Abstract
There were not any past studies about metallothionein isoforms (smtB and mt2) having anti-oxidative functions on zebrafish after Cd2+ exposure. On the other hand, the anti-oxidative enzymatic factors such as superoxide dismutase (sod), glutathione peroxidase (gpx1a), and catalase (cat) are used as references to investigate whether the smtB and mt2 have anti-oxidative responses on the gills and brain of zebrafish after 1–6 h of 0 and 1.78 μM Cd2+ exposure. The anti-oxidative system such as sod, cat, and gpx1a mRNA expressions demonstrated a cascade response upon Cd2+-induced oxidative stress in the present study. Interestingly, the smtB mRNA expression levels increased by 3.2- to 6.1-fold, and mt2 raised by 4.1- to 11.3-fold in gills at 1 and 3 h after exposure to Cd2+, respectively. On the other hand, the smtB mRNA levels increased by 10.6- to 58.6-fold, but mt2 mRNA levels increased by 2.3- to 11.1-fold in brain at 1 and 3 h after exposure to Cd2+, respectively. In addition, both tissues showed increased apoptosis levels at 3 h, and recovery after 6 h of Cd2+ exposure. From the results, we suggest that both mt2 and smtB play a role in anti-oxidation responses within 6 h after exposure to Cd2+. In conclusion, the smtB mRNA levels have a higher response than mt2 in the brain, but both mRNA expressions appear to have a similar pattern in the gill. We suggest that smtB plays an important role to defend oxidative stress in the brain of adult zebrafish upon acute Cd2+ exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metallothioneins (MTs) have been found around 60 years ago, and its physiological functions have also evolved, including the detoxification of heavy metals such as cadmium (Cd) and mercury, and the regulation of some essential metals such as copper and zinc in teleosts (Roesijadi 1992). In addition, MT may be induced in response to oxidative stress and may function to protect tissues from oxidative damage (Sato and Bremner 1993). In zebrafish (Danio rerio) larvae, the genes mt2 and smtB first appeared in 6 hpf (hours of post-fertilization) and 12 hpf of the embryonic stage, respectively (Wu et al. 2008a). Our previous studies found that both genes appeared to have different functions between larvae and adult fish. For example, the mt2 signals appeared upon ionic cadmium (Cd2+) exposure and cold shock, but smtB signals were not found under the same treatment conditions by whole-mount in situ hybridization in developmental larvae (Wu et al. 2008a). However, the mt2 and smtB mRNA levels were, respectively, increased by 4- and 3.3-fold in the brain of adult fish after cold shock (Wu et al. 2015). The mt2 mRNA expression noticeably increased after exposure to waterborne metals, but the phenomenon was not copied for smtB in the ovaries of female zebrafish (Wu et al. 2012). On the other hand, it was interesting to find that the smtB mRNA showed a tissue-specific expression, and it indicated that the brain contained 2- to 3-fold more of the smtB mRNA expression as compared to other tissues (e.g., gill, liver, and intestine). However, tissue-specific expressions relating to mt2 mRNA cannot be found in adult zebrafish (Wu et al. 2008a). Therefore, the present study was to study the physiological roles of smtB and mt2 in the brain after Cd2+ exposure.
The brain may be the most sensitive organ to environmental fluctuations, since most of the physiological acclimation responses are initiated by the central nervous system (Crawshaw et al. 1985). Therefore, the brain is a highly energy-consuming organ, and the high metabolic rate of brain cells implies a high production of ROS (Tseng et al. 2011). Thus, the brain requires a powerful anti-oxidative system in order to avoid oxidative stress and keep cellular redox state in balance. On the other hand, the gills are the first organ to be affected by exposure to waterborne trace elements (Taylor et al. 1996), and our previous study has found that the gills seem to have a powerful repairing system after exposure to metal (Wu et al. 2008b). Therefore, it is interesting to compare with the anti-oxidative function of metallothionein isoforms between the two organs. Our previous study found that the RT-PCR products of relative expressions of MT mRNA and β-actin of mt2 were down-regulated in the brain, and smtB was not induced in gills but can be induced in the brain by Cd2+ on mature zebrafish (Wu et al. 2008a). These results raise questions regarding the physiological functions of smtB and mt2 in the brain and gills of adult zebrafish after Cd2+ exposure. A particular focus is placed on the anti-oxidative functions.
