Abstract
Sulfate radical based-advanced oxidation process has received increasing interest in the remediation of wastewater and contaminated soil. In this study, degradation of 2, 4-dichlorophenol (2, 4-DCP) was investigated over peroxymonosulfate (PMS) activation by MnO2, which was prepared by liquid-phase oxidation method. The prepared MnO2 was characterized by transition electron microscopy, X-ray diffraction, N2 adsorption–desorption, and X-ray photoelectron spectroscopy. Characterization results showed that α-MnO2 exhibited the highest surface area and Mn (III) content. The PMS activation by MnO2 in 2, 4-DCP degradation followed the order of α-MnO2 > γ-MnO2 > β-MnO2, which is dependent on the properties of MnO2 including crystal structure, surface area and Mn (III) content. Influences of initial concentration of 2, 4-DCP, PMS and MnO2 dosage, pH and co-existing inorganic ions on the degradation were examined. Electron paramagnetic resonance (EPR) and quenching experiments with ethanol and tert-butanol suggested that sulfate radicals were the dominant radicals in the process. Findings in this study indicated that α-MnO2 was an attractive catalyst for activation of PMS to degrade 2, 4-DCP in aqueous solution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Chlorophenols as chemical raw materials are typically used in leather, dyes, pesticide and fungicides (Chen et al. 2013; Zhou et al. 2018), which have been listed as priority pollutants by the United States Environmental Protection Agency (Chen et al. 2018). The discharged chlorophenols in wastewater have caused serious environmental concerns due to the toxicity, persistence and carcinogenicity (Zhang et al. 2015a; Zhao et al. 2016). Hence, it is highly demanded to develop effective methods for chlorophenols removal from water.
Many treatment techniques including adsorption (Chen et al. 2012; Xu et al. 2012), hydrodechlorination (Jin et al. 2011; Zhou et al. 2014), photocatalysis (Bayarri et al. 2005; Liu et al. 2012), advanced oxidation processes (Jia et al. 2015; Karci et al. 2012) and biological methods (Eker and Kargi 2008; Wang et al. 2015c), have been used to treat 2, 4-DCP. In recent years, sulfate radical-based advanced oxidation process has attracted great attention for the treatment of a wide range of organic pollutants (Anipsitakis and Dionysiou 2003; Ghanbari and Moradi 2017; Zhou et al. 2018b). Sulfate radicals have higher redox potential (2.5–3.1 V), wider range of solution pH, and longer life time (30–40 μs) than hydroxyl radicals. Sulfate radicals could be generated from the activation of peroxymonosulfate (PMS) and peroxydisulfate (PDS) via UV, heat, carbon-based materials and ransition metals (Fang et al. 2015; Wang and Wang 2018).
Various transition metal catalysts (Co2+, Cu2+, Ag+, V3+ et al.) have been reported to activate PMS (Nfodzo and Choi 2011; Wang and Wang 2018). Cobalt-based materials have been recognized as one of the most effective options for the activation of PMS. However, the leaching of cobalt oxides from cobalt-based materials posed risks to human health and environment. Consequently, it is necessary to develop environmental-friendly catalysts for PMS activation. Manganese oxides, due to low toxicity, abundance in natural stock and environmental friendliness, have been widely used in oxidation reaction for treatment of organic pollutants (Saputra et al. 2013; Wang et al. 2015a; Zhou et al. 1998). Moreover, manganese dioxides are promising catalysts in activation persulfate for degradation of organic contaminants. Saputra et al. reported that Mn2O3 was the best catalyst in activating PMS for phenol degradation (Saputra et al. 2013). As reported, α-MnO2 showed outstanding catalytic activity in activation of PDS and PMS for degradation of phenol and dye (Liu et al. 2016; Saputra et al. 2012; Zhao et al. 2016).
However, to the best of our knowledge, there are few studies to compare the structure of MnO2 on PMS activation and chlorophenols degradation in MnO2/PMS system. In this study, three types of MnO2 with different structural properties were synthesized and their performances in activation of PMS for 2, 4-dichlorophenol (2, 4-DCP) degradation were investigated. The dominant radicals were identified by electron paramagnetic resonance (EPR) spectra and free radical quenching studies. Furthermore, several influence factors were investigated, including 2, 4-DCP concentration, MnO2 dosage, PMS concentration, pH and co-existing inorganic ions.
