Abstract
This work reports on the method optimization and application for quantitative analysis of non-steroidal anti-inflammatory drugs and anti-epileptic drug in soil and sediment samples. The analytes were extracted by ultrasonic extraction followed by solid phase extraction and quantified using liquid chromatographic coupled with photodiode array. The sensitivity of the method was determined based on the limit of detection and the limit of quantification which ranged between (0.010–0.027 µg/kg) and (0.025–0.049 µg/kg), respectively. The %recoveries of the method ranged between 74% and 112%. The concentrations obtained in real samples ranged from 0.055 to 0.426 µg/kg in sediment and 0.044–0.567 µg/kg in soil samples. The highest concentration was found for diclofenac in soil samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The occurrence and the fate of pharmaceuticals in the environment is a growing concern, especially since the consumption of pharmaceuticals is increasing due to the growth in the number of people being infected and diagnosed with various chronical diseases (Kalyva 2017). Pharmaceuticals are not completely digested in the body, therefore their residues are excreted from the body (as metabolites or parent compounds), through urine and feces. Thus they enter the sewer system and hence transported to the wastewater treatment plants (WWTPs), (Madikizela 2017). Studies have shown that WWTPs do not completely remove these residues during the treatment processes and thus they are released into the environment with the treated effluent. Some pharmaceutical compounds get adsorbed into sludge, which is thereafter used as fertilizer in agricultural lands and hence the pharmaceuticals end up entering the biosphere. Other sources of pharmaceuticals in the environment include direct disposal of unused and expired drugs, hospital discharge, pharmaceutical manufacturing industries, lack of sanitation in rural areas, leaching into the soil during heavy rainfall, agricultural irrigation, sorption, human and veterinary use (Vonkeman and van der Laar 2010; Kermia et al. 2016).
A lot of emphasis and studies have been made on the fate of pharmaceutical compounds in the water bodies, and little interest has been placed on their occurrence and fate in the terrestrial environment (soil, sediment, and sludge). Similar to the aqueous environment, pharmaceutical compounds interact with the soil and sediment depending on their physicochemical properties, and also on the environmental chemical and biological processes. Once pharmaceuticals reach the environment they tend to undergo processes such as biodegradation, biotransformation, hydrolysis and also adsorption into soil particles (Caldwel et al. 2016). Exposure to pharmaceuticals has negative effects on the aquatic and terrestrial organisms as well as the environment. The effects include hormone disruption to the aquatic organisms, the feminization of male fish, the inhibition of the cyclooxygenase in population, the adsorption into plant species and thus the uptake by terrestrial animals such as earthworm, they also affect algae and bacteria (Białk-bielińs et al. 2016). Due to the effect that pharmaceuticals have, it is important that they are investigated in various environmental matrices.
The detection and quantification of pharmaceuticals at trace levels in complex environmental samples poses an analytical problem. This is due to the lack of sensitivity in the current methods used to detect them at such low levels. Thus, sample preparation step is very important before the analysis of environmental samples for extraction and pre-concentration of the analytes of interest and also the removal of matrix interferences, which results in improved selectivity and sensitivity of the analytical method (Białk-bieliń et al. 2016; Shraim et al. 2017).
Therefore, in this work, an ultrasonic extraction (UE) method has been optimised followed by pre-concentration by solid phase extraction (SPE) for the extraction of pharmaceutical drugs in soil and sediment samples. The pharmaceutical drugs studied in this work were the non-steroidal anti-inflammatory drug (NSAIDs) which include naproxen, fenoprofen, diclofenac and ibuprofen and also an anti-epileptic drug, carbamazepine. NSAIDs have anti-inflammatory, analgesic and antipyretic properties and they are considered the most widely used drugs for the relief of chronical pain and fever (Madikizela 2017). Carbamazepine drug is used to treat epilepsy which is a neurological disease that affects the brain and causes seizes that have affected about 50 million people worldwide (Gracia-Lor 2012).
Ibuprofen (98%), fenoprofen (97%), naproxen (98%), diclofenac (98%) and carbamazepine (98%), acetonitrile (99.9%), methanol (99.9%), dichloromethane (99.9%), acetone (99.9%) and ethyl acetate (99.9%) were all purchased from Sigma Aldrich (Johannesburg, South Africa).
