Abstract
In this study we test the sensitivity of three sizes of golden mussel (Limnoperna fortunei), an introduced species in Argentina, to a 96-h exposure to \(\hbox {Cd}^{2+}\), \(\hbox {Cr}^{6+}\), and \(\hbox {Ni}^{2+}\). We also analysed the relative sensitivity of L. fortunei compared to other freshwater bivalve equivalent sensitivity data. The ANOVA results showed that both factors, heavy metal and size, had significant effects (p = 0.0013 and p = 0.0091, respectively) on the mortality of the golden mussel. Tukey’s test showed significant differences for \(\hbox {Cr}^{6+}\) treatment and the smallest size class (7 mm \(\pm 1\)). The relative sensitivity analysis showed that \(\hbox {LC}_{{50}}\) values for the smallest size class of L. fortunei exposed to \(\hbox {Ni}^{2+}\) and \(\hbox {Cd}^{2+}\) were in the low range, with values of 11.40 mg/L and 12.65 mg/L, respectively. In the case of \(\hbox {Cr}^{6+}\) (1.66 mg/L), its \(\hbox {LC}_{{50}}\) was in the medium-low range of the freshwater bivalve sensitivity distribution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The pollution in aquatic ecosystems with heavy metals is a worldwide concern given the increase in their emissions (Vareda et al. 2019). Biomonitoring is a widely implemented technique that uses organisms in order to assess environmental pollution levels (Zhou et al. 2008). Due to satisfying many of the conditions for an ideal monitoring organism, bivalve molluscs have been extensively utilized for several decades in freshwater and marine environmental monitoring programs (Gupta et al. 2011). Elder and Collins (1991) pointed out the convenience of using introduced species as monitoring organisms because of their physiological tolerance range and their wide distribution. The freshwater golden mussel, Limnoperna fortunei (Dunker, 1857) (Bivalvia, Mytilidae), was introduced by ship ballast water, and reported for the first time in South America in 1991 (Pastorino et al. 1993). Byssate juvenile and adult forms live in dense groups attached to hard surfaces, where they feed on plankton by filtration (Darrigran and Damborenea 2006). The life cycle of L. fortunei presents a planktonic larvae which facilitates a rapid dispersion, assisted by commercial ship traffic, which has enabled the species to colonise five countries in South America (Oliveira et al. 2015). L. fortunei has been tested as a suitable organism for biomonitoring, with most studies focusing on bioaccumulation and biomarkers (do Amaral et al. 2019; Belaich et al. 2006; Villar et al. 1999), but very little has been reported regarding mortality of this species exposed to contaminants (Cataldo et al. 2003; Soares et al. 2009). The objectives of the present study were to (1) analyse the sensitivity (as mortality) of three sizes of L. fortunei to the acute exposure of three heavy metals of environmental relevance: \(\hbox {Cd}^{2+}\), \(\hbox {Cr}^{6+}\), and \(\hbox {Ni}^{2+}\), and (2) establish the relative sensitivity range of L. fortunei among other freshwater bivalves used for toxicological assessment.
Materials and Methods
Individuals of L. fortunei were haphazardly collected during low tide at Palo Blanco Beach in Berisso (\(34^\circ 51'19.1''\hbox {S}\), \(57^\circ 50'17.3''\hbox {W}\)), Buenos Aires, Argentina. Individuals were acclimated in 100 L tanks with dechlorinated and oxygenated tap water (conductivity 1.0 mS/cm; hardness \(215\ \hbox {mg/L}\ \hbox {CaCO}_{{3}}\); alkalinity 180 mg/L \(\hbox {CaCO}_{{3}}\); pH range \(7.6 \pm 0.2\); temperature \(20 \pm 2\) °C; photoperiod 16:8 light:darkness) for at least 2 weeks before each assay. During acclimation, mussels were fed with a cultured Chlorophyceae algal solution. Food was supplied according to mussel’s filtering activity in order to maintain a low green tinge in the water at the end of a 24 h period. Mussels were not fed during the assays. Individuals were measured along the maximum anterior-posterior axis (total length) with a digital calliper (precision 0.01 mm) and, based on the more abundant size intervals, were arranged in three size classes (SC): SC1(\(7 \pm 1\) mm), SC2 (\(13 \pm 1\) mm), and SC3 (\(19 \pm 1\) mm). Three heavy metals of environmental relevance were selected: \(\hbox {Cd}^{2+}\), \(\hbox {Cr}^{6+}\), \(\hbox {Ni}^{2+}\). Dilutions were prepared from stock solutions using analytical grade (ACS) \(\hbox {3CdSO}_{{4}}.