Abstract
According to the Environmental Protection Agency in Taiwan, the common carp (Cyprinus carpio) is one species of fish for acute toxic test. It has been found to be extremely sensitive to the toxicity of Cd2+; Furthermore, the goldfish (Carassius auratus) has a higher resistance than common carp upon Cd2+ exposure, but both fish are the same family. The aim of the study was to compare the physiological and histo-pathological responses between goldfish and common carp under exposure to sublethal concentrations of Cd2+ in order to understand the reasons behind the Cd2+-resistance. Results showed that metallothionein (MT) protein levels in visceral tissues were exceptionally increased and elevated at an earlier time in goldfish than in common carp. Meanwhile, the amount of Cd2+ accumulation in goldfish was higher than common carp after Cd2+ exposure. The histo-pathological results revealed that the density of gill mucus cells and the thickness of gill epithelium in common carp were raised earlier than in goldfish, but the histo-pathological findings resemble each other. According to the data, we suggested the efficient response of MT proteins may contribute to goldfish with a higher Cd2+ tolerance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Cadmium (Cd2+) is a non-essential element and has severe toxic effects on aquatic organisms. The waterborne Cd2+ causes a wide range of pathological effects on fish. According the Taiwanese Annual Report from Taiwan Environmental Protection Agency (Taiwan EPA 1994), the 96 h-LC50 of Cd2+ was 3.1 μM on the common carp (Cyprinus carpio), but the resistance of Cd2+ on goldfish (Carassius auratus) was higher by 17.1 folds than common carp. Both fish are the same family, but the reason for such a difference is unknown. The present study is interested in finding the reason.
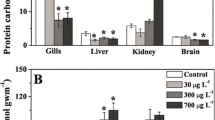
The distribution of Cd2+ in sediment from the 6 main rivers in southern Taiwan is about 31.1 µM, and the quantity of Cd2+ contained in the sediment reflect the degree of pollution for the body of water (Tsai et al. 2007). Cd2+ is easily accumulated most in the kidney, liver, gills of fish, and muscles in this order (Ambreen et al. 2015). On the other hand, metallothionein (MT) appeared most in visceral tissues including the liver, intestine, and kidney (Cretì et al. 2010). The gills are also directly exposed to an aquatic environment, and it is very sensitive to aquatic pollutants. In addition, the accumulation rate of Cd2+ in the gill was faster than that in other tissues at an earlier time, but the accumulation of Cd2+ had a limit to how much it can absorb after Cd2+ exposure (Wu et al. 2006). In fact, the accumulation rate of Cd2+ in other visceral tissues was higher compared to gills in later stages of Cd2+ exposure (Cretì et al. 2010). This phenomenon reveals that the gill might have some strategies to slow down the accumulation of Cd2+ in order to decrease the histological damage by free Cd2+. Therefore, the present study measured MT contents from visceral tissues, and analyzed and observed the histo-pathology in gills.
The continued stress induced by heavy metals exerts negative effects in fish. The several physiological responses of fish, such as the up regulation of MT and mucus secretion, were induced by heavy metal exposure (Alvarado et al. 2006). These responses in the gill have positive and/or negative effects to fish. For example, the increased mucus secretion from the fish gill had a positive effect in removing metals from the surface of the gill epithelium. In the meantime, the increased mucus secretion covered the gill epithelium, and even reduced or blocked the gas exchange. Moreover, Liu et al. (2012) indicated that Cd2+ exposure disturbed the mRNA expression of epithelial Ca2+ transporters, decreasing the ability to uptake Ca2+. On the other hand, the previous study indicated that the MT synthesis in tissues was induced by Cd2+ exposure, and it can increase the Cd2+ bioaccumulation. The increased MT was suggested to be a detoxification function for exposed tissues (Oost et al. 2003). However, its detoxification function was limited under normal physiology (Wu et al. 2016). Thus, we assumed a strategy to promote Cd2+-resistance that maintains a normal physiological function rather than decreasing pathological levels. The MT induced efficiency might be related to Cd2+ resistance of fish.