Cadmium is a nonessential and toxic metal that can be found at elevated levels in water and soil. Cuypers et al. (2010) reported that Cd2+ could indirectly generate free radicals. Cd2+ damages cells while simultaneously causing the formation of reactive oxygen species (ROS) (Leonard et al. 2004). Thus, it is suspected that the exposure of aquatic animals to Cd2+ causes biochemical or physiological changes that can protect organisms from Cd2+ toxicity. In addition, antioxidant responses will be induced by chemical and physical stresses, even for limited durations. However, the action of Cd2+ toxicity can potentially disrupt the antioxidant potential and cause oxidative stress (Bagnyukova et al. 2007). Like many other vertebrates, fish use antioxidant defense mechanisms to reduce damage from oxidative stress. There were differential types of antioxidant defense mechanisms which included anti-oxidant molecules, such as glutathione (GSH), ascorbic acid, tocopherol, and carotenoids (Alvarez et al. 2005), and antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) (Valavanidis et al. 2006). Particularly, several studies have examined the role of non-enzyme antioxidants such as metallothioneins (MTs) (Shimoda et al. 2003; Chen and Maret 2001). MTs are a small family of thiol-rich proteins that exhibit antioxidant activities and, together with GSH, are important in the regulation of redox homeostasis and protection against superoxide and hydroxyl radicals (HO.) (Chen and Maret 2001). Recently, it has been demonstrated that both genes (mt2 and smtB) appear to have an anti-oxidative function upon cold shock (Wu et al. 2015). However, it has not yet been confirmed if mt2 and smtB might have different anti-oxidative roles between tissues upon Cd2+ exposure.
Oxidative stress generated by Cd2+ and other stresses can cause DNA damage. The oxidation of DNA bases can be due to a HO. radical attack on the pyrimidines aromatic cycles. Lipid peroxidation is also a source of endogenous DNA damage via malondialdehyde (MDA) and 4-hydroxy-2-Nonenal (HNE) which can react with DNA bases. ROS reaction to proteins can also produce new radicals that can react with DNA. These examples of DNA damage are induced by lipids and protein oxidation products, which has shown to cause the indirect genotoxic effects of oxidative stress. In other words, a cascade response of oxidative stress, which was induced by ROS production, oxidative DNA damage, and lipid and protein oxidation, resulted in short-term cell apoptosis (Choi et al. 2007). However, during short periods of stress, antioxidant molecules, antioxidant enzymes, and proteins protect the organism to keep off oxidative stress, in which the antioxidant defense system, e.g., sod, cat, gpx1a, and mt2 mRNA expressions, was all up-regulated after 1 h of cold shock, and the smtB mRNA gene expression was up-regulated after 6 h of cold shock in zebrafish (Wu et al. 2015). Thus, the present study seeks to confirm that smtB and mt2 play a physiological anti-oxidative role following the up-regulation of ROS and LPO levels, sod, gpx1a, and cat levels within 6 h following Cd2+ exposure. In addition, levels of cells apoptosis in the brain and gills upon Cd2+ exposure were observed in adult zebrafish.
Materials and methods
Animals and Cd2+ exposure
Sexually mature zebrafish (D. rerio) of both sexes were obtained from the Taiwan Zebrafish Core Facility (TZCF, Miaoli, Taiwan). The fish were kept in an aquarium supplied with circulating, dechlorinated, aerated local tap water at 28 °C with a 14 h:10 h light and dark photoperiod. Fish were fed both Daphnia pluex and Artemia franciscana prior to the experiments. Following conditions from our previous study, a concentration of 1.78 μM Cd2+ was used. The experimental medium was prepared with 1.78 μM Cd2+ in dechlorinated, circulated, aerated local tap water. The water quality parameters included a total hardness of 146.6 ± 5.6 mg/L, Na+ of 35.6 ± 0.3 mg/L, K+ of 3.3 ± 0.1 mg/L, Ca2+ of 30 ± 2.3 mg/L, O2 of 5–7 mg/L, and pH of 8.2 ± 0.3; Cd2+ concentration was <1 μg/L. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC Approval No. 101003).
Experimental settings and sampling
Experiment 1
Experiment 1: Eight test tanks were used in the present study, and five male zebrafish were used in each tank to prevent inconsistent gender distributions from causing different physiological responses upon Cd2+ exposure (Sellin et al. 2007). Four tanks of control groups contained 0 μM Cd2+ water, and four tanks of treatment groups contained 1.78 μM Cd2+ water. Experiments ran for 6 h with sampling at 0, 1, 3, and 6 h after Cd2+ exposure (n = 5). Groups of five control zebrafish were sampled at each time interval. The fish were immediately anesthetized with 50 mg/L MS222 (Ethyl 3-aminobenzoate methanesulfonate salt; Sigma) after 0, 1, 3, and 6 h of Cd2+ exposure, and the gills and brain were excised from the body to determine cat, sod, gpx1a, mt2, and smtB mRNA expression after each sampling time.
