Abstract
The present study was aimed to evaluate the effect of municipal wastewater of Tung Dhab drain to freshwater fish Labeo rohita through genotoxicity assays. The idea is to explore and standardize procedures for assessment of toxic impact of pollutant at the cellular level in aquatic species. Hence, chronic, non-renewal toxicity tests were performed with sublethal concentrations (17.7, 26.6 and 35.4 %) of wastewater for exposure durations of 15, 30 and 60 days. Recovery experiments were also performed for duration of 60 days. Higher micronuclei (MN) and binuclei (BN) frequencies were recorded in fluorescently stained liver cells as compared to frequencies obtained for gill cells and erythrocytes. The induced frequencies of MN, BN and nuclear abnormalities (NA) were found in the following order: MN (liver cells) > BN (liver cells) > BN (gill cells) > MN (gill cells) > NA (erythrocytes) > MN (erythrocytes). The results pointed towards the mutagenic nature of water quality of Tung Dhab drain and recommend the incorporation of the liver and gill cells as biomarkers of cytotoxicity over erythrocytes in chronic experiments during water monitoring programs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The aquatic ecosystems are threatened due to unregulated discharge of industrial, agricultural and municipal pollutants throughout the world. Their release into the aquatic bodies not only leads to contamination of surface waters but also has the potential to disturb the structure and functioning of natural ecosystems (De Flora et al. 1993; Al-Sabti and Metcalfe 1995; Cavas and Ergene-Gozukara 2003; Cavas and Ergene-Gozukara 2005; Kaur and Dua 2015a, b). Bolognesi and Hayashi (2011) reviewed that among the various pollutants, carcinogenic and mutagenic compounds are the most dangerous as they hold their activities for several generations. The sensitivity of fish to contaminants in their surrounding environment made them suitable sentinels for genotoxic and cytotoxic monitoring of toxic chemicals. The induction of micronuclei due to cytogenetic damage in fish subjected to various genotoxic agents both under laboratory and in situ studies was reviewed by Al-Sabti and Metcalfe (1995). Till date, most of the work has been done on fish erythrocytes, whereas few studies have been conducted on gill and liver cells. The sensitivity and suitability of the gill cells in the assessment of micronucleus induction over erythrocytes was mentioned in various studies (Cavas 2008; Arslan et al. 2010). They concluded that as the gills are directly exposed to pollutants, continuously dividing and showed high sensitivity for the cytogenetic effects induced by environmental contaminants. The liver is the major site of genobiotic biotransformation in the body and is one of the most damaged organs when exposed to various chemicals. The usefulness of the liver tissue in micronucleus tests was considered by workers (Cavas and Ergene-Gozukara 2003; Cavas et al. 2005). A few studies have concluded the suitability of acridine orange staining over conventional Giemsa staining (Cavas 2008; Polard et al. 2011), although the pictorial representation of the gill and liver cells were missing for comparison both in fluorescent and Giemsa staining procedures. The present study has been undertaken to evaluate the use of micronuclei (MN), binuclei (BN) and other nuclear abnormalities (NA) in the assessment of genotoxic and cytotoxic nature of municipal wastewater of Tung Dhab drain, Amritsar, India through studies conducted on the blood, liver and gill cells in fish Labeo rohita. The focus was to explore which tissue should be incorporated in water monitoring studies to assess genotoxicity and cytotoxicity and also to see the suitability of fluorescent staining techniques in such water studies.
Materials and methods
Specimen procurement and acclimatization
Fingerlings of L. rohita (8.32 ± 0.24 g weight; 6.76 ± 0.32 cm length) were collected from the Rajasansi government fish farm Amritsar and transported to the laboratory. Fish were immediately transferred to acclimatization tanks (63 × 39 × 63) containing tap water (temperature—23.9 ± 0.32 °C, pH—7.02 ± 0.01, DO (dissolved oxygen)—6.37 ± 0.34 mg/L, EC (electrical conductivity)—458.2 ± 2.15 μS/cm, TDS (total dissolved solids)—134.8 ± 2.43 mg/L) for 30 days. Normal photoperiod (12-h light/dark cycle) was maintained both for acclimation period and experimentation. Fish were fed ad libitum on commercial fish food, and feeding was suspended 1 day prior to the experiment. L. rohita was chosen as the test organism due to its wide geographic distribution, economic importance, ready availability throughout the year, ease of maintenance, convenience of testing and relative sensitivity to pollutants.