The developmental stage of fish, waterborne factors, and fish scale functions were all related to the different LC50 of Cd2+ in fish (Zeynab et al. 2012). Hence, the aims of the study analyzed whether physiological and histo-pathological responses are both related to the different LC50 of Cd2+ in common carp (C. carpio) and goldfish (C. auratus). These parameters of physiological responses include Cd2+ accumulation, Ca2+ influx, and MT contents. The histo-pathological responses in gills includes the density of mucus cells, and the thickness of filament and hematoma levels of both fish after sublethal concentration Cd2+ exposure.
Materials and Methods
In order to avoid Cd2+ impact levels that were influenced by fish size, developmental stage, and environmental conditions in the present study, we chose juvenile common carp (C. carpio) and goldfish (C. auratus), which has a body length/body weight of 2.5–3.5 cm/0.94 ± 0.15 g, and 2.5–3.5 cm/0.98 ± 0.23 g, respectively. Both species of juvenile fish were purchased from a private aquaculture farm in Tainan County, Taiwan. Fishes were cultured in tanks, each filled with 300 L of filtered water at 27°C, and a 12 h photoperiod with 12 h of darkness. The protocol for animal use was reviewed and approved by the Chiayi University (IACUC Approval No.: 102027).
Tissues were dried at 65°C overnight, and digested with 2 mL of 13.1 M HNO3 at 100°C overnight. The digested solutions were diluted with double deionized (dd) water and subjected to Cd2+ analysis from an air/acetylene flame with atomic absorption spectrophotometry (AA, Z-8000, Hitachi, Japan). Standard solutions of Cd2+ (Merck, Germany) were used for establishing standard curves. Addition of certain amounts of standard solutions to the test samples was used for background correction to eliminate the matrix effect. The concentration of exposure media (Cd2+) were prepared from completely dried CdCl2 (Sigma, USA) dissolved in 1 mL concentrated HCl, and used with dd water to prepare stock solution. Exposure medium were used with local tap water from the stock solution, in which its concentration was measured using AA before exposure. The procedure in measuring Cd2+ followed an operation manual of the instrument and was referenced from our previous study (Wu et al. 2000). The experimental medium was changed once every day, in order to maintain the Cd2+ concentration and water quality.
The MT ELISA kit used is the same from our past research with minor modifications (Wu et al. 2000). The visceral tissues were collected as a sample and analyzed using MT ELISA. A synthetic peptide (MDPCECAKTGTCNCGGS) from the N-terminal of tilapia MT antibody was coated onto 96-well microtiter plates with coating buffer (0.05 M NaHCO3; pH 9.6). Rabbit anti-tilapia MT serum was added as the first antibody (1:2000) and peroxidase-labeled goat anti-rabbit IgG (1:4000) was added as the secondary antibody. Color development was measured at 405 nm with an automatic microtiter plate ELISA reader (Dynex MRX, USA). Total proteins were determined using a protein assay kit (Bio-Rad Lab., USA). The coefficients of intra- and inter- assay variations were 5.04% (n = 8) and 15.05% (n = 7), respectively. MT was extracted from goldfish and carp, and the original extraction of MT was diluted with 0.01 M phosphate buffer in 1, 2, 4, and 6 folds. Both the standard MT (a synthetic peptide) and the diluted extraction of MT were run by an ELISA test at the same time.
60 juvenile goldfish (C. auratus) and 60 carp (C. carpio) were separated to 10 tanks for control and treatment groups. The two species of fish were treated with a sublethal concentration of Cd2+ (44.6 μM) for 0, 1, 2, 3, and 4 days (d), and immediately anesthetized with MS222.