Materials and Methods
2, 4-Dichlorophenol, 2-chlorophenol, phenol and 5, 5-dimethyl-1-pyrrolidine N-oxide (DMPO, 97%) were purchased from Sigma-Aldrich. Sodium PMS (available as a triple potassium salt with the commercial name of Oxone®, 2KHSO5·KHSO4·K2SO4) was obtained from Alfa Aesar. Ethanol (EtOH) and acetonitrile (chromatography grade) were purchased from Merck. Tertiary butanol (TBA) was obtained from Aladdin Chemistry Co. Ltd. MnSO4, (NH4)2S2O8, (NH4)2SO4, HCl and NaOH were purchased from Nanjing Chemical Reagents Co. Ltd. All other reagents were chemical grade or higher and were used without further purification. Deionized water with a resistivity of 18 MΩ cm−1 was used for preparing aqueous solutions.
The synthesis of MnO2 was conducted based on liquid-phase oxidation method according to literature (Wang and Li 2003). Details of synthesis and characterization are listed in Text S1 in Supporting Information (SI).
The degradation of 2, 4-DCP was carried out in a 250 mL three-necked flask at 25 ± 0.5 °C with a water-bath. Briefly, 50 mg of MnO2 was added into 250 mL solution with 20 mg/L of 2, 4-DCP and 1.63 mM PMS under stirring (1400 rpm) and parallel experiment were carried out simultaneously. Control experiments with only MnO2 or PMS were performed under the same condition. A 0.5 mL of samples were collected at selected time intervals (5, 10, 15, 20, 30, 40, 50, 60, 90 and 120 min) and then mixed with 0.5 mL ethanol to quench the reaction. After filtered with 0.45 μm membrane filter, the concentration of solute in an aliquot was analyzed by high-performance liquid chromatography (Agilent 1200, USA) with an ultraviolet detector at wavelength of 270 nm using a 4.6 × 150 mm HC-C18 column. The mobile phase was 60% acetonitrile and 40% water (v/v).
Results and Discussion
Transmission electron microscope (TEM) images of three MnO2 are presented in Fig. 1. Obviously, α-MnO2 is ribbon-like nanowires with diameters around 10 nm and lengths ranging between 40 and 200 nm. TEM images of β-MnO2 demonstrated that it was nanorods with average diameters of 100 nm, while γ-MnO2 showed a nanofiber structure with the diameters of 10–30 nm. The Brunauer–Emmett–Teller (BET) surface areas were 96.9, 15.2, and 72.3 m2/g for α-, β- and γ-MnO2, respectively. Clearly, α-MnO2 has the largest BET surface area.
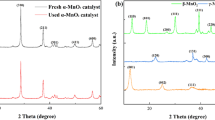
As shown in Fig. 2, the XRD patterns of the MnO2 were in according with the inorganic crystallographic database of XRD patterns of α-MnO2 (JCPDS 44-0141), β-MnO2 (JCPDS 24-0735) and γ-MnO2 (JCPDS 14-0664) (Huang et al. 2019; Wang and Li 2003; Wang et al. 2015b). For all the MnO2, the strong bands at 473, 519 and 715 cm−1 were observed in FTIR spectra (Fig. S1), which were assigned to the Mn–O lattice vibration (Kang et al. 2007; Kim and Stair 2004). The weak peak around 1105 cm−1 was assigned to the vibration of Mn (III)-O bond (Kang et al. 2007).
The XPS spectra of MnO2 in the Mn 2p3/2 regions are presented in Fig. S2. The Mn 2p3/2 spectra were divided into two peaks at binding energies of 642.4 eV and 641.3 eV, which were assigned to Mn (IV) and Mn (III) species (Peng et al. 2017), respectively. The fitting results of Mn 2p3/2 spectrums of Mn (III) and Mn (IV) are summarized in Table S1. Clearly, the abundance of Mn (III) species decreased in the order: α-MnO2 > γ-MnO2 > β-MnO2.