Experiment 2
Apoptosis cells were measured with TUNEL assay. Four test tanks were used with 1 tank containing 0 μM Cd2+ water (negative and positive control group) and 3 other tanks containing 1.78 μM Cd2+ water (treatment group). The fish were taken from the tanks, and the brain and gills were excised after 0, 3, and 6 h of various doses of Cd2+ exposure. All experiments were repeated 3 times.
Preparation mRNA and cDNA synthesis
To prepare total RNA, 5 mg of gills and brain tissues was homogenized in 800 μL of Trizol reagents (Invitrogen, Carlsbad, CA, USA) and treated according to the manufacturer’s protocols. The amount and quality of total RNA were determined by measuring the absorbance at 260/280 nm with a spectrophotometer (NanoDrop ND-1000, DE, USA) and analyzed using RNA-denatured gels. The total amount of RNA was subsequently extracted using a QuickPrep Micro mRNA purification kit (Amersham Pharmacia, Piscataway, NJ, USA). Finally, the mRNA pellets were precipitated with 0.1 mg glycogen, 1/10 the volume of 3 mM NaOAc, and 95 % ethanol, stored at −20 °C for 10 min, then centrifuged at 13,500×g for 15 min in order to do cDNA synthesis. A first-strand cDNA library for both tissues was then constructed according to the protocol of the SMART™ cDNA Library Construction Kit (Clontech, USA) and stored at −20 °C until QPCR analysis.
Quantitative real-time PCR of sod, cat, gpx1a, mt2, smtB mRNA and β-actin
Quantitative real-time PCR (qPCR) was carried out using a SYBR Green dye-based assay (Qiagen, Hilden, Germany) with an ABI Prism 7500 Sequence Detection System (Perkin-Elmer, Applied Biosystems, Wellesley, MA, USA) according to the manufacturer’s instructions. We followed Choi et al. (2010) in selecting primers targeting SOD, CAT, and GPx, and Wu et al. (2012) in selecting primers targeting smtB and mt2. The internal control gene β-actin was designed using the Primer Express 2.0 software (Applied Biosystems). In each assay, 25 ng cDNA was amplified in a 25 μL reaction containing 12.5 μL of Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen, California), 0.5 μL (200 nM) of forward and reverse primers, and nuclease free water. The primers used for the consensus of the selected genes and internal control gene (β-actin) are listed in the table below.
Gene | Forward primer (5′–3′) | Reverse primer (5′′–3′) |
|---|---|---|
SOD | GGC CAA CCG ATA GTG TTA GA | CCA GCG TTG CCA GTT TTT AG |
Cat | AGG GCA ACT GGG ATC TTA CA | TTT ATG GGA CCA GAC CTT GG |
GPx1a | ACC TGT CCG CGA AAC TAT TG | TGA CTG TTG TGC CTC AAA GC |
sMT-B | TGC TCC AAA TCT GGA TCT TG | GCA GTC CTT CTT GCC CTT AC |
MT-2 | AGA CTG GAA CTT GCA ACT GTG GT | CAG CTG GAG CCA CAG GAA TT |
β-actin | CGG AAT CCA CCA AAC CAC CTA | ATC TCC TTC TGC ATC CTG TGA |
All qPCRs were performed as follows: 1 cycle at 50 °C for 2 min and 95 °C for 2 min, followed by 40 cycles at 95 °C for 15 s and 58–60 °C for 15 s (with times depending on the standard annealing temperature of all primers). PCR products were subjected to a melting curve analysis, and representative samples were electrophoresed to verify that only a single product was present. Control reactions were conducted with sterile water to determine levels of background and genomic DNA contamination. The standard curve of each gene was confirmed to be in a linear range with β-actin as an internal control. The relative quantity formulae are as follows:
Within qPCR reactions, triplicate reactions were performed for each sample, including β-actin (internal control) and target genes. To ensure data precision, qPCR data were accepted only when the variation Ct values were less than 1 in triplicate reactions of each gene within the whole set of experiments.
Lipid hydroperoxide (LPO) assay and reactive oxygen species (ROS) assay
LPO assays were conducted using a method modified from Porter et al. (1995), while the ROS assays were conducted using a method modified from Imada et al. (1999). The 5 mg of gills and brain tissues was individually homogenized in a homogenization buffer either with 500 μL of Extract R-Saturated methanol solution for the LPO assay or with 200 μL of L-012 (8-Amino-5-chloror-7-phenylpyrido(3,4-d)pyridazine-1,4-(2H,3H) dione sodium salt) (Wako, Japan) for the ROS assay. Briefly, L-012 was dissolved into ddH2O to a final concentration at 20 mM, and then diluted with an appropriate buffer [50 mM Tris–HCl (pH 7.5)] to be an assay that has a concentration of 4 μM for O2 − radical measurements. The homogenates were incubated with the 4 μM of L-012 reaction medium for 10 min under a dark space, and then centrifuged at 12,000×g for 3 min, after which 100 μL of supernatants was used to measure the relative light units (RLU) with a luminescence instrument (Berthold, Lumat LB9507, Germany) (Imada et al. 1999).