LC-2020 equipped with Shim-Pack GIST C18-HP column (4.6 × 150 mm, 3 µm) and LC-2030/2040 PDA detector used for the analysis of the compounds was purchased from Shimadzu (Europe, Germany). The mobile phase composition of 60:40 (acetonitrile: water) and a flow rate of 0.5 mL/min were used and the data were acquired at 229 nm. Cavitek digital controlled ultrasonic water bath purchased from Science Tech (Durban, South Africa) was used for analytes extraction. The SPE bought from Sigma Aldrich (Steinheim, Germany) was used for the cleaning up of the extracts. SPE was connected to a pump manifold from Edwards (Munich, Germany). Oasis Hydrophobic-Lipophilic Balance, HLB (60 mg, 3 mL) purchased from Biotage (Uppsala, Sweden) were used as SPE sorbents.
A standard solution (100 mg/L) containing all analytes of interests (carbamazepine, naproxen, fenoprofen, diclofenac, and ibuprofen) was prepared by transferring 10 mg of each compound in a 100 mL volumetric flask and dissolved in acetonitrile. The calibration curve of each compound was obtained by analyzing the concentration of the standard solution (0.2–1 mg/L) and analyzed using liquid chromatography coupled with photodiode array (LC-PDA). All the standards were kept in the refrigerator at 4°C until used.
Soil samples were collected in farmlands and along the Msunduzi River in the Pietermaritzburg area, KwaZulu-Natal (South Africa). These included Richmond, Cedara, Donnybrook, Mngeni Valley and in four sampling points along the Msunduzi River (College Road, Camps Drift and Woodhouse). The sediment samples were collected in Pietermaritzburg along the Msunduzi River and uMngeni and Amanzimtoti Rivers. The sediments and soil samples were collected using a stainless soil auger in about 0–20 cm depth. The samples were collected in brown bottles and placed in a cooler box and then transported to the laboratory.
The soil and sediment samples were air-dried at room temperature in a fume hood. After drying they were ground and sieved prior to extraction with UE and then clean- up and pre-concentration by SPE. The optimum UE procedure was 5 g of sample spiked with pharmaceutical standards mixture to make the final concentration of 1 mg/kg. 5 mL of distilled water was added to the sample to ensure even the distribution of the analyte on the soil and then ultra-sonicated for 15 min. Thereafter, 20 mL acetonitrile: methanol (1:1, v/v) was added to the samples and further ultra-sonicated for 20 min. The samples were left to settle and the supernatant was collected and centrifuged for 10 min, in order to remove any soil traces left. The extract was then reduced to 1 mL using a rotavapor which was then reconstituted to 50 mL with distilled water. The 50 mL sample was then passed through the SPE cartridge conditioned with 2 mL acetonitrile. It was then washed with 2 mL of distilled water to remove impurities and the adsorbed analytes were eluted with 2 mL of methanol. The analytes were then concentrated to 1 mL under nitrogen and analyzed using LC-PDA.
The extraction procedure reported by Gumbi et al. (2017) was initially used and further optimized to obtain optimum extraction conditions that will give high recoveries for all analytes of interest from soil/sediment samples. The UE parameters investigated were extraction solvent, solvent volume, extraction time and sample mass.
The optimized method was validated in terms of the limit of detection (LOD), limit of quantification (LOQ), precision, linearity and percentage recoveries. The LOD and LOQ were calculated as 3 and 10 times the signal to noise ratio, respectively, using the equation, LOD = s × 3.3, where ‘s’ is the standard deviation of the replicates (Carlson et al. 2014). The precision of the method was validated in terms of reproducibility and repeatability represented as relative standard deviation (RSDs). The linearity of the method was studied using five standard point calibration ranging from 0.1 to 1.0 mg/L. The percentage recoveries were determined using 5 g of soil sample spiked with the mixture of the analytes of interest (carbamazepine, naproxen, fenoprofen, diclofenac and ibuprofen) to make the final concentration of 1 mg/kg. Concentrations of 0.01 and 0.1 mg/kg were then analysed under optimum conditions for soil and sediment samples to assess the effect of concentration on the percentage recoveries of the analytes. All the extractions were done in triplicates.
Results and Discussion
Investigating the effect of the extraction solvent is an important step because the effectiveness of the UE procedure depends on the extraction solvents capacity to absorb and transmit the energy of the ultrasound onto the soil sample to be extracted (Kong et al. 2014). The effect of extraction solvents was examined using methanol, acetone, methanol: acetonitrile (1:1), methanol: acetonitrile: ethyl acetate (1:1:1) and methanol: acetonitrile: water (1:1:1), methanol: acetonitrile: acetone (1:1:1).