Batch experiments were performed to investigate the degradation of 2, 4-DCP in MnO2/PMS system. The result is presented in Fig. 3. Clearly, only about 4.6% of 2, 4-DCP was degraded in PMS system within 120 min, suggesting that 2,4-DCP could not be degraded effectively by PMS without activation. In the system with α-MnO2 alone, approximately 37.3% of 2, 4-DCP was removed from the solution, indicating that α-MnO2 has direct oxidation ability for degradation of 2, 4-DCP. The degradation of 2, 4-DCP with different dosages of α-MnO2 were compared in Fig. S3. The degradation efficiency increased from 52.2% to 88.1% as the dosage of α-MnO2 increasing from 0.20 to 1.0 g/L within 720 min, which further validated the direct oxidation of 2, 4-DCP by MnO2. The direct oxidation mechanisms of MnO2 have been well studied in previous studies (Im et al. 2015; Lin et al. 2009; Saputra et al. 2013; Septian and Shin 2019; Zhang et al. 2008; Zhang and Huang 2003; Zhu et al. 2019b). Thus, direct oxidation will not be discussed in our study. It is note that 99% of 2, 4-DCP was degraded in α-MnO2/PMS system within 120 min, which is significantly higher than the reported degradation efficiency (around 75%) for 2, 4-DCP with persulfate activation by α-MnO2 nanowire (Zhao et al. 2016). The results indicated that MnO2 exhibited an excellent catalytic ability to activate PMS to degrade 2, 4-DCP.
Degradation of 2, 4-DCP using α-, β- and γ-MnO2 as activation reagents were compared in Fig. 4. The degradation efficiency of 2, 4-DCP was 88.6%, 76.6% and 85.5% for α-, β- and λ-MnO2, respectively. This phenomenon may be related to the Mn (III) content and crystal structure of MnO2. The sulfate radicals (\({\text{SO}}_{4}^{\bullet -}\)) and hydroxyl radicals (\(\bullet{\text{OH}}\)) are formed when PMS is activated by MnO2 according to previous studies (Huang et al. 2019; Liu et al. 2016, 2015; Wang et al. 2018) as following reaction Eqs. (1–3)
Obviously, \({\text{SO}}_{4}^{{ \bullet - }}\) is formed from PMS activation by Mn(III) and \({\text{HSO}}_{5}^{{\bullet - }}\) is formed by Mn(IV). Moreover, and \({\text{SO}}_{4}^{{\bullet - }}\) has a higher redox potential than \({\text{HSO}}_{5}^{{\bullet - }}\) (Oh et al. 2016). Hence, the higher the content of Mn (III), the more \({\text{SO}}_{4}^{{\bullet - }}\) were produced for degradation reaction. As mentioned above, α-MnO2 has the highest content (62.3%) of Mn (III), leading to the highest degradation efficiency. Additionally, Mn (III) forms weaker and more flexible Mn–O bonds due to the occupation of the antibonding eg orbital, which are more catalytically reactive (Robinson et al. 2013). In contrast, β- and γ-MnO2 with stronger Mn(IV)-O bonds are more stable and inflexible, leading to the low oxidation potential. This further explained why α-MnO2 showed the highest catalytic activity.
The unique tunnel structure of α-MnO2 is also responsible for the extraordinarily strong catalytic reactivity (Huang et al. 2019, 2018; Zhang et al. 2015b). α-MnO2 with (2 × 2) tunnel structure displayed larger tunnel sizes than γ-MnO2 with (1 × 1) and (1 × 2) tunnel structure, and β- MnO2 with (1 × 1) tunnel structure. Additionally, α-MnO2 exhibited the largest surface area, which would provide more active sites and enhance the catalytic performance.
EPR studies were performed to identify the reactive radical species in MnO2/PMS system. EPR spectroscopy coupled with DMPO as a spin-trapping agent was used to detect free radicals. As shown in Fig. 5, DMPO-OH (four lines, 1:2:2:1) signal with hyperfine splitting constants of aH = aN = 4.7G was observed in PMS system. The generation of DMPO-OH signals suggested \(\bullet{\text{OH}}\) was formed in PMS solution at ambient temperature without activator, which was consistent with previous studies (Shukla et al. 2010).
However, no DMPO-OH and DMPO-SO4 signals were observed in MnO2/PMS system. Instead, a typical seven line spectrum with the intensity ratio of 1:2:1:2:1:2:1 signal was detected, which was identified the characters of 5-tert-butoxycarbonyl-methyl-2-oxo-pyrroline-1-oxyl (DMPOX). The generation of DMPOX was possibly oxidation of DMPO by many strong oxidizing substances as reported in previous studies (Du et al. 2019; Xie et al. 2019). It is should be noted that the formation of DMPOX did not indicated that \(\bullet{\text{OH}}\) and \({\text{SO}}_{4}^{{\bullet - }}\) were absent. It is mainly because that the DMPO oxidation is difficult to be detected due to its low sensitivity or short life time (Wang et al. 2017; Xie et al. 2019; Zhu et al. 2019a).
