Abstract
An accurate analytical method was developed to determine selected per- and polyfluorinated alkyl substances (PFAS) at the level of parts per trillion (ppt or ng/L) in drinking water. The method included a concentration step using solid phase extraction (SPE) approach in combination with a liquid chromatography-tandem mass spectrometry system (LC-MS/MS). This method was optimized and validated for the common PFAS contaminants in drinking water. An initial demonstration of capability was established with an acceptable initial calibration, minimum reporting limit (MRL), limit of detection (LOD), initial demonstration of low system background, and initial demonstration of precision (IDP). Isotopically labeled internal standards were used for quantification. Surrogate standards were used to monitor method performance. The current method will help in better understanding of PFAS crisis by providing an efficient measurement of PFAS in water. In this study, the recoveries of four surrogates were between 84 and 113%, and calculated limit of detection (DL) and minimum reporting limits (MRL) were generally 1.0–3.0 and 5–10 ng/L, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Per- and polyfluoroalkyl substances (known as PFAS) are synthetic organic substances in which the hydrogen atoms are completely or partially substituted by fluorine atoms (Buck et al. 2011; Wang et al. 2022). PFAS are widely used, long-lasting chemicals and found at various levels in water, air, fish and meat, crops, and soil as well as in the blood of people and animals over the world. PFAS substances are stable and persistent pollutants that can be hardly degraded because of the strong carbon-fluorine bond (Pan et al. 2016; Podder et al. 2021; Wang et al. 2022). PFAS are also known as “forever chemicals” (Kempisty et al., 2021). Because of the highly valued properties of water-resistant, oil-resistant, and heat-resistant, since the 1950s, PFAS have been used in a large number of industrial applications and consumer products such as non-stick coating, surfactants, food-packaging materials, aqueous film forming foam (AFFF), kitchenware, cleaning products (Chu et al. 2016; Jurikova et al. 2022; Place et al., 2012; Schaider et al. 2017; Tokranov et al. 2019; Trier et al. 2011; Wang et al. 2017; Y.-Q. Wang et al. 2022; Young et al. 2022). Many PFAS substances are released from industrial sources, agricultural sites, and consumer products into the environment, and have the potential to accumulate in food chains (Ahrens et al., 2014; Jurikova et al. 2022; Müller et al. 2011; Pérez et al. 2013). PFAS production sites are major point sources of various water bodies, including surface water, groundwater, drinking water, and wastewater contamination in the United States and in other countries (Babayev et al. 2022; Houtz et al. 2016; Pan et al. 2016; Xu et al. 2021). It was reported that PFAS have been associated with adverse effects on human growth, implicated effects on birth weight, carcinogenesis, early menopause, fertility disorders, and thyroid malfunction (Crone et al. 2019). The use of perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) has been significantly reduced in the U.S. since the manufacturers stopped production in 2006 (Brennan et al. 2021). However, the high stability of PFOA and PFOS makes their persistence in the environment for an extended period of time and it becomes an ongoing issue (Hernandez et al. 2022). Analytical methodology for the monitoring and determination of PFAS substances is important to support environmental fate studies, enhance environmental regulation and promote contamination remediation (Jia et al. 2022). Solid-phase extraction (SPE) is a commonly used technique for PFAS sample preparation, including SPE cartridge extraction, solid-phase microextraction (SPME), and dispersive solid-phase extraction (DSPE) that can be used to concentrate PFAS prior to instrumental analysis (Jia et al. 2022; Lorenzo et al. 2018; Tröger et al. 2018). SPE cartridge extraction is one of the most popular techniques for sample preparation (Jia et al. 2022; Taniyasu et al. 2022). For example, an inter-laboratory trial was performed to validate ISO 21,675 method for the measurement of PFAS in water samples using the solid phase extraction method and LC-MS/MS (Taniyasu et al. 2022). A total of 27 laboratories from 11 countries worked on the same PFAS analytical method on river water, seawater, and wastewater (Taniyasu et al. 2022). Another recent study reported that further extract clean-up using weak anion exchange SPE (WAX SPE) did not seem to be necessary because it readily led to lower fortification recoveries and thus resulted in lower precisions and higher LODs (Groffen et al. 2021). Most PFAS contaminants existing in the environments are at low concentrations. The objective of the present study aimed to develop an analytical method for the accurate determination of 16 PFAS at part per trillion (ppt or ng/L) levels in drinking water.