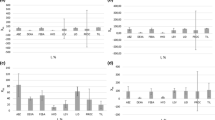
Acetonitrile showed the lowest recoveries for all the analytes and fenoprofen, diclofenac and ibuprofen were not extracted (Fig. 1). When methanol and acetonitrile were mixed, they produced similar results as those obtained with methanol alone. This further indicated that acetonitrile does not have much effect on the extraction of the analytes. The mixture of methanol, acetonitrile and ethyl acetate or acetone showed an improvement in the percentage recoveries for all analytes, however, they were still below 70% except carbamazepine. The mixture of water + methanol + acetonitrile gave the highest recoveries (74%–112%). This indicated that mixing these solvents enhanced their penetration into the sample matrix which resulted in the efficient diffusion of the analyte from the soil core to the extraction solvent (Kong et al. 2014). Therefore, a mixture of water + methanol + acetonitrile was taken as the optimum extraction solvent.
The influence of extraction solvent on the pharmaceuticals recoveries. Extraction conditions were – extraction mass: 5 g of soil was spiked with 1 ppm of the pharmaceuticals mixture, extraction time: 40 min, extraction solvent volume: 20 mL. ACN acetonitrile, MeOH: methanol, ACT acetone, EA ethyl acetate, H2O water
The effect of extraction time was investigated at 10, 20 and 40 min, using water + methanol + acetonitrile as the extraction solvents. The recoveries showed an increase as the extraction time increases and 40 min gave the recoveries ranging from 74% to 112% (Fig. 2). The reason for the increase in recoveries could be due to that the longer extraction time results in more time available for the extraction solvent to penetrate the soil matrix and thus break the bonds between the soil and the analyte resulting to effective extraction and hence higher analyte recoveries. This also indicated that 40 min was not too long to results in the degradation of the analytes (Ntombela and Mahlambi 2019) and hence it was adopted as the efficient extraction time.
The sample mass was investigated by extracting 1, 3 and 5 g of sediment sample using water + methanol + acetonitrile and the extraction time of 40 min. From the results obtained it was observed that the recoveries increased with the increase in sample mass and 5 g sample gave the recoveries which ranged from 74% to 112% (Fig. 3). The increase in the recoveries could be due to that as the mass increases the amount of analytes available to dissolve into the extraction solvent also increases thus resulting in more analytes to be recovered (Ntombela and Mahlambi 2019). Therefore 5 g was taken as the optimum sample mass.
The performance of the UE-LC-PDA method was inspected based on LODs, LOQs, %recoveries, and precision. The LOQs obtained ranged between 0.025 and 0.049 µg/kg and the LODs were between 0.010 and 0.027 µg/kg (Table 1). The recoveries ranged between 74% and 112%. The RSDs obtained for all the analytes ranged between 1% and 3% which indicated good precision of the optimized method (Table 2). The recoveries obtained were almost similar in different spike concentrations used which shows that they are independent of the concentration present in the samples. This indicate the direct proportionality of the amount extracted to that in the sample which is important for accurate quantification.
The LOD and LOQ results obtained in this work are lower than those reported by Matongo et al. (2015) on the analysis of pharmaceuticals in sediments where LODs, LOQs and RSDs ranging between 0.054–0.49 µg/kg, 1.8–1.6 µg/kg and 0.82%–6.8%, respectively. They are also lower than those obtained by Gumbi et al. (2017) where LODs ranged from 0.30 to 1.03 µg/kg, LOQs were 0.048–0.092 µg/kg and RSDs were 7%–20%. The recoveries obtained in this work are that higher than those obtained by Gumbi et al. (2017), (66%–103%). This indicates the good precision and accuracy of the optimized method.
The optimized UE-LC-PDA method was then applied to soil and sediment samples obtained around KwaZulu-Natal. The soil samples were collected from Richmond, Cedara, Donny Brook and Mngeni Valley, Woodhouse, Bishopstowe, Camps Drift. Sediment samples were collected along with the Msunduzi River (Camps Drift, Woodhouse and College Road).
The effect of seasonal variation was investigated using sediment samples collected in Woodhouse and Bishopstowe during the winter and spring season (Table 3). In Woodhouse, naproxen was detected in both seasons and the concentrations obtained are comparable. In Bishopstowe carbamazepine and diclofenac were detected in both seasons with higher concentrations detected in the winter season, and in spring they were detected below the quantification limits. Diclofenac was detected with the highest concentration of 0.426 µg/kg in Bishopstowe during the winter season. Diclofenac has a high adsorption coefficient (Koc) of 830, which means that it has a high affinity for sediment hence it is captured by the sediment pores thus reducing its solubility in water (Díaz and Pena 2017). The reason for higher concentrations being detected in winter could be due to lower temperatures which results in the decrease or stop of the reproduction of micro-organisms, therefore, the bio-degradation and photo-degradation of these compounds are decreased resulting in higher concentrations to be detected in the sediments. Similar findings were reported by Varga et al. (2010), where higher concentrations of ibuprofen, diclofenac, and naproxen were detected during the winter season in sediment samples collected in the Danube River in Budapest (Hungary) with maximum concentration (29–38 µg/kg) which are higher than those obtained in this study. Gumbi et al. (2017), also investigated the occurrence of naproxen and ibuprofen pharmaceuticals in the Mgeni River (KwaZulu Natal), and higher concentrations of ibuprofen (4.31–13.4 µg/kg) were obtained during the winter season.