Radical quenching experiments by adding EtOH and TBA were conducted to examine the main reactive species. EtOH is a well-known quenching agent for both \(\bullet{\text{OH}}\) (\(k_{\bullet{\text{OH}}}\) = 1.2–2.8 × 109 M−1 s−1) and \({\text{SO}}_{4}^{{\bullet - }}\) (\(k_{{{\text{SO}}_{4}^{ \bullet - } }}\) = 1.6–7.7 × 107 M−1 s−1). TBA is used to quench \({\bullet{\text{OH}}}\) (\(k_{\bullet{\text{OH}}}\) = 3.8–7.6 × 108 M−1 s−1, \(k_{{{\text{SO}}_{4}^{ \bullet - } }}\) = 4–9.1 × 105 M−1 s−1) (Huang et al. 2017; Liang and Su 2009). As shown in Fig. S4, the degradation of 2, 4-DCP was significantly inhibited after the addition of 0.02 M EtOH, but only slightly inhibited after addition of 0.02 M TBA. The results indicated that \({\text{SO}}_{4}^{{\bullet - }}\) was the dominant reactive species for PMS activation by crystalline MnO2, which was consistent with previous reports (Liu et al. 2016; Luo et al. 2015; Wang and Chen 2015).
Reactant adsorption on catalyst surface is considered as the prerequisite step for heterogeneous catalysis reaction. Therefore, concentration of adsorbed reactants is related to the reaction rate. Degradation reactions with different initial 2, 4-DCP concentration were compared in Fig. 6a. Clearly, the reaction rate was positively related to the concentration of 2, 4-DCP adsorbed on MnO2. The results are fitted by the Langmuir–Hinshelwood model (Dong et al. 2019; Konstantinou and Albanis 2004) as following reaction Eqs. (4, 5)
where r0 is the initial reaction rate, C0is the initial 2,4-DCP concentration, θs is the surface coverage of 2,4-DCP adsorption, k is the reaction rate constant, and b is the adsorption constant for 2,4-DCP. As shown in Fig. 6b, the plot of 1/r0 versus 1/C0 presented a linear relation with a higher R2 (0.9989), reflecting that the degradation of 2, 4-DCP well follows the Langmuir–Hinshelwood model.
The impact of α-MnO2 dosage on 2, 4-DCP degradation is displayed in Fig. S5. The degradation of 2, 4-DCP was remarkably influenced by the dosage of α-MnO2. The degradation efficiency increased from 89.0% to 99.2% with the increase of α-MnO2 dose from 0.04 to 0.20 g/L. The enhancement of the degradation efficiency should be ascribed to more generation of radicals due to increased reactive sites with MnO2 addition. The effect of PMS concentration for the degradation of 2, 4-DCP is present in Fig. S6. As shown in Fig. S6, the degradation efficiency of 2, 4-DCP increased with the increasing of PMS concentration. This owes to more \({\text{SO}}_{4}^{{ \bullet - }}\) generated with the increasing concentration of PMS, when the reactive sites on the surface of catalyst are sufficient to activate PMS (Feng et al. 2015; Liu et al. 2016; Tan et al. 2014).
The effect of solution pH on 2, 4-DCP degradation in α-MnO2/PMS system is presented in Fig. 7. The degradation efficiency was 96.3% at pH 7. However, the degradation efficiency were decreased to 85.7% and 24.7% at pH 3.0 and pH 10.0 in the same reaction process. Solution pH governed the dissociation of 2, 4-DCP (pKa = 7.85 (Schwarzenbach et al. 2003)) and PMS (pKa of 9.4 for H2SO5 (Guan et al. 2011)). Dissociated 2, 4-DCP may enhance electron donating strength for oxidants under acidic and neutral conditions. Similarly, PMS is mainly presented in the form of HSO5−, which is favorable for the reaction Eq. (1). In addition, solution pH affected surface charge of the α-MnO2 (point of zero charge of 4.5, (Prélot et al. 2003)), and then affected interaction between α-MnO2 surface and PMS (Wang et al. 2018). The electrostatic repulsions interaction between the MnO2 surface and HSO5− increased at alkaline condition, leading to suppressed generation of reactive free radicals (Liu et al. 2015), because. Moreover, PMS was unstable and self-decomposition of PMS occurred in the presence of high concentration of hydroxide ions (Ahmadi and Ghanbari 2019; Ghanbari and Martínez-Huitle 2019). Therefore, the degradation of 2, 4-DCP decreased significantly under alkaline conditions.