Sampling site, collection and analysis of municipal wastewater
The sampling site chosen for the present study lies near village Mahal of Tung Dhab drain (31°67′612″ N and 74°74′280″ E) Amritsar, India (Fig. 1). The drain is actually a storm water drain but is being used by food, leather and chemical industries along with paper mill and iron foundries to dump their untreated waste. It is 20 km long, has a catchment area of 208.83 km2, capacity of 53 m3/min and bed width of 13.72 m (at outfall) and 1.22 m (at starting point). The drain receives effluents from the other two drains—Gumtala drain (carrying paper mill and textile processing mill effluents), the Verka drain (carrying milk plant, iron foundries and woollen dyeing mill effluents) and also receives sewage of the Amritsar city. The level of pollution inside the drain is extremely high as no treatment plant is yet installed along the drain. It covers many areas and villages such as Fatehgarh Shukarchak, Verka, Othian, Nashera, Gumtala, Mahal and Wadala Bhitewadh along its course and after that it joins with Hudiara drain near Khiala Khurd and finally enters the river Ravi near the international border.
Map showing Tung Dhab Drain and Hudiara Drain. a The sampling site is marked by a star ( ) origin of drains is shown by (
) origin of drains is shown by ( ); confluence of Tung Dhab drain and Hudiara drain is marked by (
); confluence of Tung Dhab drain and Hudiara drain is marked by ( ). b Map showing main industries and sewer outfalls along Tung Dhab drain. (
). b Map showing main industries and sewer outfalls along Tung Dhab drain. ( ) is indicating sewer outfalls; (
) is indicating sewer outfalls; ( ) metal foundries; (
) metal foundries; ( ) paper mill; (
) paper mill; ( ) food; (
) food; ( ) leather; (
) leather; ( ) chemical industries. Source: adapted from Google Earth Maps, accessed August 2014
) chemical industries. Source: adapted from Google Earth Maps, accessed August 2014
Municipal wastewater samples were collected (between 05:00 and 07:00 h) both for setting the experiments (100 L capacity polypropylene cans) and for physicochemical analysis (pre-treated and well labelled plastic bottles). The wastewater samples were chilled in cool packs, immediately brought to the laboratory and refrigerated further till analysis. The procedures for water analysis were followed as previously given by APHA (American Public Health Association, American Water Works Association, and Water Environment Federation (2005). Mean ± SE values were calculated for wastewater using Minitab statistical software (Minitab, version 14).
Chronic toxicity bioassays
For chronic studies, fish were divided into five groups: one control (n = 6; total 18) and three exposed groups (n = 6; total 54) for chronic toxicity (15, 30 and 60 days) and recovery (n = 6; total 18) bioassays (60 days). Static chronic toxicity tests (non-renewal) were conducted with three sublethal concentrations of municipal wastewater (40, 60 and 80 % of LC50) that is 17.7, 26.6 and 35.4 % (v/v) collected from Tung Dhab drain. The experiments were previously conducted for determination of 96 h LC50, and the value came out to be 44.24 % (Kaur and Dua, 2014). All the test exposures were carried out in triplicates. The wastewater was thoroughly mixed and concentrations of the quantity 80 L (v/v) were prepared in experimental tanks (42 × 57 × 42) using tap water for dilutions (temperature—23.6 ± 0.30 °C, pH—7.01 ± 0.01, DO—6.42 ± 0.22 mg/L, TH (total hardness)—138.4 ± 1.68 mg/L, TDS—126.4 ± 1.96 mg/L and EC—463.3 ± 1.42 μS/cm). Control groups were simultaneously maintained using tap water. Fish were removed from experimental tanks on completion of 60 days and transferred to recovery tanks containing tap water for additional 60 days. Fish were not given any food during the tests except for recovery experiments. Chronic toxicity tests were conducted in accordance with standard methods (U.S. Environmental Protection Agency 2002; APHA/AWWA/WEF 2005).