Cell death detection
Cell death was detected using TUNEL assays to label DNA strand breaks. The TUNEL assay was performed on 5-μm sections and reacted with an ApopTag® Peroxidase In Situ Apoptosis Detection Kit S7100 (Millipore, USA). To remove the paraffin, the sections were washed in three changes of xylene over a period of 5 min each time and were then immersed in two changes of absolute ethanol. They were then washed twice in ethanol (95 and 70 % ethanol) for 5 min each, followed by rinsing with 0.5 % Triton X-100 (Triton X-100, JT Baker, USA) for 10 min, and finally twice with clean water for 2 min each. The sections were then immersed in 0.01 M PBS (Na2HPO4, NaCl, KCl, KH2PO4, and H2O, BSA 1 %; pH 7.4) buffer containing 3 % H2O2 (30 % H2O2, Sigma, USA) for 5 min. The specimens were then rinsed with 65 μL of equilibration buffer and incubated for at least 10 s at room temperature. Then 55 μL of TdT enzyme (reaction buffer: TdT enzyme = 7:3) was administered by pipette, followed by 1 h incubation in a humidified chamber at 37 °C. The slide was placed in a working strength stop/wash buffer, agitated for 15 s, and incubated for 10 min at room temperature. The slide was washed in three changes of PBS for 1 min each time, reacted with an anti-digoxigenin conjugate solution on the slide, and incubated in a humidified chamber for 30 min at room temperature. The slide was then washed in four changes of 0.01 M PBS before being completely covered in working strength peroxidase substrate (DBA dilution buffer: DBA substrate = 50:1) for 5 min at room temperature. Finally, the slide was stained with hematoxylin and covered with ClearMount (Invitrogen, USA, California). The positive control used DNAse I (1U/μl) to induce DNA breakage in the initial step, and then followed the same steps described above. The positive control sample was treated with DNase solution (1U/μl) for 5 min at room temperature, while negative control staining was performed without active TdT, but was incubated with proteinase K digestion to control for the nonspecific incorporation of nucleotides or for the nonspecific binding of enzyme conjugates.
Statistical analysis
Values are presented as the mean ± SD (n = 5). Control and treatment groups at the same time points were compared with Student’s t test with p < 0.05 being accepted as a significant difference.
Results
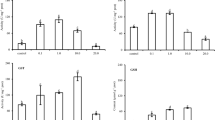
In the gills, the ROS significantly increased, but the LPO levels decreased significantly after exposure to Cd2+ for 3 h as compared to the control group (Fig. 1). The sod and cat mRNA expressions were significantly up-regulated after the 1st h of Cd2+ exposure as compared to the control group at the same treatment time, but both mRNA expressions recovered quickly. In addition, gpx1a mRNA levels significantly increased after Cd2+ exposure for 3 h. Three anti-oxidative genes (sod, cat, and gpx1a) nearly recovered to their initial levels after exposure to Cd2+ for 6 h (Fig. 2).
ROS (a) and LPO (b) levels in the gills after 0, 1, 3, and 6 h of exposure to 1.78 μM Cd2+ (treatment), with 0 being the control. Data are given as mean ± SD (n = 5), and data were analyzed by Student’s t test to compare the control and treatment groups at the same treatment time (*p < 0.05; ***p < 0.001)
Changes of sod (a), cat (b), and gpx1a (c) mRNA relative expressions in gills after 0, 1, 3, and 6 h of exposure to 1.78 μM Cd2+ (treatment), with 0 being the control. Data are given as mean ± SD (n = 5), and data were analyzed by Student’s t test to compare the control and treatment groups at the same treatment time (*p < 0.05)
In the brain, the ROS increased significantly within 6 h after Cd2+ exposure. However, LPO levels did not change significantly following Cd2+ exposure (Fig. 3). SOD levels were not significantly changed after exposure to Cd2+, but CAT and GPx levels were significantly heightened at 1–3 h after Cd2+ exposure (Fig. 4).