Effect of influencing factors for 2, 4-DCP degradation in α-MnO2/PMS process a Dose of α-MnO2. Reaction conditions: [2, 4-DCP] = 20 mg/L, [PMS] = 1.63 mM, pH 7.0, T = 25 °C. b Concentration of PMS. Reaction conditions: [2, 4-DCP] = 20 mg/L, [α-MnO2] = 0.04 g/L, pH 7.0, T = 25 °C. c Initial pH, Reaction conditions: [2, 4-DCP] = 20 mg/L, [PMS] = 0.33 mM, [α-MnO2] = 0.04 g/L, T = 25 °C
The impacts of selected inorganic ions (Cl− and NO3−), which commonly occurr in the environment, on 2, 4-DCP degradation were examined. As shown in Fig. 8, the degradation efficiency of 2, 4-DCP was enhanced with the addition of Cl− and NO3−. Cl− has different effects on the degradation of contaminants. For instance, Qin et al. reported that the presence of Cl− greatly inhibited the degradation of DDT (Qin et al. 2016). While, Anipsitakis et al. reported opposite conclusion that Cl− facilitated the degradation of phenolic compounds by \({\text{SO}}_{4}^{{ \bullet - }}\) (Anipsitakis et al. 2006). As a fact, Cl− could be oxidized by \({\text{SO}}_{4}^{{ \bullet - }}\) to form \({\text{Cl}}^{{ \bullet}}\) according to following reaction (Eq. (6)), which favored the degradation of 2, 4-DCP (Liang et al. 2006).
In addition, the degradation efficiency of 2, 4-DCP significantly increased from 85.7% to 94.6% after adding NO3−. The enhanced effect in the presence of NO3− is owing to the active oxygen produced in the degradation process (Hu and Long 2016; Huang et al. 2009; Zhu et al. 2019a).
In this study, MnO2 with different structures were prepared and activation of PMS for the removal of 2, 4-DCP from water was studied systematically. The prepared α-MnO2 showed excellent activity to activate PMS for 2, 4-DCP degradation because of the larger tunnel size, higher BET surface area and Mn (III) content. The degradation mechanisms included direct oxidation by MnO2 and catalytic oxidation by radicals (\({\bullet{\text{OH}}}\) and \({\text{SO}}_{4}^{{ \bullet - }}\)). The degradation of 2, 4-DCP was facilitated at lower solution pH and the presence of Cl− and NO3−. Additionally, sulfate radicals were the main reactive species responsible for the degradation of 2, 4-DCP in MnO2/PMS system.
References
Ahmadi M, Ghanbari F (2019) Organic dye degradation through peroxymonosulfate catalyzed by reusable graphite felt/ferriferrous oxide: mechanism and identification of intermediates. Mater Res Bull 111:43–52
Anipsitakis GP, Dionysiou DD (2003) Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt. Environ Sci Technol 37:4790–4797
Anipsitakis GP, Dionysiou DD, Gonzalez MA (2006) Cobalt-mediated activation of peroxymonosulfate and sulfate radical attack on phenolic compounds implications of chloride ions. Environ Sci Technol 40:1000–1007
Bayarri B, Giménez J, Curcó D, Esplugas S (2005) Photocatalytic degradation of 2,4-dichlorophenol by TiO2/UV: kinetics, actinometries and models. Catal Today 101:227–236
Chen A et al (2012) Novel thiourea-modified magnetic ion-imprinted chitosan/TiO2 composite for simultaneous removal of cadmium and 2,4-dichlorophenol. Chem Eng J 191:85–94
Chen G et al (2013) Cadmium removal and 2,4-dichlorophenol degradation by immobilized phanerochaete chrysosporium loaded with nitrogen-doped TiO2 nanoparticles. Appl Microbiol Biotechnol 97:3149–3157
Chen Z, Jin J, Song X, Zhang G, Zhang S (2018) Redox conversion of arsenite and nitrate in the UV/quinone systems. Environ Sci Technol 52:10011–10018
Dong CD, Chen CW, Tsai ML, Chang JH, Lyu SY, Hung CM (2019) Degradation of 4-nonylphenol in marine sediments by persulfate over magnetically modified biochars. Bioresour Technol 281:143–148
Du J, Bao J, Liu Y, Kim SH, Dionysiou DD (2019) Facile preparation of porous Mn/Fe3O4 cubes as peroxymonosulfate activating catalyst for effective bisphenol A degradation. Chem Eng J 376:119193