Tissue processing and extraction of gill and liver cells
For the micronucleus test, fish gill and liver tissues were dissected and placed into plastic vials in Carnoy’s fixative (C.F.) prepared in the ratio of 3:1 (methanol/acetic acid) and were stored in refrigerator until processing.
Processing of gill tissue
-
1.
The gills were transferred from C.F. into 25 % acetic acid for 10–15 min for tissue maceration.
-
2.
The epithelial cells were then scraped off the gills with the help of the forceps placing the whole tissue in a petri dish into the acetic acid solution.
-
3.
The scraped cells were then poured into the centrifuge tubes; tissue clumps and gill arches were removed and discarded.
-
4.
The cell suspension was agitated with help of dropper for 10 min, and any leftover tissue was removed.
-
5.
The free cells were collected by centrifugation on 1000 rpm at 4 °C for 10 min. Then distilled water is added to the pellet and centrifuged again; pellet containing free cells was then fixed in three consecutive changes of chilled Carnoy’s fixative along with centrifugation at the same revolutions and time. Finally C.F. was added to pellet, and contents were lightly mixed with Pasteur pipette and refrigerated till slide preparation.
Processing of the liver tissue
-
1.
The liver tissue pieces were transferred from C.F. into 15 mL tube having 30 % acetic acid solution for 20 min for tissue softening.
-
2.
The contents of the vial were gently mixed with the help of Pasteur pipette for 5 min.
-
3.
The free cells were collected by centrifugation on 500 rpm at 4 °C for 10 min. Then distilled water is added to the pellet and centrifuged again; pellet containing free cells was then fixed in three successive changes of freshly prepared chilled Carnoy’s fixative along with centrifugation at the same revolutions and time. Finally C.F. was added to pellet, and contents were lightly mixed with Pasteur pipette and refrigerated till slide preparation.
Slide preparation, staining and scoring
The caudal peduncle was cut, and blood drop was smeared immediately on a clean labelled glass slide. The slides were fixed in methanol for 10 min, air dried and stored for staining. Cells were dropped on clean and properly labelled glass slides with the help of 10 μl pipette, air dried and stored. The slides were stained with 10 mg/100 mL of acridine orange in Sorenson’s buffer (7.2 ± 0.1). Only 2–3 drops were placed on a slide, covered with a cover glass and scored within the same day. Microscopic slides (4 per tissue; 24 per treatment and duration) were prepared. To detect the frequencies of binucleated (BN), micronucleated (MN) cells and nuclear abnormalities (NA), 1000 cells were scored under ×100 magnification from each slide (24,000 per treatment and duration) using fluorescent microscope (Nikon Eclipse E200) and photographed with Nikon D 3100 digital camera. For scoring purposes, the standard terminologies were used for identification of MN, BN and NA. Small, non-refractive, circular or ovoid chromatin bodies showing the same staining pattern as the main nucleus were considered as MN. The cells with two nuclei were considered as binuclei (BN). The nuclei showing relatively small evagination of the nuclear membrane are considered as blebbed nuclei (BL). The nuclei showing evaginations larger than the blebbed nuclei and having several lobes are classified as lobed nuclei (LB). Notched nuclei (NT) are distinguished from others having vacuoles and appreciable depth into a nucleus that does not contain nuclear material (Carrasco et al. 1990; Al-Sabti and Metcalfe 1995; Cavas and Ergene-Gozukara 2005). The data from MN, BN and NA studies was subjected to one-way ANOVA using Minitab (version 14) software to compare significant differences (p ≤ 0.01) between control-treatment groups and treatment-recovery group (60 days). The significant results were further analyzed with Tukey’s post hoc test (Assistat, version 7.7 beta).