The visceral tissues were excised from the body and MT was measured by ELISA following MT extraction. The Cd contents of the whole body were then measured. Both species of juvenile fish were separately treated with water containing 44.6 μM Cd2+ for 24 and 48 h. Six individual fish were rinsed briefly in deionized water, and then transferred to a 1 L 45Ca2+ (Amersham, Piscataway, NJ: with a final working specific activity of 5 mCi/mmol)-containing medium for a subsequent 3 h incubation. After incubation, juveniles were washed several times in an isotope-free medium in order to sufficiently remove all surface-bound calcium. Every larva was put into one vial, anesthetized with MS-222, and then digested with tissue solutions supplemented with counting solutions. Finally, samples were counted with a liquid scintillation beta counter (1211 Rackbeta, LKB, Finland). The Ca2+ influx was calculated using the following formula: Jin = Qlarva·X−1out·t−1·W−1, where Jin is the influx (mmol/mg/h); Qlarvae is the radioactivity of the larva and each individual larva is counted per minute (cpm) at the beginning and end of incubation; Xout is the specific activity of the incubation medium (cpm/nmol); t is the incubation time (h); and W is the average body wet weight of larvae from different stages (mg).
The gills were fixed, dehydrated, embedded, and then sectioned horizontally, perpendicular to the filaments and parallel to the lamellae for the histo-pathological observation upon fish treated with 44.6 μM Cd2+ for 0–4 days. Slides were stained with periodic acid Schiff (PAS). Mucus cell (MC) numbers in gills were determined with a light microscope at 200 × for each individual by examining the following: 10 filaments per section were collected from a single individual, and four sections were separated by at least two intervening sections in every individual. Three individuals in the same treatment group were collected for one sample. This represented a total of 30 measurements per treatment. Microscopic images were captured with an image analysis system (Image Pro Plus, Version 4.5, Nikon) using a color tri-charged coupled device video camera. The method for measuring the thickness of epitheliocystis in the gills were referenced from Monteiro et al. (2009), and it was assayed by the Image- Pro Plus 4.5 software (Media Cybernetics).
Data for Figs. 2 and 5B were done by static assay through one-way ANOVA analysis with Tukey’s comparison, and static analysis was done by student’s t-test for Figs. 3 and 4.
Results and Discussion
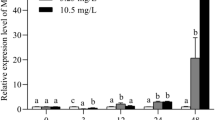
A typical dose-related standard curve and MT extracted from the two species of fish by competitive ELISA are shown in Fig. 1. The displacement curve for Cd-induced MT extracted from goldfish and carp were parallel to that of the MT standard, indicating that the ELISA is suitable for measuring Cd-induced MT in goldfish and carp. According to the mortality from both species of fish, we find that the goldfish had a higher Cd2+ resistance than that in the common carp (Table s1). We also wanted to test the same Cd2+ treatment for both goldfish and common carp in which both species of fish have a similar mortality rate. Since there is no data for the mortality rate of goldfish between 35.7 and 53.5 μM Cd2+, we estimated that the concentration of 44.6 μM Cd2+ would have a similar mortality (13%) for goldfish and common carp (Table s1). In the same family, the common carp exhibited a more similar level of LC50 with grass carp (Ctenopharyngodon idellus) at 84.1 μM (Yorulmazlar and Gul 2003). Indeed, 96 h LC50 was observed from 1.25–159 μM Cd2+ in common carp (Delahaut et al. 2020). Therefore, the concentration of 44.6 μM Cd2+ was used in the present study. MT mRNA was a useful biomarker for aquatic organisms upon metal exposure. Actually, the MT mRNA expression levels are positively correlated with MT protein contents following the treatment time in common carp (Castaldo et al. 2020). The present study used a synthetic peptide from the N-terminal of tilapia (Oreochromis mossambicus) MT to produce a rabbit anti tilapia MT antibody. The homology of the amino acid sequence of tilapia MT was around 80% to goldfish (C. auratus) or common carp (C. carpio). The amino acid sequence of MT in goldfish and common carp were around 92% identical (Chan 1995). We conducted the competitive binding curves of Cd2+ induced MT with various concentrations in goldfish and common carp, and standard MT (Log10) by ELISA. The coefficient of correlation (r2) values were 0.93 and 0.94. The slope of the MT standard curve was 0.301, 0.3066 in goldfish, and 0.2234 in common carp (Fig. 1). The data showed that the MT protein levels from both species of fish could be recognized by a synthetic peptide of tilapia MT antibody.