Eker S, Kargi F (2008) Biological treatment of 2,4-dichlorophenol containing synthetic wastewater using a rotating brush biofilm reactor. Bioresour Technol 99:2319–2325
Fang G, Liu C, Gao J, Dionysiou DD, Zhou D (2015) Manipulation of persistent free radicals in biochar to activate persulfate for contaminant degradation. Environ Sci Technol 49:5645–5653
Feng Y, Liu J, Wu D, Zhou Z, Deng Y, Zhang T, Shih K (2015) Efficient degradation of sulfamethazine with CuCo2O4 spinel nanocatalysts for peroxymonosulfate activation. Chem Eng J 280:514–524
Ghanbari F, Martínez-Huitle CA (2019) Electrochemical advanced oxidation processes coupled with peroxymonosulfate for the treatment of real washing machine effluent: a comparative study. J Electroanal Chem 847:113182
Ghanbari F, Moradi M (2017) Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: review. Chem Eng J 310:41–62
Guan Y-H, Ma J, Li X-C, Fang J-Y, Chen L-W (2011) Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/Peroxymonosulfate system. Environ Sci Technol 45:9308–9314
Hu P, Long M (2016) Cobalt-catalyzed sulfate radical-based advanced oxidation: a review on heterogeneous catalysts and applications. Appl Catal B 181:103–117
Huang G-X, Wang C-Y, Yang C-W, Guo P-C, Yu H-Q (2017) Degradation of bisphenol A by peroxymonosulfate catalytically activated with Mn1.8Fe1.2O4 nanospheres: synergism between Mn and Fe. Environ Sci Technol 51:12611–12618
Huang J, Dai Y, Singewald K, Liu CC, Saxena S, Zhang H (2019) Effects of MnO2 of different structures on activation of peroxymonosulfate for bisphenol A degradation under acidic conditions. Chem Eng J 370:906–915
Huang J, Zhong S, Dai Y, Liu CC, Zhang H (2018) Effect of MnO2 phase structure on the oxidative reactivity toward bisphenol A degradation. Environ Sci Technol 52:11309–11318
Huang YH, Huang YF, Huang CI, Chen CY (2009) Efficient decolorization of azo dye reactive black B involving aromatic fragment degradation in buffered Co2+/PMS oxidative processes with a ppb level dosage of Co2+-catalyst. J Hazard Mater 170:1110–1118
Im J, Prevatte CW, Campagna SR, Loffler FE (2015) Identification of 4-hydroxycumyl alcohol as the major MnO2-mediated Bisphenol A transformation product and evaluation of its environmental fate. Environ Sci Technol 49:6214–6221
Jia L, Shen Z, Guo W, Zhang Y, Zhu H, Ji W, Fan M (2015) QSAR models for oxidative degradation of organic pollutants in the Fenton process. J Taiwan Inst Chem Eng 46:140–147
Jin Z, Yu C, Wang X, Wan Y, Li D, Lu G (2011) Liquid phase hydrodechlorination of chlorophenols at lower temperature on a novel Pd catalyst. J Hazard Mater 186:1726–1732
Kang L, Zhang M, Liu ZH, Ooi K (2007) IR spectra of manganese oxides with either layered or tunnel structures. Spectrochim Acta Part A Mol Biomol Spectrosc 67:864–869
Karci A, Arslan-Alaton I, Olmez-Hanci T, Bekbölet M (2012) Transformation of 2,4-dichlorophenol by H2O2/UV-C, fenton and photo-fenton processes: oxidation products and toxicity evolution. J Photochem Photobiol A 230:65–73
Kim H-S, Stair PC (2004) Bacterially produced manganese oxide and todorokite: UV raman spectroscopic comparison. J Phys Chem B 108:17019–17026
Konstantinou IK, Albanis TA (2004) TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: a review. Appl Catal B Environ 49:1–14
Liang C, Su H-W (2009) Identification of sulfate and hydroxyl radicals in thermally activated persulfate. Ind Eng Chem Res 48:5558–5562
Liang C, Wang ZS, Mohanty N (2006) Influences of carbonate and chloride ions on persulfate oxidation of trichloroethylene at 20 °C. Sci Total Environ 370:271–277
Lin K, Liu W, Gan J (2009) Oxidative removal of Bisphenol A by manganese dioxide: efficacy, products, and pathways. Environ Sci Technol 43:3860–3864