Results and discussion
The wastewater samples like total suspended solids (TSS), biological oxygen demand (BOD), nitrate as N (NO3-N), oil and grease (O and G) and heavy metals like chromium Cr (VI), manganese (Mn) and lead (Pb) did not meet the discharge standards of effluents into inland surface waters as previously described in one of our manuscript (Kaur and Dua 2015c). The recorded values showed deteriorated water quality of Tung Dhab drain, hence supporting the higher incidence of MN, BN and NA in the liver, gill and erythrocytes in the experimental fish.
Micronucleus and total nuclear abnormalities in erythrocytes
Statistically significant changes in the frequencies of micronucleated (MN) and total (NA) were recorded in the erythrocytes of L. rohita on exposure to municipal wastewater for durations of 15, 30 and 60 days. The results of MN and NA tests with fish L. rohita are presented in Figs. 2 and 3. Higher NA frequencies in comparison to MN frequencies were recorded in fish erythrocytes. The values of MN frequencies ranged from 0.88 ± 0.08, 1.05 ± 0.12, 1.15 ± 0.08 and NA frequencies varied from 1.12 ± 0.10, 1.27 ± 0.08 and 1.31 ± 0.17, respectively, in control fish. The treated fish showed higher MN frequencies that varied from 1.02 ± 0.12 (17.7 %, 15 days) to 1.38 ± 0.19 (35.4 %, 60 days) than control. On the other hand, NA frequencies were increased much in comparison to both control and MN frequencies. These ranged from 1.43 ± 0.08 (17.7 %, 15 days) to 3.02 ± 0.19 (35.4 %, 60 days) (Figs. 3a, c). The erythrocyte MN and NA frequencies increased in concentration and duration dependent manner. Highly significant values (p ≤ 0.01) for MN and NA frequencies were obtained for all tested concentrations and exposure durations when compared to control (Fig. 3). In the recovery fish, the frequencies of MN and BN showed a significant decline (p ≤ 0.01) when compared with the treated fish for 60 days (Fig. 3b–d). The erythrocyte frequencies ranged from 1.12 ± 0.12 to 1.21 ± 0.15 (MN) and 1.65 ± 0.16 to 1.95 ± 0.18 (NA) in recovered fish as compared to values of 1.27 ± 0.14 to 1.38 ± 0.19 (MN) and 2.45 ± 0.08 to 3.02 ± 0.19 (NA) observed for treated fish exposed to toxicant for 60 days.
Graphs showing erythrocytes MN a and total NA c frequencies in fish L. rohita subjected to municipal wastewater experiments for 15, 30 and 60 days, and comparison of MN b and NA d frequencies obtained in recovered and treated fish for 60 days. Values are mean ± SE (vertical bars); means followed by different letters are significantly different from each other both in a concentration and time duration dependent manner (Tukey’s post hoc test, p ≤ 0.01); T1, T2 and T3 are the 17.7, 26.6 and 35.4 % wastewater concentrations
MN and NA were found to be statistically significant (both for concentration and duration) in case of treated fish erythrocytes when compared to control. The frequencies were found to be parallel to the values detected in in situ experiments conducted by other workers (Minissi et al. 1996; Matsumoto et al. 2006; Polard et al. 2011).