Materials and Methods
The organic solvent (methanol) used in the study was LC-MS grade (Thermo Fisher Scientific, Waltham, MA, USA) and the water was reagent water of LC-MS grade (VWR, USA). They were tested free for PFAS contamination before use. Ammonium acetate optima and acetic acid optima (1 mL glass ampoule) were of LC-MS grade and they were purchased from Thermo Fisher Scientific (Waltham, MA, USA). An ammonium acetate solution (100 mM) was prepared by dissolving 770 mg of ammonium acetate in 100 mL of the LC-MS grade reagent water. TRIZMA preset crystals were purchased from Fisher Scientific (Waltham, MA, USA). Trizma was used as a buffer to maintain the pH of the tested water near 7.0 at 25 °C and also to remove free chlorine if present. Argon was used as collision gas in MS/MS instruments. PFAS analytical standards were purchased from Wellington Laboratories (Ontario, Canada). PFAS analytical standards were received in multiple ampules A mixture of twenty-seven (27) analytical standards of native PFAS and isotopically labeled analogs covering a range of 5 compound classes were acquired at high purity (> 98%). A list of the native PFAS and isotopically labeled internal standards properties is presented in Table 1.
PFAS Primary Dilution Standards (250 ng/mL) were prepared by dilution of stock standard (2000 ng/mL) 1:8 with 96% MeOH. This standard was used to prepare calibration standards. This method used four (4) surrogate compounds listed in Table 2. These surrogate analytes were defined as pure chemicals (i.e., isotopically labeled PFAS compounds) which were chemically similar to but different from the tested PFAS substances. These chemicals were added to each sample with a known amount (20 µL of Surrogate Primary Dilution Standards) before processing and were measured using the same analytical method. The purpose of using selected surrogates was to monitor method performance from the beginning of extraction to the completion of analysis. Surrogate Primary Dilution Standards were prepared by dilution of surrogate standard stock solution 1:10 with 96% MeOH.
The current method used three isotopically labeled internal standard compounds (13C2-PFOA, 13C4-PFOS, and d3-NMeFOSAA) with stock solution concentrations of 1, 3, and 4 µg/mL, respectively. Internal Standard Primary Dilution Standards were prepared by dilution The Internal Standards stock solution (from Wellington) 1:10 with 96% MeOH. 20 µL Internal Standard Primary Dilution Standards were added to each sample before analysis.
Sample Collection and Preparation
Sample collection of tap water samples was performed at Michigan State University, East Lansing, Michigan. Samples of drinking water were collected in polypropylene bottles fitted with a polypropylene screw cap. The bottles were rinsed twice with 20 mL of methanol and twice with 20 mL of reagent water and air-dried prior to use. Each polypropylene bottle contained 1.25 g of Trizma as a preservative. A minimum of 250 mL of water samples was collected. The samples were kept in a refrigerator at 4 °C until the extraction.