Changes of sod (a), cat (b), and gpx1a (c) mRNA relative expressions in the brain after 0, 1, 3, and 6 h of exposure to 1.78 μM Cd2+ (treatment), with 0 being the control. Data are given as mean ± SD (n = 5), and data were analyzed by Student’s t test to compare the control and treatment groups at the same treatment time (*p < 0.05; **p < 0.01; ***p < 0.001)
The mt2 and smtB gene expressions in the gills increased significantly (4.1- to 11.3-fold and 3.2- to 6.1-fold, respectively) within 3 h after Cd2+ exposure. In addition, mt2 mRNA expression in the gills still exceeds the control 6 h after Cd2+ exposure (Fig. 5). In the brain, mt2 and smtB mRNA levels followed a similar pattern, respectively, increasing 2.3- and 10.6-fold after exposure to Cd2+ for 1 h and 11.1- and 58.6-fold after exposure to Cd2+ for 3 h. However, both genes’ mRNA levels recovered 6 h after Cd2+ exposure (Fig. 6). In the brain and gills, the levels of MT genes (mt2 and smtB) both showed a time-dependent increase after Cd2+ exposure for 1 and 3 h (Figs. 5, 6).
Changes of smtB (a) and mt2 (b) levels in gills after 0, 1, 3, and 6 h of exposure to 1.78 μM Cd2+ (treatment), with 0 being the control. Data are given as mean ± SD (n = 5), and data were analyzed by Student’s t test to compare the control and treatment groups at the same treatment time (*p < 0.05; **p < 0.01; ***p < 0.001)
Changes of smtB (a) and mt2 (b) levels in the brain after 0, 1, 3, and 6 h of exposure to 1.78 μM Cd2+ (treatment), with 0 being the control. Data are given as mean ± SD (n = 5), and data were analyzed by Student’s t test to compare the control and treatment groups at the same treatment time (*p < 0.05; **p < 0.01; ***p < 0.001)
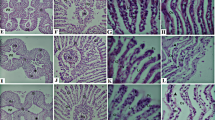
No mortality was observed following Cd2+ exposure. The highest levels of cell apoptosis signals appeared after exposure to Cd2+ for 3 h, and recovery took place during a period of 3–6 h after 1.78 μM of Cd2+ exposure in the gills (Fig. 7). Similar results were found in the brain, where the cell apoptosis reached its highest level 3 h after Cd2+ exposure, followed by recovery after 6 h (Fig. 8).
Discussion
Previous studies have reported that heavy metals induce ROS in fish, and others have examined antioxidant gene expressions to decrease oxidative damage (Firat et al. 2009; Cao et al. 2010). Such defense mechanisms have been proposed as biomarkers following pro-oxidant challenge (Regoli et al. 2002). In addition, many studies have sought to monitor environmental pollution by tracking an organism’s antioxidant biomarkers. For example, the GPX gene has emerged as a basic research tool for studying antioxidants and is used as an indicator for measuring environmental pollution or stress in fish (Choi et al. 2007). The present study found that Cd2+ exposure exerted a range of impact such as cell apoptosis and the increase in mRNA expressions of antioxidant enzyme genes in the brain and gills of adult zebrafish.
Exposure to Cd2+ has been found to cause a time- and dose-dependent increase in Cd2+ contents in various fish species (Wu et al. 2006; Firat et al. 2009). Accumulation of Cd2+ in fish could stimulate the production of ROS, which then reacts with susceptible biological macromolecules, resulting in changes to LPO and antioxidant defenses (Firat et al. 2009). In the present study, ROS levels of gills did not significantly change over various exposure times, but were elevated in the treatment group as compared to the control group 3 h after Cd2+ exposure (Fig. 1a). However, results in the brain were significantly different, with ROS contents increasing significantly during 1–6 h after Cd2+ exposure, but no change was observed in the LPO levels upon Cd2+ exposure (Fig. 3). Increased ROS levels as a result of Cd2+ exposure probably produce antioxidant enzymes involved in protecting against oxidative stress. Increased LPO levels in fish following Cd2+ exposure are usually due to oxidative stress (Cao et al. 2010). Thus, the present study showed that the LPO level did not change in the brain even when the ROS were increased during exposure of Cd2+ for 1–6 h. These results were to prove that the brain has a powerful anti-oxidative system under Cd2+ exposure. On the other hand, ROS level significantly increased around 1.5-fold and LPO levels on the treatment group was lower than the control following 3 h of Cd2+ exposure in the gills. The LPO level seems to be either a compensative physiological response or a yet identified reason, which could be investigated in the future.