Ibuprofen was only detected in Woodhouse in spring with a concentration of 0.128 µg/kg, which could be due to its high water solubility and low affinity for sediment matrices because of its moderate octanol–water coefficient (Kow) (3.97), (Madikizela 2017). Bishopstowe was the most polluted site which could be due to that it is situated just after the Darvill WWTP, therefore the treatment plant could be the possible source of contamination when it discharges its treated effluent into the river.
The concentration of pharmaceuticals obtained in soil ranged from 0.044 to 0.567 µg/kg (Table 4). Diclofenac was detected with the highest concentrations of 0.567 µg/kg in Donny Brook and 0.557 µg/kg in Cedara samples. Detection of diclofenac could be due to its high affinity for the soil matrices and hence it easily gets absorbed into the soil matrix. Naproxen was detected with the lowest concentrations compared to other analytes in most of the samples which could mean that it has low affinity for the soil matrix and hence poorly absorbed into the soil. However, naproxen was the only detected analyte in Bishopstowe but below the quantification limits. Soil samples obtained from Richmond, Cedara and Curry Post were found to be contaminated with all the analytes of interest. Sources of pharmaceutical residues mainly in the soil are sewage sludge as it used as manure as well as irrigation using treated wastewater which may contain pharmaceutical contaminants, also animal manure if it is applied in the land (Białk-bielińs et al. 2016). These sampling areas can also be contaminated due to illegal dumping on or near the sampling site, which could be the point source of pharmaceuticals. In Woodhouse, none of the analytes were detected. The factors that may contribute to the absence of pharmaceuticals in the environment are if they undergo the processes of biodegradation and photodegradation.
The maximum concentrations obtained in this study are lower than those obtained by Gibson et al. (2010), on the analysis of pharmaceuticals in soil samples obtained from Mexico which were (0.3–16.4 µg/kg) as well as those obtained in Spain farmers for ibuprofen (1.5–3.2 µg/kg), naproxen (nd-5.9 µg/kg), fenoprofen (nd-3.2 µg/kg) and carbamazepine (nd-2.8 µg/kg), (Roca, 2016). However, the concentrations obtained in this study raise the concern due to the ecological and environmental effects that pharmaceutical compounds have.
UE-LC-PDA method has been successfully developed and applied for the analysis of pharmaceutical compounds in soil and sediments. The LODs and LOQs ranged from 0.025 to 0.049 µg/kg and 0.010 to 0.027 µg/kg respectively. The percentage of recoveries ranged from 74% to 112%.
The concentrations obtained in sediment ranged from 0.055 to 0.426 µg/kg and in the soil they ranged from 0.044 to 0.567 µg/kg. The highest concentration (0.567 µg/kg) was detected for diclofenac in the Donny Brook soil sample. Higher concentrations were obtained during winter (0.055–0.426 µg/kg) compared to spring season (0.075–0.128 µg/kg), however, the highest number of pharmaceuticals were detected in the spring season. This is due to that photo-degradation is higher during the hot season which results in lower concentrations of pharmaceuticals being found in environmental samples. The concentrations detected in this study were higher than those detected from previous studies, which is a great concern, because of ecological, biological effect and health risks associated with pharmaceuticals in the environment. Also, there are no set allowable concentrations for pharmaceuticals in the environment. Therefore, this research will contribute to the knowledge of the concentration of pharmaceuticals being detected in the South African environment and thus enable the policymakers to set the allowable concentrations for the South African environment.
