the remaining 761 subjects, 106 were transfused with four or more PRBCs. Clinical characteristics, admission physiology and laboratory parameters are detailed in Table 1. The majority of the bleeding patients were severely injured (94 % ISS > 15) and had sustained blunt injury (87 %). Over 40 % were coagulopathic on admission by CA5 or PTr. On average patients required 8 U of PRBCs with 38 % requiring a massive transfusion of ten or more PRBCs.
In total, there were 181 4-U PRBC intervals available for analysis (ESM Table 1). Few blood components other than PRBC were delivered in the first 4-U window as emergency blood is delivered from stocks held in emergency departments. On average, patients received 2 U of FFP with the first 4 U of PRBC. Patients received significantly more of all components in subsequent 4-U intervals—on average (mean) 3.4 U of FFP, 0.5 pools of platelets and 0.8 pools of cryoprecipitate, equivalent to 4.4 g of fibrinogen (ESM Table 1).
Forty percent of bleeding patients were coagulopathic upon admission (Table 1). This proportion rose to 58 % after 4 PRBC units and 81 % after 8 U (Fig. 1a). Similarly, more patients developed a prolonged PTr during hemorrhage despite damage control resuscitation and high plasma ratios (Fig. 1b; ESM Table 1). All functional clotting parameters deteriorated during hemorrhage with the most significant changes seen in clot generation and clot firmness (Fig. 1c–f). Regardless of overall transfusion requirements, there was a worsening in clotting parameters throughout the acute bleeding episode. Damage control resuscitation protocols were not associated with correction or improvement of TIC during the active hemorrhage.
The evolution of TIC during hemorrhage. Bars are mean with 95 % CI. + signifies comparison of admission vs. minor injury. Minor injury are those patients with an ISS ≤4 from the ACIT II cohort. *p < 0.05 signifies comparison to admission only. Minor injury values and upper/lower threshold for normal (dotted line) ROTEM values for reference. a Incidence of coagulopathy (CA5 ≤35) during trauma hemorrhage. b Proportion of patients with PT(r) >1.2 during trauma hemorrhage. c Mean CT on ExTEM. d Mean alpha angle on ExTEM. e Mean MCF on ExTEM. f Mean MCF on FibTEM
There was little discernable effect on clotting function regardless of the type of blood component administered. The mean dose of FFP administered per interval (3.2 U) had no effect on clotting time, clot generation or maximum clot firmness (Fig. 2a–d). Adding cryoprecipitate (average 1.6 pools per interval) and platelets (average 1 pool per interval) showed some protection of clotting function across the interval, but only an improvement in CT time was significant (+7.6 vs. −19.2 s, p < 0.05). There were very few intervals where platelets or cryoprecipitate was transfused in isolation so we could not identify the individual effect of these products. Component therapy in average doses appears to have little effect on the functional clotting profile during acute hemorrhage.
Effect of blood component therapy on coagulopathy when administered during damage control resuscitation. Bars are mean interval change in ROTEM parameters with 95 % CI. a Interval change in CT on ExTEM. b Interval change in alpha angle on ExTEM. c Interval change in MCF on ExTEM. d Interval change in MCF on FibTEM
There was sufficient variation in FFP administered between intervals to allow analysis of the effect of the FFP dose on coagulation function. Overall less than 10 % of coagulopathic patients had their coagulopathy corrected at the end of the 4-U interval, regardless of the amount of FFP administered (Fig. 3a). Increasing doses of FFP also did not prevent patients from becoming coagulopathic, with 56 % of intervals that started with CA5 >35 mm becoming coagulopathic with 1:1 FFP:PRBC administration compared to 35 % with 1:4 ratios (Fig. 3a). Increasing doses of FFP had a slightly better effect on the PTr, with 60 % of intervals that started with a PTr >1.2 normalizing with a 1:1 ratio compared to less than 20 % with a 1:4 ratio (Fig. 3b). However, on average all parameters of coagulation function deteriorated in all intervals regardless of the dose of FFP administered (Fig. 3c–f). FFP in PRBC ratios of 1:2 or higher generally preserved levels of fibrinogen and procoagulant coagulation factors II, V, IX, X, XI and XIII. However the difference in these factor levels between low and high FFP ratios was small, varying by only 5–10 % of baseline (ESM Table 2).