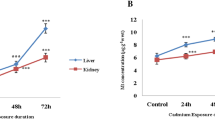
Cadmium is a MT inducer, but the sensitivity appears to be depended on the tissue and the exposure time. Thus, MT can reduce the amount of Cd2+ entering the cell, thereby limiting the toxicity (Divya et al. 2018). The present results showed that the Cd2+ contents increased over time in both goldfish and common carp under Cd2+ exposure (Fig. 2B). Although both goldfish and common carp had similar Cd2+ accumulation levels, the changing trend of Cd2+ content was more drastic in carp (increased by 5.9 folds; 44.6/7.5 µg/g of Cd2+) than goldfish (increased by 2.9 folds; 49.3/16.8 µg/g of Cd2+) from day 1 to day 4 after exposing to Cd2+ (Fig. 2B). Meanwhile, the MT content was significantly increased in goldfish after 1 d of exposure to Cd2+, but back to base levels on the 4th day. Compared to goldfish, the MT content of carp was only up-regulated on the 3rd day after exposure to Cd2+ (Fig. 2A). Our previous study found a similar profile that fishes had a positive relationship between MT expression and metal contents under normal physiological circulation (Wu et al. 2000, 2016).
Metallothionein (MT) contents and Cd contents in goldfish and common carp exposed to 44.6 μM of Cd for 0, 1, 2, 3, and 4 days. Data shown as mean ± SE (n = 6). Data comparison of the same species by one-way ANOVA analysis with Tukey’s comparison. Different letters indicate a significant difference among treatment time
Goldfish and common carp both had around a 30%–40% decrease in Ca2+ uptake upon 24–48 h of 44.6 μM of Cd2+ exposure (Fig. 3). The density of mucus cells in the gills of carp and goldfish highly increased after exposure to Cd2+ for 1 and 2-day, respectively (Fig. 4). On the histo-pathological profile, the gill filament of both fish had a morphological change upon exposure to 44.6 μM Cd2+ for 0–4 days. The thickness of gill epitheliums in carp and goldfish was up-regulated after 1-day and 2-day of Cd2+ exposure, respectively (Fig. 5B). In addition, 36.9% hematoma was found in the gill filaments of goldfish after 4-day of Cd2+ exposure, but only 14.8% hematoma was observed in common carp (Fig. 6).
Calcium ion influx in goldfish (A) and common carp (B) exposed to 0 (control) and 44.6 μM of Cd2+ for 24 and 48 h. Data shown as mean ± SE (n = 5–6). Comparison of data at the same treatment time by student’s t-test (* indicates a significant difference between the control and treatment group, p < 0.05)
Density of mucus cells in gill filaments of goldfish (A) and common carp (B) exposed to 0 (control) and 44.6 μM of Cd2+ for 0, 1, 2, 3, and 4 days. Data shown as mean ± SE (n = 3–6). Comparison of data at the same treatment time by student’s t-test (* indicates a significant difference between control and treatment group, p < 0.05)
A Schematic diagram of gill lamella (L), central venous sinus (cvs), and thickness of gill epitheliocystis (*). The scale is 12.5 μm; (B) Comparison of the thickness of gill epitheliocystis between goldfish and common carp exposed 44.6 μM of Cd2+ for 0, 1, 2, 3, and 4 days. Data shown as mean ± SE (n = 6). Data by one-way ANOVA analysis with Tukey’s comparison for the same species. Different letters indicate a significant difference among treatment time (p < 0.05)
According to the data, the histo-pathological responses of both fish resemble each other. Therefore, we suggested that the goldfish appeared to have a higher tolerance upon Cd2+ exposure than common carp because the MT in visceral tissues was induced earlier in goldfish compared to common carp.