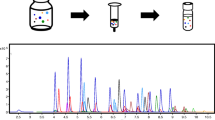
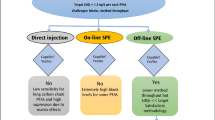
Fortification samples at levels 5, 10, 16, and 80 ng/L were prepared by spiking reagent water with known amounts of PFAS. Before sample extraction, all samples were fortified with surrogates. The extraction was performed by well mixing the sample and passing the sample through an SPE cartridge containing polystyrene divinylbenzene (PSDVB). Solid phase extraction (SPE) was performed using a vacuum manifold (Fig. 1). The SPE steps are as follows: eluate 3–5 mL of methanol, 3–5 mL of water, 250 mL of the sample, and 2 × 7.5 mL of reagent water (to rinse the polypropylene bottle) and dropwise pass them through the SPE cartridge column under the vacuum. The flow rate was controlled to be 2–4 drops per second. Do not let the SPE column go dry. Finally, the PFAS compounds were eluted from the SPE sorbent with 4 mL of methanol by gravity. The extract was concentrated to dryness on an E-NVAP under a stream of nitrogen (water bath 60℃). Adjust the final volume to 1.00 mL volume with methanol:water (96:4, vol/vol). A known amount of the internal standards was added before sample analysis.
LC-MS/MS analysis was carried out using a Thermo Scientific Vanquish UHPLC system that included PFAS Upgrade Kit (Vanquish Flex Binary) in combination with TSQ Altis Triple-Stage quadrupole mass spectrometer. The mass spectrometry system was operated in a negative ionization mode and the separation of PFAS analytes was performed using an Acclaim RSLC 120 C18 column (2.1 × 100 mm, 2.2 μm). During the current study, the separation of Accucore RP-MS column (2.1 × 100 mm, 2.1 μm) was evaluated as well. The analytical LC Column (Acclaim RSLC 120 C18) provided higher retention times for PFAS analytes than the Accucore RP-MS column. Both columns gave similar results. A comparison of the chromatograms is given in Fig. 2.
A delay LC column (Hypersil Gold 3.0 × 50 mm, 1.9 μm) was used to separate the target PFAS from interferences from the LC system. The separation of the PFAS compounds took place within a 20-min gradient elution program (Table 3) using gradient A (water, containing 2 mM ammonium acetate and 2% MeOH and 0.1% acetic acid) and gradient B (methanol, containing 2 mM ammonium acetate and 2% H2O and 0.1% acetic acid) as mobile phase.
The flow rate was 400 µL/min. Injection volume 5 µL. Sample compartment set at 10 °C. MS/MS method conditions/parameters of HESI-MS were: negative polarity, spray voltage 1500 V, sheath gas 50 (Arb), Aux gas 12 sweep gas 0.5, ion transer tube temp. 250 °C, vaporizer temp. 225 °C, desolvation heated nitrogen gas. The Selective Reaction Monitoring (SRM, also known as MRM) transition of quantitation are given in Table 4. A second set of SRMs are monitored to ensure/confirm the identities of PFAS. The confirmative SRMs are not listed in Table 4.
Results and Discussion
Initial demonstration of capability quality control requirements was established (Shoemaker et al., 2018). An acceptable initial calibration was obtained by performing ESI-MS/MS tune. That included running the check mass calibration and the Electron Multiplier Gain to maintain mass accuracy and mass resolution of the instrument. Obtaining stable stability was obtained prior to performing mass calibration. Compound optimization was performed by infusing approximately 250 ng/mL of each analyte directly into the MS/MS at flow rate of 0.4 mL/min. LC operating parameters were established to optimize resolution and peak shape. A set of ten calibration standards were prepared as described in Table 5. The concentration of the lowest calibration standard was below the minimum reporting limit (MRL). MRL was defined as a minimum concentration of quantitation that can be reported for the analyte of interest in the sample following the analytical method. This concentration can only be used if acceptable quality control parameters (QC criteria) are met.
The LC-MS/MS system was calibrated using the internal standard (IStd). The software of LC-MS/MS data system was Thermo Chromeleon which was used to generate a linear regression calibration curve for each of the PFAS analytes. The initial calibration was validated by calculating the concentration ratio of each PFAS analyte to the pre-set IStd as an unknown against its regression equation. For the calibration levels that are less than the minimum reporting limit (MRL), the results for each analyte were within ± 50% of the true values. All other points of the calibration were within ± 30% of their true values.