The effect of increasing doses of FFP on TIC. Bars are mean change in ROTEM parameters with 95 % CI. a The effect of increasing FFP:PRBC on the proportion of trauma patients with coagulopathy corrected (CA5 ≥ 35) and coagulopathy worsened (CA5 ≤ 35). b The effect of increasing FFP:PRBC compared to the proportion of patients with corrected PT(r) and worsening PT(r). c Mean CT on ExTEM. d Mean alpha angle on ExTEM. e Mean MCF on ExTEM. f Mean MCF on FibTEM. There was no difference between the FFP:PRBC ratio groups in any measure of coagulation response
There were moderately more consistent findings for the effect of total dose of fibrinogen administered within the blood components. Fibrinogen doses of 6 g or more achieved correction of CA5 and PTr in 36 % and 29 % of patients, respectively (Fig. 4a, b). There were statistically significant improvements in functional clotting parameters when 6 g or more of fibrinogen was administered within a 4-U PRBC interval (Fig. 4c–f). However, 57 % of intervals that started with normal functional clotting developed coagulopathy despite this dose of fibrinogen (Fig. 4a). Although there was wide variability in the fibrinogen response, high-dose replacement had the most consistent improvements in functional clot measures during hemorrhage.
Discussion
In this international multicenter clinical study, we prospectively examined the effects of blood component therapy in the actively bleeding trauma patient. A large proportion of trauma patients was coagulopathic upon arrival, and more developed TIC during hemorrhage despite modern damage control resuscitation. High-dose blood component therapy was generally able to maintain coagulation parameters but was not associated with correction of TIC. Only at the highest dose combinations of products was a consistent, although small, effect observed.
Despite the widespread use of blood components to treat TIC, their effects on the coagulation profile when administered during damage control resuscitation remain unknown. Previous studies have investigated the effect of varying FFP:PRBC on outcomes, but there has been conflicting evidence regarding the use of high ratios. High-dose plasma and platelets have both been associated with improved outcomes [17], but our previous pilot study found that increasing ratios of FFP:PRBC had a variable effect on the coagulation profile during hemorrhage [7]. In this study, we have shown that there was no consistent correction of any measure of clot function nor any large increases in the procoagulant factor level when FFP was delivered during the acute phase of on-going bleeding. Furthermore, FFP:PRBC ratios above 1:2 were not associated with any major hemostatic benefit.
Normal hemostasis is critically dependent on fibrinogen as a substrate for clot formation. The fibrinogen level is decreased in the severely injured bleeding patient on admission and is associated with poor outcomes [18]. Our group has previously shown that fibrinogen depletion occurs in TIC and progresses during trauma hemorrhage [18]. Fibrinogen levels do not normalize during damage control resuscitation despite the provision of high ratios of plasma and platelets. There was a small but inconsistent response to high doses of fibrinogen in the current study. This may be because of the volume of blood components that have to be administered to achieve such high doses in a standard damage control resuscitation protocol. For example, 4 U of FFP, one pool of platelets and one pool of cryoprecipitate would provide 6 g of fibrinogen, but diluted into a volume of approximately 3 l (when given with 4 U of PRBCs). It is possible that more concentrated delivery may be more effective, and clinical trials of early high-dose cryoprecipitate therapy and fibrinogen concentrate are underway.
There are several limitations to this study. First, this was an observational study, so results can only show the effect of blood components as administered by the MHPs in our institutions. Patients received different blood components across the different PRBC intervals and were more likely to receive 1:1 FFP:PRBC ratio between PRBC units 8 and 12. These interval cohorts may be influenced by confounding from our transfusion protocols and practices, rate of blood loss or early hemorrhage control. The effect of immediate balanced transfusion therapy with plasma, platelets and RBCs was not evaluated in the present study. Randomized control trials of plasma and other blood component therapies in TIC are highly warranted [19]. While we aimed to reduce the impact of survival bias in this study, there may still be differences in these parameters that may have influenced the observed efficacy of the component therapies. Furthermore, patients that bleed at a higher rate are more likely to have worse coagulation profiles during resuscitation. Precise timing of hemorrhage control is difficult to ascertain; therefore, we used completion of PRBC transfusion as a surrogate measure for control of bleeding. We did not examine the fibrinolytic component of TIC in this study, although from 2010 and the publication of the CRASH-2 trial, major hemorrhage protocols at all sites specified early administration of tranexamic acid. Finally, 28 patients were impossible to sample as the high rate of transfusion meant we were unable to sample every 4 U of PRBCs; this is clearly an important group of patients who we were not able to study.