The reason for variability in frequencies lies in the nature of the toxic agent used, type of species as well as the speed of the hemopoietic cycle. As micronuclei are small, intracytoplasmic masses of chromatin formed as a result of clastogenic action (chromosome breaks) or as a whole chromosome that do not migrate during anaphase (aneugenic effect). The cells that divide abnormally due to blocking of cytokinesis resulted in binucleated cells. But the frequency of MN and NA varies with factors such as interspecies differences in xenobiotic metabolism, nature of the toxic agent and the exposed species, DNA repair and cell proliferation in the target organ. All these factors affect the sensitivity of fish species to genotoxicity as these are related to pharmacokinetics of the drugs/pollutants and speed of the hemopoietic cycle (Al-Sabti and Metcalfe 1995; Palhares and Grisolia 2002). Jiraungkoorskul et al. (2007) concluded that genotoxic chemicals form reactive oxygen species as well as free-radical metabolites that interact with DNA to cause disruptive changes. They suggested that heavy metal exposure in fish produced electrophilic ions and chemicals, which interacted with nucleophilic sites in DNA and resulted in breaks and other damage in DNA. The higher frequencies of MN and NA in erythrocytes as compared to the control pointed towards the genotoxic effect of heavy metals (chromium, manganese and lead) and organics present in water of Tung Dhab drain.
Gill and liver binucleated and micronucleated studies
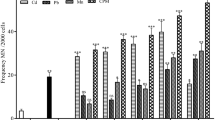
Significant changes in the frequencies of MN and BN parameters in the gill and liver cells were recorded in the present study on exposure to municipal wastewater for durations of 15, 30 and 60 days (Figs. 4, 5, 6 and 7). Higher MN and BN frequencies were recorded in the liver cells as compared to the frequencies obtained for the gill cells in wastewater treated fish. The values of MN and BN frequencies in the gill cells from control fish ranged from 0.98 ± 0.11 to 1.07 ± 0.07 and 1.37 ± 0.06 to 1.40 ± 0.09 (Figs. 4a, b). The obtained values varied from 1.28 ± 0.07 to 1.35 ± 0.08 and 1.53 ± 0.07 to 1.58 ± 0.11 for MN and BN in the case of liver cells (Figs. 5a, b) in control fish. In comparison to control, the treated fish showed elevated frequencies in the gill cells that varied from 1.45 ± 0.11 (17.7 %, 15 days) to 3.47 ± 0.07 (35.4 %, 60 days) and ranged from 2.15 ± 0.17 (17.7 %, 15 days) to 4.37 ± 0.20 (35.4 %, 60 days) in the case of MN and BN, respectively, (Figs. 4a, b). The MN frequencies increased both concentration and duration dependent manner from 2.77 ± 0.18 (17.7 %, 15 days) to 6.17 ± 0.09 (35.4 %, 60 days) in the case of liver cells, whereas the high BN frequencies (5.10 ± 0.13) were recorded at lowest concentration of 17.7 % for 15 days (Figs. 5a, b). The MN frequencies in the gill and liver cells increased dose and time dependently whereas BN frequencies decreased time dependently for the liver cells and increased time as well as in a concentration dependent manner in the case of gill cells. Highly significant values (p ≤ 0.01) for MN and BN cell frequencies (liver and gill) were obtained for all tested concentrations and exposure durations when compared to control.
Graphs showing gill MN a and BN b frequencies in fish L. rohita subjected to municipal wastewater experiments for 15, 30 and 60 days, and comparison of MN c and BN d frequencies obtained in recovered and treated fish for 60 days. Values are mean ± SE (vertical bars); means followed by different letters are significantly different from each other both in a concentration and time duration dependent manner (Tukey’s post hoc test, p ≤ 0.01)
Graphs showing liver MN a and BN b frequencies in fish L. rohita subjected to municipal wastewater experiments for 15, 30 and 60 days, and comparison of MN c and BN d frequencies obtained in recovered and treated fish for 60 days. Values are mean ± SE (vertical bars); means followed by different letters are significantly different from each other both in a concentration and time duration dependent manner (Tukey’s post hoc test, p ≤ 0.01)
In the recovery fish, the frequencies of MN and BN showed a significant decrease (p ≤ 0.01) when compared with the treated fish for 60 days. The frequencies ranged from 1.42 ± 0.10 to 1.90 ± 0.30 (MN) and 2.20 ± 0.16 to 2.93 ± 0.13 (BN) in the recovered gill tissue as compared to values of 2.02 ± 0.18 to 3.47 ± 0.07 (MN) and 2.93 ± 0.24 to 4.37 ± 0.20 (BN) observed for treated fish exposed to toxicant for 60 days (Figs. 4c and 3d). In the case of liver cells, the values varied from 1.93 ± 0.15 to 2.40 ± 0.08 (MN) and 2.27 ± 0.11 to 2.45 ± 0.06 (BN) in recovered fish as compared to values obtained for treated fish that ranged from 3.48 ± 0.17 to 6.17 ± 0.09 (MN) and 3.70 ± 0.08 to 3.63 ± 0.08 (BN) (Figs. 5c, d).