On Agust 3, 2020, the State of Michigan officially passed laws regarding maximum contaminant levels (MCLs) for seven different types of per- and polyfluoroalkyl substances (hexafluoropropylene oxide dimer acid (HFPO-DA), perfluorobutane sulfonic acid (PFBS), perfluorohexane sulfonic acid (PFHxS), perfluorohexanoic acid (PFHxA), perfluorononanoic acid (PFNA), perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA)) with MCL’s values 370, 420, 51, 400,000, 6, 16 and 8 ng/L respectively. For this reason, two values (5 and 10 ng/L) were selected and tested to be the minimum reporting limit (MRL). MRL confirmation was established by spiking, extracting, and analyzing seven replicates LFBs (Shoemaker et al., 2018).
The mean measured concentration and standard deviation for these replicates were calculated. Half Range for the prediction interval of results (HRPIR) was calculated by.
HRPIR = 3.963 s.
where s is the standard deviation, 3.963 is a constant value for seven replicates.
Prediction Interval of Result (PIP) is calculated by.
PIR = Mean + HRPIR.
The requirements for upper and lower limits for PIP are:
-
Upper PIR Limit ≤ 150% recovery.
-
(Mean + HRPIR)/ (Fortified Concentration) × 100% ≤150%.
-
Lower PIR Limit ≥ 50% recovery.
-
(Mean-HRPIR)/ (Fortified Concentration) × 100% ≥50%.
The minimum reporting limit (MRL) is considered validated if both the Upper and Lower PIR Limits meet the criteria described above.
The results showed that 5 ng/L of the spike level was validated as MRL for the following PFAS substances: PFHxA, PFHpA, ADONA, PFOA, PFNA, PFTrDA, 9-Cl-PF3ONS, NmeFOSAA, and PFTA (Table 6). Furthermore, 10 ng/L of the spike level was validated to be a minimum reporting limit for the following PFAS compounds: PFBS, HFPO-DA-CO2, 9-Cl-PF3ONS, PFDA, PFUnA, 11Cl-PF3OUdS, PFDoA, PFHxS, PFOS and NEtFOSAA (Table 6). Detection limit (DL) can be estimated by fortifying, extracting, and analyzing seven replicate LFBs at 5 ng/L (or the lowest fortification level). DL is calculated by:
DL = s × t (n−1, 1−α =0.99).
where s = standard deviation of replicate analyses, t (n−1, 1−α=0.99) = Student’s t value for the 99% confidence level with n-1 degrees of freedom, and n = number of replicates, respectively. Table 6 shows the estimated detection limits (DLs) and minimum reporting limits (MRLs) for each PFAS.
Laboratory reagent blank (LRB) was defined as reagent water, tab or surface water that was treated in the same manner as the sample including exposure to sample container, lab glassware, processing equipment, solvents and reagents, sample preservation, internal standard and surrogate, and analytical instrument that may be used in the analysis batch. Initial demonstration of low system background was performed by analyzing LRB with each extraction batch to confirm that background contamination is not interfering with the detection of analytes of interest. The results of the analysis of LRBs during the current study were within the acceptable range and the background from any contaminants that interfere with the measurement of PFAS compounds was below 1/3 of the minimum reporting limit (MRL). Keeping a record of LRBs data is very important to monitor background contamination which is considered a significant problem for several PFAS analytes. Laboratory fortified blank (LFB) was defined as reagent water, tab water, or surface water spiked with known amounts of the tested PFAS substances, preservation compounds, surrogates, and internal standards in the laboratory. The main objective of LFB is to make sure that measurements are accurate, and that the analytical method is under control. LFB was analyzed exactly like a sample. Initial demonstration of precision (IDP) was performed by Preparation, extraction, and analysis of a minimum of four replicates LFBs fortified by 16 and 80 ng/L.