The present study measured the mRNA expression of these antioxidants with real-time RT-PCR (qPCR) and normalization by β-actin. In fact, β-actin has been the most commonly used reference gene for normalizing qRT-PCR data in zebrafish (Tang et al. 2007). In addition, our previous study has reported that the zebrafish β-actin gene appeared suitable as a reference gene on Cd2+ exposure study (Liu et al. 2012). These antioxidant enzymes, including mRNA levels of sod, cat, and gpx1a, respectively, increased 2.2-, 1.6-, and 3.1-fold after 1 h of Cd2+ exposure as compared to the control. Our previous study reported that Cd2+ temporarily accumulates in the gills before being transferred to the digestive organs via the circulatory system or enterohepatic circulation, resulting in lower concentrations of Cd2+ in the gills than in other organs (Wu et al. 2007). This is the likely cause of the rise in ROS levels in the gills, 1.3- to 1.5-fold after Cd2+ exposure, which were lower than in the brain.
The uptake of Cd by living cells usually induce ROS to rise, cause oxidative stress (Hsu et al. 2013), and normally leads to cell apoptosis depending on the Cd dose and time of exposure (Chen et al. 2014). However, it should be mediated by some antioxidants. In the present study, ROS levels, respectively, increased 8.0-, 7.1- and 6.2-fold in the brain 1, 3, and 6 h after Cd2+ exposure, respectively. Therefore, the sod, cat, and gpx1a mRNA levels increased by 1.2-, 16.5-, and 65.6-fold after exposure to Cd2+ for 1 h, and by 2.4-, 9.2- and 29.5-fold after Cd2+ exposure for 3 h. We suggest that the LPO levels did not significantly increase since these expressions of antioxidant (sod, cat, and gpx1a) increase in the brain. Observably, these antioxidant expressions also provided a dominant antioxidant system in the brain after Cd2+ exposure.
Both SOD and CAT directly decrease the toxicity of ROS. SOD is the enzyme which deals with oxygen toxicity. It can remove O2− through the process of dismutation (super oxide free radical to hydrogen peroxide and oxygen) (An et al. 2010). CAT can reduce H2O2 into water and oxygen to prevent oxidative stress and maintain cell homeostasis (Kashiwagi et al. 1997). GPx activity is believed to play an important role in cellular antioxidant defense by using glutathione (GSH) as a reducing agent to reduce H2O2 and various hydroperoxides (Wendel 1980). Similar to previous studies, the present study measured how some anti-oxidative enzymes such as sod, cat, and gpx1a defend the organism against oxidative stress through the Cd2+ toxicity induction. The three enzyme antioxidants are all interrelated, with the cat and gpx1a expressions increasing, followed by an increase in sod mRNA levels within 3 h of exposure time. Atli and Canli (2010) reported that the stimulation of CAT activity is associated with an effective antioxidant defense system acting against oxidative stress and compensating for the decrease in other antioxidant enzymes, such as SOD and GPx. The present study confirmed the cascade reaction of anti-oxidative system upon Cd2+ exposure. However, a lack of significant change or decrease in the cat mRNA may be attributed to the increase in gpx1a and mt. In the gills, cat showed no significant change but gpx1a and mt mRNA levels increased significantly at 3 h after Cd2+ exposure. In the brain, all antioxidants including cat, sod, gpx1a, smtB, and mt2 mRNA expression increased 3 h following Cd2+ exposure, but then decreased after 6 h. The LPO level did not significantly change within 6 h after Cd2+ exposure.
Aside from SOD, CAT, and GPx, MT is the best-known antioxidant providing protection against metal toxicity (Choi et al. 2007). The Cyprinidae MT gene family includes two major groups: D. rerio mt2 and MT-A. smtB; an expressed sequence tag (EST) of D. rerio was submitted to GenBank in 1999, and its physiological functions have been gradually explored. Our previous study hypothesized that mt2 is more important during larval stages, but smtB may perform certain physiological functions in the brain of mature zebrafish (Wu et al. 2008a). Moreover, transcripts of smtB in the brain increased significantly by 3.5-fold at 1 h after cold shock, which had a higher mRNA expression than that in the gill and liver (Wu et al. 2015). The present study showed that the smtB mRNA levels were significantly increased, respectively, by 6.5-fold (0.13/0.02) in the gills and 59.4-fold (15.44/0.26) in the brain at 3 h after Cd2+ exposure. In contrast, the mt2 mRNA levels expression increased 11-fold in gills and brain after 3 h of Cd2+ exposure. These results represent that the physiological function of smtB might be more important than mt2 in the brain of the adult zebrafish upon stress.