The statistically significant values (p ≤ 0.01) of MN and BN in the liver and gill cells on all the exposure concentrations and durations pointed towards the genotoxic and cytotoxic nature of municipal wastewater of Tung Dhab drain. Till date, there are very few studies that use gill and liver cells for MN and BN studies although many workers have worked on liver erythrocytes and gill erythrocytes. Higher values of heavy metals and organics in wastewater resulted in inhibition of segregation of chromosomes as well as leads to apoptosis of the liver and gill cells, thus resulting in higher MN and BN frequencies in the liver and gill cells. The results are in agreement with the previously conducted studies by authors that worked with heavy metals (Cavas and Ergene-Gozukara 2003; Cavas and Ergene-Gozukara 2005; Cavas et al. 2005). Rao et al. (1997) concluded that MN in the liver cells was caused through the activity of chemical ‘spindle poisons’ in the extract or was caused by chemically induced apoptosis of hepatocytes. Cavas and Ergene-Gozukara (2003) concluded the genotoxic potential of textile effluent and supported the view that the gill cells were more sensitive than erythrocytes for MN testing. Cavas et al. (2005) confirmed induction of micronuclei and binuclei in the liver cells on heavy metal exposure.
Conclusions
The elevated frequencies observed in the present study pointed towards the mutagenic nature of drain water contaminated with heavy metals and organics. The induced frequencies of MN, BN and NA were found to be in the following order: MN (liver cells) > BN (liver cells) > BN (gill cells) > MN (gill cells) > NA (erythrocytes) > MN (erythrocytes). The study suggests the preferred use of the liver and gill cells in comparison to erythrocytes, as biomarkers in micronuclei and binuclei assays for chronic toxicological studies. The study also proposes the use of fluorescent nucleic acid dye—the acridine orange over Giemsa. This helps in scoring purposes as the dye only stains nuclear material, hence helps to reduce errors during counting, avoiding the artifacts and also reduces the scoring time.
References
Al-Sabti K, Metcalfe CD (1995) Fish micronuclei for assessing genotoxicity in water. Mutat Res 343:121–135
American Public Health Association, American Water Works Association, and Water Environment Federation (2005) Standard methods for the examination of water and wastewater. American Public Health Association, Washington, DC
Arslan OC, Katalay HPC, Boyacioglu M, Karaaslan MA, Guner H (2010) Detecting micronuclei frequency in some aquatic organisms for monitoring pollution of Izmir Bay (Western Turkey). Environ Monit Assess 165:55–66
Bolognesi C, Hayashi M (2011) Micronucleus assay in aquatic animals—review. Mutagenesis 26:205–213
Carrasco KR, Tilbury KL, Myers MS (1990) Assessment of the piscine micronucleus test as an in situ biological indicator of chemical contaminant effects. Can J Fish Aquat Sci 47:2123–2136
Cavas T (2008) In vivo genotoxicity of mercury chloride and lead acetate: micronucleus test on acridine orange stained fish cells. Food Chem Toxicol 46:352–358
Cavas T, Ergene-Gozukara S (2003) Micronuclei, nuclear lesions and interphase silver-stained nucleolar organizer regions (AgNORs) as cytogenotoxicity indicators in Oreochromis niloticus exposed to textile mill effluent. Mutat Res 538:81–91
Cavas T, Ergene-Gozukara S (2005) Induction of micronuclei and nuclear abnormalities in Oreochromis niloticus following exposure to petroleum refinery and chromium processing plant effluents. Aquat Toxicol 74:264–271