\hbox {8H}_{{2}}\hbox {O}\) (Merck), \(\hbox {Cr}_{{2}}\hbox {O}_{{7}}\hbox {K}_{{2}}\) (Analar), and \(\hbox {Ni}^{0}\) granules (Biopack) previously dissolved in \(\hbox {HNO}_{{3}}\). Concentrations were tested and run in triplicate in polyethylene containers. Sets of samples were randomly taken at different days of renewal. Actual metal concentrations of the samples were measured using a Varian Spectr AA 330 atomic absorption spectrophotometer with air-acetylene flame (APHA 1998 Method 3111 B). Quality assurance and control comprised the calibration of equipment using certified reference materials from Accustandard Inc. (Cd:AA08N-1, Cr:AA13N-1, Ni:AA37N-5), blanks and replicates of analytical samples, and bidistilled water. The detection limit for all three metals was 0.005 mg/L. All labware was previously cleaned in a 10% \(\hbox {HNO}_{{3}}\) bath, and assay samples were all refrigerated and acidified with \(\hbox {HNO}_{{3}}\) (Analar) analytical grade for storage. Since not every assay replicate and concentration was sampled at each medium renewal event, data of measured and nominal concentrations from each set of samples was used to calculate estimated concentrations by linear regression methods for each assay. Nominal concentrations in mg/L were: 5, 8, 14, 23, 39, 64 for \(\hbox {Cd}^{2+}\), 1, 2, 4, 8, 16, 32, 64 for \(\hbox {Cr}^{6+}\), and 7, 12, 20, 35, 60, 100, 165 for \(\hbox {Ni}^{2+}\). Negative control contained the same water that was used to make dilutions. For each replicate, 15 mussels were allocated in 0.5 L of dechlorinated and mechanically aerated water and kept for 24 h. After this settlement period, only 10 mussels that had attached to the walls of each container were kept for the assays. Those individuals that had failed to attach were considered unhealthy and therefore discarded. Finally, containers were emptied and refilled with 0.5 L of the corresponding dilution; each of the assays were 96-h static-renewal tests. Test dilutions were renewed every 24 h. Mortality was the selected endpoint. Mussels were considered dead if they remained with opened valves when removed from the container or if they did not show signs of activity in response to physical stimuli with a plastic stick. The integrity of all individuals was assessed under stereo microscope. Statistical endpoints \(\hbox {LC}_{{50}}\) and \(\hbox {LC}_{{10}}\) were estimated by fitting measured concentration data to Finney’s Probit model (Finney 1971) using the Probit software from USEPA (1993), or the Trimmed Spearmen-Karber method (TSK USEPA 1993) where needed. A two-way ANOVA without replication (Microsoft Excel) was performed to assess the effects of the two factors, size class and heavy metal, on the sensitivity (Log \(\hbox {LC}_{{50}}\)) of L. fortunei. Post-hoc Tukey HSD test for multiple comparisons was applied to evaluate the differences between the levels of the two factors. In order to calculate the relative sensitivity (RS) of L. fortunei, we conducted a bibliography search and consulted the USEPA ECOTOX database. Only mortality \(\hbox {LC}_{{50}}\) values from 96 h exposure to the same heavy metals, and from freshwater bivalves in juvenile and adult stage were considered. The toxicity of \(\hbox {Cd}^{2+}\) and \(\hbox {Ni}^{2+}\) are water hardness dependent, however, there is no evidence for this effect on the toxicity of \(\hbox {Cr}^{6+}\) (USEPA 1996). Neither the data from ECOTOX, nor the original publications provided consistently hardness-adjusted \(\hbox {LC}_{{50}}\) values. And in some cases, water hardness was not reported at all. Due to these limitations, no adjustments were made to data in our analysis. We calculated the \(\text{RS}\,=\,\text{Log}(\hbox {LC}_{50\textit{L}f}/\hbox {LC}_{50\textit{i}})\) (Santos-Medrano and Martinez 2019), where ‘\(\hbox {LC}_{50\textit{L}f}\)’ corresponds to the calculated lethal concentration for L. fortunei, and ‘\(\hbox {LC}_{50\textit{i}}\)’ is any one species’ \(\hbox {LC}_{{50}}\) value recorded from our search. In those cases where there was more than one record for a given ‘\(\hbox {LC}_{50\textit{i}}\)’, the arithmetic mean was calculated. With respect to ‘\(\hbox {LC}_{50\textit{L}f}\)’, only those from the size class (SC) that had yielded significant differences in the ANOVA were included in the relative sensitivity analysis. Finally, we charted the frequency distribution of all Log \(\hbox {LC}_{{50}}\) values, and graphically showed where the sensitivity of the three size classes of L. fortunei lie for each heavy metal.