It is well known that ROS can cause severe damage to cellular macromolecules, especially DNA (Barzilai and Yamamoto 2004). Cell death can be detected using a TUNEL assay to label DNA strand breaks. The present data showed the strongest levels of cell apoptosis in the gills and brain after exposure to Cd2+ for 3 h, and both tissues showed that the cells were repaired 3–6 h after exposure to Cd2+ (Figs. 7, 8). Depending on the changes over time, the results of cell apoptosis were found to correspond to the anti-oxidative responses. Furthermore, three enzymatic antioxidant gene expressions (sod, cat, and gp1ax) and two non-enzymatic antioxidant gene expressions (mt2 and smtB) were confirmed to serve as biomarkers for oxidative stress on fish. The transcripts of the three gene expressions showed a noticeable increase during 1–3 h of Cd2+ exposure, with an increase in cell apoptosis during that time. In addition, the three genes’ transcripts decreased at 6 h, and the apoptosis levels in the brain and gills also decreased. According to these data, we suggested that mt2 and smtB both genes might have two functions upon the fish’s exposure to metals: binding metals and defending against oxidative stress. However, under Cd2+ exposure, smtB might play more important roles than mt2 in defending oxidative stress in brain of adult zebrafish.
Conclusion
The results of the present investigation show that ROS can be induced by 1.78 μM Cd2+ exposure and do not cause significant oxidative stress in the brain or gill tissue because many antioxidant genes act to defend against oxidative stress during a short time after Cd2+ exposure. The brain and gill tissue still exhibit cell apoptosis 3 h after Cd2+ exposure and recovery after 3–6 h. The present study is the first attempt to compare the antioxidant functions of mt2 and smtB following Cd2+ exposure between the brain and gills and finds that smtB seems to play a more significant antioxidant role in the brain than in the gills, while mt2 serves a similar function between the brain and gills.
References
Alvarez RM, Morales AE, Sanz A (2005) Antioxidant defenses in fish: biotic and abiotic factors. Rev Fish Biol Fish 15:75–88
An KW, Kim NN, Shin HS, Kil GS, Choi CY (2010) Profiles of antioxidant gene expression and physiological changes by thermal and hypoosmotic stresses in black porgy (Acanthopagrus schlegeli). Comp Biochem Physiol 156A:262–268
Atli G, Canli M (2010) Response of antioxidant system of freshwater fish Oreochromis niloticus to acute and chronic metal (Cd, Cu, Cr, Zn, Fe) exposures. Ecotoxicol Environ Saf 73:1884–1889
Bagnyukova TV, Lushchak OV, Storey KB, Lushchak VI (2007) Oxidative stress and antioxidant defense responses by goldfish tissues to acute changes of temperature from 3 to 23 °C. J Therm Biol 32:227–234
Barzilai A, Yamamoto KI (2004) DNA damage responses to oxidative stress. DNA Repair 3:1109–1115
Cao L, Huang W, Liu J, Yin X, Dou S (2010) Accumulation and oxidative stress biomarkers in Japanese flounder larvae and juveniles under chronic cadmium exposure. Comp Biochem Physiol 151C:386–392
Chen Y, Maret W (2001) Catalytic selenols couple the redox cycles of metallothionein and glutathione. Eur J Biochem 268:3346–3353
Chen YY, Zhu JY, Chan KM (2014) Effects of cadmium on cell proliferation, apoptosis, and proto-oncogene expression in zebrafish liver cells. Aquat Toxicol 157:196–206
Choi CY, An KW, Nelson ER, Habibi HR (2007) Cadmium affects the expression of metallothionein (MT) and glutathione peroxidase (GPX) mRNA in goldfish, Carassius auratus. Comp Biochem Physiol 145C:595–600
Choi JE, Kim J, Ahn JH, Youn P, Kang JS, Park K, Yi J, Ryu DY (2010) Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish. Aquat Toxicol 100:151–159
Crawshaw L, Grahn D, Wollmuth L, Simpson L (1985) Central nervous regulation of body temperature in vertebrates: comparative aspects. Pharmacol Ther 30:19–30
Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H (2010) Cadmium stress: an oxidative challenge. Biometals 23:927–940
Firat O, Cogun HY, Aslanyavrusu S, Kargin F (2009) Antioxidant responses and metal accumulation in tissues of Nile tilapia Oreochromis niloticus under Zn, Cd and Zn+Cd exposures. J Appl Toxicol 29:295–301