For spiking level 16 ng/L (four replicates), the average recoveries ranged from 79 to 117 ng/L with relative standard deviation (RSD) ranged between 1 and 10%. The recoveries and RSD results were within the acceptable range 70–130% and ≤ 20% respectively. The surrogate recoveries ranged from 84 to 113% with RSD ranging between 3 and 9%. The surrogate recoveries and RSD results were within the acceptable range 70–130% and ≤ 20% respectively (Table 7). For spiking level 80 ng/L (four replicates), the average recoveries ranged from 73 to 113 ng/L with relative standard deviation (RSD) ranging between 7 and 14%. The recoveries and RSD results were within the acceptable range 70–130% and ≤ 20% respectively. The surrogate recoveries ranged from 72 to 100% with RSD ranging between 4 and 9%. The surrogate recoveries and RSD results were within the acceptable range 70–130% and ≤ 20% respectively (Table 7). The current method was tested using two different water matrixes (tab and surface water). Tap water was collected from Michigan State University water facilities and surface water was collected from lake Lansing Lake, East Lansing, Michigan, USA. Initial demonstration of precision (IDP) and initial demonstration of accuracy (IDA) for tap and surface water were performed by preparation, extraction, and analyzing four replicates, fortified by 5 ng/L.
For tap water, the average recoveries were found to be from 80 to 105 ng/L with relative standard deviation (RSD) ranging between 2 and 11%. The recoveries and RSD results were within the acceptable range 70–130% and ≤ 20% respectively except for PFHpA, the average recovery was 132%. The surrogate recoveries ranged from 87 to 104% with RSD ranging between 7 and 10%. The surrogate recoveries and RSD results were within the acceptable range 70–130% and ≤ 20% respectively (Table 8). For surface water, the average recoveries ranged from 72 to 114 ng/L with a relative standard deviation (RSD) ranging between 1 and 8%. The recoveries and RSD results were within the acceptable range 70–130% and ≤ 20% respectively except for PFTrDA and PFTA, the average recoveries were 62 and 57%. The surrogate recoveries ranged from 82 to 101% with RSD ranging between 2 and 7%. The surrogate recoveries and RSD results were within the acceptable range of 70–130% and ≤ 20% respectively (Table 8).
Conclusion
This method was developed and validated to determine 16 PFAS at part per trillion levels. An initial demonstration of capability was performed with an acceptable initial calibration, Initial demonstration of peak asymmetry factor, minimum reporting limit (MRL), detection limits, initial demonstration of low system background, and initial demonstration of precision (IDP). The method provides better and more efficient detection and accurate measurement of PFAS in drinking water.
References
Ahrens L, Bundschuh M (2014) Fate and effects of poly- and perfluoroalkyl substances in the aquatic environment: a review. Environ Tox Chem 33(9). https://doi.org/10.1002/etc.2663
Babayev M, Capozzi SL, Miller P, McLaughlin KR, Medina SS, Byrne S, Zheng G, Salamova A (2022) PFAS in drinking water and serum of the people of a southeast Alaska community: a pilot study. Environ Pollut 305:119246. https://doi.org/10.1016/j.envpol.2022.119246
Brennan NMB, Evens AT, Fritz MK, Peak SA, von Holst HE (2021) Trends in the regulation of per- and polyfluoroalkyl substances (PFAS): a scoping review. Int J Environ Res Public Health 18(20):10900. https://doi.org/10.3390/ijerph182010900
Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SPJ (2011) Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Managem 7(4):513–541. https://doi.org/10.1002/ieam.258
Chu S, Letcher RJ, McGoldrick DJ, Backus SM (2016) A new fluorinated surfactant contaminant in biota: Perfluorobutane sulfonamide in several fish species. Environ Sci Technol 50(2):669–675. https://doi.org/10.1021/acs.est.5b05058
Crone BC, Speth TF, Wahman DG, Smith SJ, Abulikemu G, Kleiner EJ, Pressman JG (2019) Occurrence of per- and polyfluoroalkyl substances (PFAS) in source water and their treatment in drinking water. Crit Rev Environ Sci Technol 49:24. https://doi.org/10.1080/10643389.2019.1614848
Groffen T, Bervoets L, Jeong Y, Willems T, Eens M, Prinsen E (2021) A rapid method for the detection and quantification of legacy and emerging per-and polyfluoroalkyl substances (PFAS) in bird feathers using UPLC-MS/MS. J Chromatogr B 1172:122653
Hernandez ET, Koo B, Sofen LE, Amin R, Togashi RK, Lall AI, Gisch DJ, Kern BJ, Rickard MA, Francis MB (2022) Proteins as adsorbents for PFAS removal from water. Environ Sci Water Res Technol 8:1188–1194. https://doi.org/10.1039/D1EW00501D
Houtz EF, Sutton R, Park JS, Sedlak M (2016) Poly- and perfluoroalkyl substances in wastewater: Significance of unknown precursors, manufacturing shifts, and likely AFFF impacts. Water Res 95. https://doi.org/10.1016/j.watres.2016.02.055
Jia S, Dos Santos M, Li C, Snyder SA (2022) Recent advances in mass spectrometry analytical techniques for per- and polyfluoroalkyl substances (PFAS). Anal Bioanal Chem 414:2795–2807. https://doi.org/10.1007/s00216-022-03905-y
Jurikova M, Dvorakova D, Pulkrabova J (2022) The occurrence of perfluoroalkyl substances (PFAS) in drinking water in the Czech Republic: a pilot study. Environ Sci Pollut Res 29(40):60341–60353. https://doi.org/10.1007/s11356-022-20156-7
Kempisty DM, Racz L (2021) Forever Chemicals: Environmental, Economic, and Social Equity Concerns with PFAS in the Environment. ISBN 978-1-003-02452-1. CRC Press. Boca Raton, FL
Lorenzo M, Campo J, Picó Y (2018) Analytical challenges to determine emerging persistent organic pollutants in aquatic ecosystems. Trends in Anal Chem 103:137–155. https://doi.org/10.1016/j.trac.2018.04.003
Müller CE, de Silva AO, Small J, Williamson M, Wang X, Morris A, Katz S, Gamberg M, Muir DCG (2011) Biomagnification of perfluorinated compounds in a remote terrestrial food chain: Lichen-Caribou-Wolf. Environ Sci Technol 45(20). https://doi.org/10.1021/es201353v
Pan CG, Liu YS, Ying GG (2016) Perfluoroalkyl substances (PFASs) in wastewater treatment plants and drinking water treatment plants: Removal efficiency and exposure risk. Water Res 106. https://doi.org/10.1016/j.watres.2016.10.045
Pérez F, Nadal M, Navarro-Ortega A, Fàbrega F, Domingo JL, Barceló D, Farré M (2013) Accumulation of perfluoroalkyl substances in human tissues. Environm Int 59. https://doi.org/10.1016/j.envint.2013.06.004
Place BJ, Field JA (2012) Identification of novel fluorochemicals in aqueous film-forming foams used by the US military. Environ Sci Technol 46(13). https://doi.org/10.1021/es301465n
Podder A, Sadmani AHMA, Reinhart D, Chang N, bin, Goel R (2021) Per and poly-fluoroalkyl substances (PFAS) as a contaminant of emerging concern in surface water: a transboundary review of their occurrences and toxicity effects. J Hazard Mater 419:126361. https://doi.org/10.1016/j.jhazmat.2021.126361