Liu C, Pan D, Tang X, Hou M, Zhou Q, Zhou J (2016) Degradation of rhodamine B by the α-MnO2/peroxymonosulfate system. Water Air Soil Pollut 227:92
Liu J, Zhao Z, Shao P, Cui F (2015) Activation of peroxymonosulfate with magnetic Fe3O4–MnO2 core–shell nanocomposites for 4-chlorophenol degradation. Chem Eng J 262:854–861
Liu L, Chen F, Yang F, Chen Y, Crittenden J (2012) Photocatalytic degradation of 2,4-dichlorophenol using nanoscale Fe/TiO2. Chem Eng J 181–182:189–195
Luo S, Duan L, Sun B, Wei M, Li X, Xu A (2015) Manganese oxide octahedral molecular sieve (OMS-2) as an effective catalyst for degradation of organic dyes in aqueous solutions in the presence of peroxymonosulfate. Appl Catal B Environ 164:92–99
Nfodzo P, Choi H (2011) Triclosan decomposition by sulfate radicals: effects of oxidant and metal doses. Chem Eng J 174:629–634
Oh W-D, Dong Z, Lim T-T (2016) Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: current development, challenges and prospects. Appl Catal B Environ 194:169–201
Peng X et al (2017) Double-exchange effect in two-dimensional MnO2 nanomaterials. J Am Chem Soc 139:5242–5248
Prélot B, Poinsignon C, Thomas F, Schouller E, Villiéras F (2003) Structural–chemical disorder of manganese dioxides: 1. Influence on surface properties at the solid–electrolyte interface. J Colloid Interface Sci 257:77–84
Qin W, Fang G, Wang Y, Wu T, Zhu C, Zhou D (2016) Efficient transformation of DDT by peroxymonosulfate activated with cobalt in aqueous systems: kinetics, products, and reactive species identification. Chemosphere 148:68–76
Robinson DM et al (2013) Photochemical water oxidation by crystalline polymorphs of manganese oxides: structural requirements for catalysis. J Am Chem Soc 135:3494–3501
Saputra E, Muhammad S, Sun H, Ang H, Tade M, Wang S (2013) Different crystallographic one-dimensional MnO2 nanomaterials and their superior performance in catalytic phenol degradation. Environ Sci Technol 47:5882–5887
Saputra E, Muhammad S, Sun H, Patel A, Shukla P, Zhu ZH, Wang S (2012) α-MnO2 activation of peroxymonosulfate for catalytic phenol degradation in aqueous solutions. Catal Commun 26:144–148
Schwarzenbach RP, Gschwend PM, Imboden DM (2003) Environmental organic chemistry, 2nd edn. Wiley-Inter-science, New York
Septian A, Shin WS (2019) Oxidative removal of sulfadiazine using synthetic and natural manganese dioxides. Environ Technol 1:1–13
Shukla P, Wang S, Singh K, Ang HM, Tadé MO (2010) Cobalt exchanged zeolites for heterogeneous catalytic oxidation of phenol in the presence of peroxymonosulphate. Appl Catal B 99:163–169
Tan C, Gao N, Deng Y, Deng J, Zhou S, Li J, Xin X (2014) Radical induced degradation of acetaminophen with Fe3O4 magnetic nanoparticles as heterogeneous activator of peroxymonosulfate. J Hazard Mater 276:452–460
Wang C, Ma J, Liu F, He H, Zhang R (2015a) The effects of Mn2+ precursors on the structure and ozone decomposition activity of cryptomelane-type manganese oxide (OMS-2) catalysts. J Phys Chem C 119:23119–23126
Wang J, Wang S (2018) Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem Eng J 334:1502–1517
Wang L et al (2018) Oxidation of bisphenol A by nonradical activation of peroxymonosulfate in the presence of amorphous manganese dioxide. Chem Eng J 352:1004–1013
Wang X, Li Y (2003) Synthesis and formation mechanism of manganese dioxide nanowires/nanorods. Chem A Eur J 9:300–306
Wang Y, Chen S (2015) Droplets impact on textured surfaces: mesoscopic simulation of spreading dynamics. Appl Surf Sci 327:159–167
Wang Y, Indrawirawan S, Duan X, Sun H, Ang H, Tadé M, Wang S (2015b) New insights into heterogeneous generation and evolution processes of sulfate radicals for phenol degradation over one-dimensional α-MnO2 nanostructures. Chem Eng J 266:12–20