Hsu T, Huang KM, Tsai HT, Sung ST, Ho TN (2013) Cadmium (Cd)-induced oxidative stress down-regulates the gene expression of DNA mismatch recognition proteins MutS homolog 2 (MSH2) and MSH6 in zebrafish (Danio rerio) embryos. Aquat Toxicol 126:9–16
Imada I, Sato EF, Miyamoto M, Ichimori Y, Minamiyama Y, Konaka R, Inoue M (1999) Analysis of reactive oxygen species generated by neutrophils using a chemiluminescence probe L-012. Anal Biochem 271:53–58
Kashiwagi A, Kashiwagi K, Takase M, Hanada H, Nakamura M (1997) Comparison of catalase in diploid and haploid Rana regosa using heat and chemical inactivation techniques. Comp Biochem Physiol 118B:499–503
Leonard SS, Harris GK, Shi X (2004) Metal-induced oxidative stress and signal transduction. Free Radic Biol Med 37:1921–1942
Liu CT, Chou MY, Lin CH, Wu SM (2012) Effects of ambient cadmium with calcium on mRNA expression of calcium uptake related transporters in zebrafish (Danio rerio) larvae. Fish Physiol Biochem 38:977–988
Porter NA, Mills KA, Caldwell SE (1995) Mechanisms of free radical oxidation of unsaturated lipids. Lipids 30:277–290
Regoli F, Gorbi S, Frenzilli G, Nigro M, Corsi I, Focardi S, Winston GW (2002) Oxidative stress in ecotoxicology: from the analysis of individual antioxidants to a more integrated approach. Mar Environ Res 54:419–423
Roesijadi G (1992) Metallothioneins in metal regulation and toxicity in aquatic animals. Aquat Toxicol 22:81–114
Sato M, Bremner I (1993) Oxygen free radicals and metallothionein. Free Radic Biol Med 14:325–337
Sellin MK, Eidem TM, Kolok AS (2007) Cadmium exposures in fathead minnows: Are there sex-specific differences in mortality, reproductive success, and Cd accumulation? Arch Environ Contam Toxicol 52:535–540
Shimoda R, Achanzar WE, Qu W, Nagamine T, Takagi H, Mori M, Waalkes MP (2003) Metallothionein is a potential negative regulator of apoptosis. Toxicol Sci 73:294–300
Tang R, Dodd A, Lai D, Mcnabb WC, Love DR (2007) Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochem Biophys Sin 39:384–390
Taylor EW, Beaumont MW, Butler PJ, Mair J, Mujallid MSI (1996) Lethal and sublethal effects of copper upon fish: a role for ammonia toxicity. In: Taylor EW (ed) Toxicology of aquatic pollution: physiological, cellular and molecular approaches. Cambridge University Press, Cambridge, pp 85–113
Tseng YC, Chen RD, Lucassen M, Schmidt MM, Dringen R, Abele D, Hwang PP (2011) Exploring upcoupling proteins and antioxidant mechanisms under acute cold exposure in brain of fish. PLoS One 6:e18180
Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M (2006) Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf 64:178–189
Wendel A (1980) Glutathione peroxidase. In: Jakoby WB (ed) Enzymatic basis of detoxification, vol 1. Academic Press, New York, pp 333–353
Wu SM, Jong KJ, Lee YJ (2006) Relationships among metallothionein, cadmium accumulation, and cadmium tolerance in three species of fish. Bull Environ Contam Toxicol 76:595–600
Wu SM, Shih MJ, Ho YC (2007) Toxicological stress response and cadmium distribution in hybrid tilapia (Oreochromis sp.) upon cadmium exposure. Comp Biochem Physiol 145C:218–226
Wu SM, Zheng YD, Kuo CH (2008a) Expression of mt2 and smt-B upon cadmium exposure and cold shock in zebrafish (Danio rerio). Comp Biochem Physiol 148C:184–193
Wu SM, Ding HR, Lin LY, Lin YS (2008b) Juvenile tilapia (Oreochromis mossambicus) strive to maintain physiological functions after waterborne copper exposure. Arch Environ Contam Toxicol 54:482–492
Wu SM, Tsai PR, Yan CJ (2012) Maternal cadmium exposure induces mt2 and smtB mRNA expression in zebrafish (Danio rerio) females and their offspring. Comp Biochem Physiol 156C:1–6
Wu SM, Liu JH, Shu LH, Chen CH (2015) Anti-oxidative responses of zebrafish (Danio rerio) gill, liver and brain tissues upon acute cold shock. Comp Biochem Physiol 187A:202–213
Acknowledgments
This study was financially supported by the National Science Council, Taiwan (NSC101-2313-B-415-003-MY3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, S.M., Shu, LH. & Liu, JH. Anti-oxidative functions of mt2 and smtB mRNA expression in the gills and brain of zebrafish (Danio rerio) upon cadmium exposure. Fish Physiol Biochem 42, 1709–1720 (2016). https://doi.org/10.1007/s10695-016-0251-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-016-0251-1