Cavas T, Garanko NN, Arkhipchuk VV (2005) Induction of micronuclei and binuclei in blood, gill and liver cells of fishes subchronically exposed to cadmium chloride and copper sulphate. Food Chem Toxicol 43:569–574
De Flora S, Viganò L, D’Agostini F, Camoirano A, Bagnasco M, Bennicelli C, Melodia F, Arillo A (1993) Multiple genotoxicity biomarkers in fish exposed in situ to polluted river water. Mutat Res 319:167–177
Jiraungkoorskul W, Kosai P, Sahaphong S, Kirtputra P, Chawlab J, Charucharoen S (2007) Evaluation of micronucleus test’s sensitivity in freshwater fish species. Res J Environ Sci 1:56–63
Kaur R, Dua A (2014) Acute toxicity, behavioural and morphological alterations in Indian carp, Labeo rohita H., on exposure to municipal wastewater of Tung Dhab drain, Punjab, India. Int J Sci Res 5:1716–1720
Kaur R, Dua A (2015a) Scales of freshwater fish Labeo rohita as bio indicators of water pollution in Tung Dhab Drain, Amritsar, Punjab, India. J Toxicol Environ Health A 78:388–396
Kaur R, Dua A (2015b) Histopathological alterations in Labeo rohita gills on chronic exposure to municipal wastewater of Tung Dhab drain—a SEM study. Toxicol Environ Chem 97:1099–1112
Kaur R, Dua A (2015c) Colour changes in Labeo rohita (Ham.) due to pigment translocation in melanophores, on exposure to municipal wastewater of Tung Dhab drain, Amritsar, India. Environ Toxicol Pharm 39:747–757
Matsumoto ST, Mantovani MS, Malaguttii MIA, Dias AL, Fonseca IC, Marin-Morales MA (2006) Genotoxicity and mutagenicity of water contaminated with tannery effluents, as evaluated by the micronucleus test and comet assay using the fish Oreochromis niloticus and chromosome aberrations in onion root-tips. Genet Molec Biol 29(1):148–158
Minissi S, Ciccotti E, Rizzoni M (1996) Micronucleus test in erythrocytes of Barbus plebejus (Teleostei, Pisces) from two natural environments: a bioassay for the in situ detection of mutagens in freshwater. Mutat Res 367:245–251
Palhares D, Grisolia CK (2002) Comparison between the micronucleus frequencies of kidney and gill erythrocytes in tilapia fish, following mitomycin c treatment. Genet Molec Biol 25:281–284
Polard T, Jean S, Gauthier L, Laplanche C, Merlina G, Sanchez-Perez JM, Pinelli E (2011) Mutagenic impact on fish of runoff events in agricultural areas in south-west France. Aquat Toxicol 101:126–134
Rao SS, Neheli T, Carey JH, Vairns VW (1997) Fish hepatic micronuclei as an indication of exposure to genotoxic environmental contaminants. Environ Toxicol Water Qual 12:217–222
U.S. Environmental Protection Agency (2002) Short-term methods for estimating the chronic toxicity of effluents and receiving waters to freshwater organisms. U.S. Environmental Protection Agency, Washington, DC
Acknowledgments
The authors would like to thank the Department of Zoology, Guru Nanak Dev University for providing necessary infrastructural facilities for the execution of this work. The authors would also like thank University Grants Commission, New Delhi for providing the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible editor: Henner Hollert
Rights and permissions
About this article
Cite this article
Kaur, R., Dua, A. Fish liver and gill cells as cytogenotoxic indicators in assessment of water quality. Environ Sci Pollut Res 23, 18892–18900 (2016). https://doi.org/10.1007/s11356-016-7080-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7080-8