Wang Y, Ren H, Pan H, Liu J, Zhang L (2015c) Enhanced tolerance and remediation to mixed contaminates of PCBs and 2,4-DCP by transgenic alfalfa plants expressing the 2,3-dihydroxybiphenyl-1,2-dioxygenase. J Hazard Mater 286:269–275
Wang Y, Zhao X, Cao D, Wang Y, Zhu Y (2017) Peroxymonosulfate enhanced visible light photocatalytic degradation bisphenol A by single-atom dispersed Ag mesoporous g-C3N4 hybrid. Appl Catal B Environ 211:79–88
Xie M, Tang J, Kong L, Lu W, Natarajan V, Zhu F, Zhan J (2019) Cobalt doped g-C3N4 activation of peroxymonosulfate for monochlorophenols degradation. Chem Eng J 360:1213–1222
Xu J, Lv X, Li J, Li Y, Shen L, Zhou H, Xu X (2012) Simultaneous adsorption and dechlorination of 2,4-dichlorophenol by Pd/Fe nanoparticles with multi-walled carbon nanotube support. J Hazard Mater 225–226:36–45
Zhang C, Zhou M, Ren G, Yu X, Ma L, Yang J, Yu F (2015a) Heterogeneous electro-Fenton using modified iron–carbon as catalyst for 2,4-dichlorophenol degradation: influence factors, mechanism and degradation pathway. Water Res 70:414–424
Zhang H, Chen W-R, Huang C-H (2008) Kinetic modeling of oxidation of antibacterial agents by manganese oxide. Environ Sci Technol 42:5548–5554
Zhang H, Huang C-H (2003) Oxidative transformation of triclosan and chlorophene by manganese oxides. Environ Sci Technol 37:2421–2430
Zhang J, Li Y, Wang L, Zhang C, He H (2015b) Catalytic oxidation of formaldehyde over manganese oxides with different crystal structures. Catal Sci Technol 5:2305–2313
Zhao Y, Zhao Y, Zhou R, Mao Y, Tang W, Ren H (2016) Insights into the degradation of 2,4-dichlorophenol in aqueous solution by α-MnO2 nanowire activated persulfate: catalytic performance and kinetic modeling. RSC Adv 6:35441–35448
Zhou H, Shen YF, Wang JY, Chen X, O’Young C-L, Suib SL (1998) Studies of decomposition of H2O2 over manganese oxide octahedral molecular sieve materials. J Catal 176:321–328
Zhou J, Wu K, Wang W, Xu Z, Wan H, Zheng S (2014) Pd supported on boron-doped mesoporous carbon as highly active catalyst for liquid phase catalytic hydrodechlorination of 2,4-dichlorophenol. Appl Catal A 470:336–343
Zhou P et al (2018) Degradation of 2,4-dichlorophenol by activating persulfate and peroxomonosulfate using micron or nanoscale zero-valent copper. J Hazard Mater 344:1209–1219
Zhou Y, Wang X, Zhu C, Dionysiou DD, Zhao G, Fang G, Zhou D (2018) New insight into the mechanism of peroxymonosulfate activation by sulfur-containing minerals: role of sulfur conversion in sulfate radical generation. Water Res 142:208–216
Zhu J, Wang J, Shan C, Zhang J, Lv L, Pan B (2019a) Durable activation of peroxymonosulfate mediated by Co-doped mesoporous FePO4 via charge redistribution for atrazine degradation. Chem Eng J 375:122009
Zhu S, Li X, Kang J, Duan X, Wang S (2019b) Persulfate activation on crystallographic manganese oxides: mechanism of singlet oxygen evolution for nonradical selective degradation of aqueous contaminants. Environ Sci Technol 53:307–315
Acknowledgements
This work was supported by the National Key Research and Development Program of China (NO. 2018YFC1803100), Scientific Research Project of Nanjing Xiaozhuang University (NO. 2017NXY46), Excellent Science and Technology Innovation Group of Jiangsu Province, Innovative Practice of Environmental Engineering Subject Based on New Engineering Construction.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, J., An, F., Li, M. et al. Efficient Degradation of 2,4-Dichlorophenol on Activation of Peroxymonosulfate Mediated by MnO2. Bull Environ Contam Toxicol 107, 255–262 (2021). https://doi.org/10.1007/s00128-021-03109-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-021-03109-7