References
Białk-bielińska A, Kumirska J, Borecka M, Caban M, Paszkiewicz M, Pazdro K, Stepnowski P (2016) Selected analytical challenges in the determination of pharmaceuticals in drinking/marine waters and soil/sediment samples. J Pharm Biomed Anal 121:271–296. https://doi.org/10.1016/j.jpba.2016.01.016
Caldwell DJ (2016) Sources of pharmaceutical residues in the environment and their control. Environ Sci Technol 41:92–119. https://doi.org/10.1039/9781782622345-00092
Carlson J, Wysoczanski A, Voigtman E (2014) Limits of quantitation—yet another suggestion. Spectrochim Acta Part B 96:69–73
Díaz A, Peña-Alvarez A (2017) A simple method for the simultaneous determination of pharmaceuticals and personal care products in river sediment by ultrasound-assisted extraction followed by solid-phase microextraction coupled with gas chromatography-mass spectrometry. J Chromatogr Sci 55:946–953. https://doi.org/10.1093/chromsci/bmx058
Gibson R, Durán-Álvarez JC, Estrada KL, Chávez A, Cisneros BJ (2010) Accumulation and leaching potential of some pharmaceuticals and potential endocrine disruptors in soils irrigated with wastewater in the Tula Valley, Mexico. Chemosphere 81:1437–1445. https://doi.org/10.1016/j.chemosphere.2010.09.006
Gracia-Lor E, Sancho JV, Serrano R, Hernández F (2012) Occurrence and removal of pharmaceuticals in wastewater treatment plants at the Spanish Mediterranean area of Valencia. Chemosphere 87:453–462. https://doi.org/10.1016/j.chemosphere.2011.12.025
Gumbi BP, Moodley B, Birungi G, Ndungu PG (2017) Assessment of nonsteroidal anti-inflammatory drugs by ultrasonic-assisted extraction and GC-MS in Mgeni and Msunduzi river sediments, KwaZulu-Natal, South Africa. Environ Sci Pollut Res Int 24:20015–20028. https://doi.org/10.1007/s11356-017-9653-6
Kalyva M (2017) Fate of pharmaceuticals in the environment—a review, MSc thesis, Umeå University, Umeå, Sweden
Kermia AEB, Fouial-Djebbar D, Trari M (2016) Occurrence, fate and removal efficiencies of pharmaceuticals in wastewater treatment plants (WWTPs) discharging in the coastal environment of Algiers. C R Chim 19:963–970. https://doi.org/10.1016/j.crci.2016.05.005
Kong W, Liu N, Zhang J, Yang Q, Hua S, Song H, Xia C (2014) Optimization of ultrasound-assisted extraction parameters of chlorophyll from Chlorella Vulgaris residue after lipid separation using response surface methodology. Int J Food Sci Technol 51:2006–2013. https://doi.org/10.1007/s13197-012-0706-z
Madikizela LM (2017) Determination of selected acidic pharmaceutical compounds in wastewater treatment plants. PhD thesis, University of the Witwatersrand, Johannesburg, South Africa. https://www.hdl.handle.net/10539/22741
Matongo S, Birungi G, Moodley B, Ndungu P (2015) Pharmaceutical residues in water and sediment of Msunduzi River, KwaZulu-Natal, South Africa. Chemosphere 134:133–140. https://doi.org/10.1016/j.chemosphere.2015.03.093
Ntombela SC, Mahlambi PN (2019) Method development and application for triazine herbicides analysis in water, soil and sediment samples from KwaZulu-Natal. J Environ Sci Heal B 54:569–579
Roca RA (2016) Determination of emerging contaminants in environmental matrices. PhD thesis, Universidad Politécnica de Madrid, Madrid, Spain
Shraim A, Diab A, Alsuhaimi A, Niazy E, Metwally M, Amad M, Sioud S, Awoud A (2017) Analysis of some pharmaceuticals in municipal wastewater of Almadinah Almunawarah. Arab J Chem 10:S719–S729. https://doi.org/10.1016/j.arabjc.2012.11.014
Varga M, Dobor J, Helenkár A, Jurecska L, Yao J, Záray G (2010) Investigation of acidic pharmaceuticals in river water and sediment by microwave-assisted extraction and gas chromatography-mass spectrometry. Microchem J 95:353–358. https://doi.org/10.1016/j.microc.2010.02.010
Vonkeman HE, van de Laar MA (2010) Nonsteroidal anti-inflammatory drugs: adverse effects and their prevention. Semin Arthritis Rheum 39:294–312. https://doi.org/10.1016/j.semarthrit.2008.08.001
Acknowledgements
The authors acknowledge the National Research Funding (NRF) of South Africa (Thuthuka Grant Number: 107091) and University of KwaZulu Natal for funding the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hlengwa, N.B., Mahlambi, P.N. Ultrasonic Followed by Solid Phase Extraction and Liquid Chromatography-Photodiode Array for Determination of Pharmaceutical Compounds in Sediment and Soil. Bull Environ Contam Toxicol 104, 464–470 (2020). https://doi.org/10.1007/s00128-020-02829-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-020-02829-6