Schaider LA, Balan SA, Blum A, Andrews DQ, Strynar MJ, Dickinson ME, Lunderberg DM, Lang JR, Peaslee GF (2017) Fluorinated compounds in U.S. fast food packaging. Environ Sci Technol Lett 4(3). https://doi.org/10.1021/acs.estlett.6b00435
Shoemaker J, Tettenhorst D (2018) EPA Document #: EPA/600/R-20/006 Method 537.1 determination of selected per- and polyfluorinated alkyl substances in drinking water by solid phase extraction and liquid chromatography/Tandem mass spectrometry (LC/MS/MS). Center for Environmental Solutions & Emergency Response Office of Research and Development, U. S. Environmental Protection Agency Cincinnati. Ohio 45168 (Vol 537)
Taniyasu S, Yeung LWY, Lin H, Yamazaki E, Eun H, Lam PKS, Yamashita N (2022) Quality assurance and quality control of solid phase extraction for PFAS in water and novel analytical techniques for PFAS analysis. Chemosphere 288(1):132440. https://doi.org/10.1016/j.chemosphere.2021.132440
Tokranov AK, Nishizawa N, Amadei CA, Zenobio JE, Pickard HM, Allen JG, Vecitis CD, Sunderland EM (2019) How do we measure poly- and perfluoroalkyl substances (PFASs) at the surface of consumer products? Environ Sci Technol Lett 6(1). https://doi.org/10.1021/acs.estlett.8b00600
Trier X, Granby K, Christensen JH (2011) Polyfluorinated surfactants (PFS) in paper and board coatings for food packaging. Environ Sci Pollut Res 18(7). https://doi.org/10.1007/s11356-010-0439-3
Tröger R, Klöckner P, Ahrens L, Wiberg K (2018) Micropollutants in drinking water from source to tap - Method development and application of a multiresidue screening method. Sci Tot Environ 627. https://doi.org/10.1016/j.scitotenv.2018.01.277
Wang YQ, Hu LX, Liu T, Zhao JH, Yang YY, Liu YS, Ying GG (2022) Per- and polyfluoralkyl substances (PFAS) in drinking water system: Target and non-target screening and removal assessment. Environ Int 163. https://doi.org/10.1016/j.envint.2022.107219
Wang Z, Dewitt JC, Higgins CP, Cousins IT (2017) A never-ending story of per- and polyfluoroalkyl substances (PFASs)? Environ Sci Technol 51(5):2508–2518. https://doi.org/10.1021/acs.est.6b04806
Xu B, Liu S, Zhou JL, Zheng C, Weifeng J, Chen B, Zhang T, Qiu W (2021) PFAS and their substitutes in groundwater: occurrence, transformation and remediation. J Hazard Mater 412:125159. https://doi.org/10.1016/j.jhazmat.2021.125159
Young RB, Pica NE, Sharifan H, Chen H, Roth HK, Blakney GT, Borch T, Higgins CP, Kornuc JJ, McKenna AM, Blotevogel J (2022) PFAS analysis with ultrahigh resolution 21T FT-ICR MS: suspect and nontargeted screening with unrivaled mass resolving power and accuracy. Environ Sci Technol 56(4). https://doi.org/10.1021/acs.est.1c08143
Acknowledgements
The authors would like to thank the PFAS Center, College of Agriculture and Natural Resources, Michigan State University (MSU) for the financial support (MSU’s GREEEN Project). Special thanks to Dr. Dan Jones for his comments and guidance. Dr. Mamy Rakotondravelo participated in part of the work such as sample extraction. We thank Thermo Fisher Scientific for partnering with this PFAS lab. We thank Thermo field applications scientist Dr. Mandy Zhu for the technical and application support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdelraheem, E., Wise, J., Murphy, C. et al. Triple-stage Quadrupole Mass Spectrometer to Determine Ubiquitously Present Per- and Polyfluorinated Alkyl Substances in Drinking Water at Part Per Trillion Levels Using Solid Phase Extraction Approach. Bull Environ Contam Toxicol 110, 32 (2023). https://doi.org/10.1007/s00128-022-03686-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00128-022-03686-1