The purpose of this study was to address the knowledge gap that exists regarding the efficacy of blood component therapy on TIC during active bleeding. We have shown that severely injured trauma patients were coagulopathic on arrival to the ED and that coagulopathy worsened during hemorrhage and damage control resuscitation. Early replacement of increasing doses of plasma and average doses of other blood component therapy had little effect on the deranged ROTEM coagulation parameters or coagulation factor concentration during the acute phase of care. Better understanding of the pathogenesis of TIC is required to delineate the limitations of current damage control resuscitation strategies in the treatment of major trauma hemorrhage and identification of novel therapeutic targets. There is an important opportunity to improve the efficacy of damage control resuscitation by improved management of TIC during hemorrhage.
References
Holcomb JB, Del Junco DJ, Fox EE et al (2012) The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. Arch Surg 15:1–10
Holcomb JB, Jenkins D, Rhee P et al (2007) Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma 62(2):307–310
Schöchl H, Maegele M, Solomon C et al (2012) Early and individualized goal-directed therapy for trauma-induced coagulopathy. Scand J Trauma Resusc Emerg Med 20(1):15
Zink KA, Sambasivan CN, Holcomb JB et al (2009) A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. Am J Surg 197(5):565–570
Borgman MA, Spinella PC, Perkins JG et al (2007) The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma 63(4):805–813
Morrison JJ, Ross JD, Poon H et al (2013) Intra-operative correction of acidosis, coagulopathy and hypothermia in combat casualties with severe haemorrhagic shock. Anaesthesia 68:846–850
Davenport R, Curry N, Manson J et al (2011) Hemostatic effects of fresh frozen plasma may be maximal at red cell ratios of 1:2. J Trauma Acute Care Surg 70(1):90–96
Snyder CW, Weinberg JA, McGwin G Jr et al (2009) The relationship of blood product ratio to mortality: survival benefit or survival bias? J Trauma 66:358–362
Geeraedts LM Jr, Demiral H, Schaap NP et al (2007) ‘Blind’ transfusion of blood products in exsanguinating trauma patients. Resuscitation 73:382–388
Cotton BA, Reddy N, Hatch QM et al (2011) Damage control resuscitation is associated with a reduction in resuscitation volumes and improvement in survival in 390 damage control laparotomy patients. Ann Surg 254(4):598–605
Rourke C, Curry N, Khan S et al (2012) Fibrinogen levels during trauma hemorrhage, response to replacement therapy, and association with patient outcomes. J Thromb Haemost 10(7):1342–1351
Khan S, Brohi K, Chana M et al (2014) Hemostatic resuscitation is neither hemostatic nor resuscitative in trauma hemorrhage. J Trauma Acute Care Surg 76(3):561–568
NHSBT,UK Average Standardised Doses
Ganter MT, Hofer CK (2008) Coagulation monitoring: current techniques and clinical use of viscoelastic point-of-care coagulation devices. Anesth Analg 106:1366–1375
Davenport R, Manson J, De’Ath H et al (2011) Functional definition and characterization of acute traumatic coagulopathy. Crit Care Med 39(12):2652–2658
Frith D, Goslings JC, Gaarder C et al (2011) Definition and drivers of acute traumatic coagulopathy: clinical and experimental investigations. J Thromb Haemost 8(9):1919–1925
Baker SP, O’Neill B, Haddon W Jr et al (1974) The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma 14(3):187–196
Holcomb J, Del Junco D, Fox E et al (2013) Resuscitation strategies in trauma. JAMA 309(21):2270–2271
Holcomb J (2014) Pragmatic randomized optimal platelets and plasma ratios (PROPPR). Injury 45(9):1287–1295
Acknowledgments
Funded in part by the National Institute for Health Research (UK) Program Grant for Applied Research (RP-PG-0407-10036).
Conflicts of interest
TEM Innovations (ROTEM): unrestricted support in the form of equipment and reagents for the ACIT study. SK and RD have received honoraria as invited speakers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Take home message: Severe hemorrhage remains a leading cause of mortality in trauma despite improvements in our understanding of trauma-induced coagulopathy. Our findings from an international prospective cohort study of severely injured bleeding trauma patients at three major trauma centers indicate that damage control resuscitation with standard doses of blood components does not consistently correct trauma-induced coagulopathy during hemorrhage.
On behalf of the International Trauma Research Network (INTRN).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khan, S., Davenport, R., Raza, I. et al. Damage control resuscitation using blood component therapy in standard doses has a limited effect on coagulopathy during trauma hemorrhage. Intensive Care Med 41, 239–247 (2015). https://doi.org/10.1007/s00134-014-3584-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-014-3584-1