References
NE Alvarado I Quesada K Hylland I Marigómez M Soto 2006 Quantitative changes in metallothionein expression in target cell-types in the gills of turbot (Scophthalmus maximus) exposed to Cd, Cu, Zn and after a depuration treatment Aqua Toxicol 77 64 77
F Ambreen M Javed U Batool 2015 Tissue specific heavy metals uptake in economically important fish, Cyprinus carpio at acute exposure of metals mixtures Pak J Zool 47 399 407
G Castaldo M Pillet B Slootmaekers L Bervoets RM Town R Blust G Boeck De 2020 Investigating the effects of a sublethal metal mixure of Cu, Zn, and Cd on bioaccumulation and ionoregulation in common carp, cyprinus carpio Aqua Toxicol. 218 105363
KM Chan 1995 Metallothionein: potential biomarker for monitoring heavy metal pollution in fish around Hong Kong Mar Pollut Bull 31 411 415
P Cretì F Trinchella R Scudiero 2010 Heavy metal bioaccumulation and metallothionein content in tissues of the sea bream Sparus aurata from three different fish farming systems Environ Monit Assess 165 321 329
V Delahaut B Raskovic MS Salvado L Bervoets R Blust G Boeck De 2020 Toxicity and bioaccumulation of cadmium, copper and zinc in a direct comparison at equitoxic concentrations in common carp (Cyprinus carpio) juveniles Plos One 15 e0220485
TV Divya C Pallavi A Celin 2018 NmtA, a novel metallothionein of Anabaena sp. strain PCC 7120 imparts protection against cadmium stress but not oxidative stress Aqua Toxicol 199 152 161
CT Liu MY Chou CH Lin SM Wu 2012 Effects of ambient cadmium with calcium on mRNA expression of calcium uptake related transporters in zebrafish (Danio rerio) larvae Fish Physiol Biochem 38 977 988
SM Monteiro E Rocha JM Mancera A Fontaínhas-Fernandes M Sousa 2009 A stereological study of copper toxicity in gills of Oreochromis niloticus Ecotoxicol Environ Saf 72 213 223
RV Oost J Beyer NPE Vermeil 2003 Fish bioaccumulation and biomarkers in environmental risk assessment: a review Environ Toxicol Pharmacol 13 57 149
Taiwan EPA (1994) The study of acute toxic test on fish. Annual Report, Environmental Protection Administration of Taiwan
LJ Tsai KC Yu ST Ho 2007 Cadmium distribution in sediment profiles of the six main rivers in southern Taiwan J Hazard Mater 148 630 639
SM Wu CF Weng JC Hwang PP Hwang 2000 Metallothionein induction in early larval stages of tilapia (Oreochromis mossambicus) Physiol Biochem Zool 73 531 537
SM Wu KJ Jangg YJ Lee 2006 Relationship among metallothionein, cadmium accumulation, and cadmium tolerance in three species of fish Bull Environ Contam Toxicol 76 595 600
SM Wu LH Shu JH Liu 2016 Anti-oxidative functions of mt2 and smtB mRNA expression in the gills and brain of zebrafish (Danio rerio) upon cadmium exposure Fish Physiol Biochem 42 1709 1720
E Yorulmazlar A Gül 2003 Investigation of acute toxicity of cadmium sulfate (CdSO4. H2O) and behavioral changes of grass carp (Ctenopharyngodon idellus Val., 1844) Chemosphere 53 1005 1010
A Zeynab KM Kazem KE Sohrab R Hossein 2012 Comparison of lethal concentrations (LC50-96h) of CdCl2, CrCl3, and pB(NO3)2 in common carp (Cyprinus carpio)and sutchi Ccatfish (Pangasius hypophthalmus) Iran J Toxicol 18 672 680
Acknowledgements
This work was supported by Grants (NSC101-2311-B-415-001-MY3) from the National Science Council, Taiwan.
Funding
This study was supported by Ministry of science and technology, Taiwan
Author information
Authors and Affiliations
Contributions
FMC and SMW perform the literature search and data analysis, and Lin had a technological cooperation on the Ca2+ influx test. All authors read and approved the final version of the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chang, F.M., Lin, C.H. & Wu, S.M. Duration of Induced Metallothionein is a Critical Factor in the Cadmium-Resistance Among Goldfish (Carassius auratus) and Common Carp (Cyprinus carpio). Bull Environ Contam Toxicol 108, 725–730 (2022). https://doi.org/10.1007/s00128-021-03449-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-021-03449-4