Results and Discussion
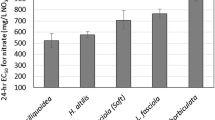
Details of the heavy metal estimated concentration adjusted by linear regression are shown in Table 1. Estimations averaged 58%, 103%, and 86% of the respective \(\hbox {Cd}^{2+}\), \(\hbox {Cr}^{6+}\), and \(\hbox {Ni}^{2+}\) nominal values. There was no mortality in the negative control for all replicates. The estimated \(\hbox {LC}_{{50}}\) and \(\hbox {LC}_{{10}}\) values with their respective 95\(\%\) confidence limits for each heavy metal assay are shown in Table 2. In the case of \(\hbox {Ni}^{2+}\), mortality data for SC1 did not show a monotonically increased response to the treatments, and thus was fitted to a TSK method. \(\hbox {Ni}^{2+}\)\(\hbox {LC}_{{10}}\) values for SC1 could not be calculated by TSK method. The \(\hbox {LC}_{{50}}\) values for SC1 showed the toxicity trend was \(\hbox {Cr}^{6+}>\hbox {Ni}^{2+}>\hbox {Cd}^{2+}\), whereas for SC2 and SC3 the trend was \(\hbox {Cr}^{6+}>\hbox {Cd}^{2+}>\hbox {Ni}^{2+}\). \(\hbox {LC}_{{10}}\) values for SC2 and SC3 showed a consistent toxicity trend: \(\hbox {Cr}^{6+}>\hbox {Cd}^{2+}>\hbox {Ni}^{2+}\). The two-way ANOVA results indicated significant effects (\(\hbox {p}<0.05\)) on the mortality of L. fortunei for metals (p= 0.0013) and mussel size class (p= 0.0095). Tukey HSD test yielded significant differences between mussels from SC1 and those of SC2 and SC3 (p = 0.0216 and p = 0.0091, respectively). Heavy metal comparisons showed that sensitivity to \(\hbox {Cr}^{6+}\) was significantly different to that of \(\hbox {Cd}^{2+}\) (p = 0.0036), and \(\hbox {Ni}^{2+}\) (p = 0.0016). The combination of data from the USEPA ECOTOX database and bibliography searches yielded 29 \(\hbox {Cd}^{2+}\)\(\hbox {LC}_{{50}}\) entries corresponding to 13 species, 12 \(\hbox {Cr}^{6+}\)\(\hbox {LC}_{{50}}\) entries for 9 species, and 16 \(\hbox {Ni}^{2+}\)\(\hbox {LC}_{{50}}\) entries for 11 species. The calculations of the relative sensitivity of L. fortunei SC1 compared to that of other freshwater bivalves are shown in Table 3. In regard to \(\hbox {Cd}^{2+}\) and \(\hbox {Ni}^{2+}\), it can be observed that L. fortunei SC1 is less sensitive than all freshwater bivalves. However, data for these two metals could not be normalised for water hardness. Villorita cyprinoides cochinensis is the organism with the highest RS scores of 3.67 and 2.27 for \(\hbox {Cd}^{2+}\) and \(\hbox {Ni}^{2+}\), respectively. In the case of \(\hbox {Cr}^{6+}\), L. fortunei SC1 showed to be more sensitive than Diplodon chilensis (RS -1.09) and Hyriopsis cumingi (RS -0.81), but scored a lower relative sensitivity than the rest of the test organisms included in the analysis. V. c. cochinensis (RS 2.16) was the most sensitive compared to L. fortunei SC1. A graphic representation of the RS values from Table 3 is depicted in Fig. 1. When compared to data from other freshwater bivalves, the sensitivity range of L. fortunei to \(\hbox {Cd}^{2+}\) (Fig. 2a) and \(\hbox {Ni}^{2+}\) (Fig. 2c) sits in the lower end of the distribution of sensitivities. Furthermore, the golden mussel’s sensitivity to \(\hbox {Cr}^{6+}\) falls within the medium-low range (Fig. 2b). Small juveniles of L. fortunei, such as those of SC1 in this study, represent the most numerous size class in natural populations of the golden mussel (Bonel and Lorda 2015). They are easy to collect in the field, and given their encrusting nature they can establish colonies on artificial substrates that can be used as artificial units of habitat for manipulative experiments. L. fortunei presents a short life cycle (2/3 years) with a planktonic larval development, and a long actively reproductive period with external fecundation (Darrigran and Damborenea 2006). Because of the aforementioned characteristics, and the ability of L. fortunei to tolerate a wide range of environmental conditions (Ricciardi 1998), this species is a versatile test organism for different experimental scenarios and rearing in the laboratory. Conversely, most of the native species of freshwater bivalve families in Argentina live buried in sediments, with the exception of some epifaunal species such as Byssanodonta paranensis and Eupera platensis which have more specific distribution patterns (Darrigran and Lagreca 2005). Their life cycles include ectoparasitic larval stages (e.g. glochidia in Hyriidae, or lasidia in Mycetopodidae), or are ovoviviparous with less prolific reproductive periods (e.g. Euperinae) (Ezcurra de Drago et al. 2006; Ituarte 1988). Previous studies on L. fortunei tested for bioaccumulation and biomarkers in response to heavy metals such as mercury, copper, and cadmium (Belaich et al. 2006; do Amaral et al. 2019; Soares et al. 2009) and organic compounds (Iummato et al. 2018; Pereyra et al. 2011, 2012). This study provides new sensitivity data for L. fortunei under acute exposure to \(\hbox {Cd}^{2+}\), \(\hbox {Cr}^{6+}\), and \(\hbox {Ni}^{2+}\). It also shows that the juveniles of the golden mussel present a range of sensitivity suitable for a sentinel species (e.g. studies with biomarkers). Its encrusting epifaunal nature that enables simple sampling methods, widespread distribution (which allows inter-regional comparisons), and easy maintenance in the laboratory, make L. fortunei a suitable candidate for biomonitoring programs.
Graphic representation of the relative sensitivity (RS) calculations from Table 3 for three heavy metals: \(\hbox {Cd}^{2+}\), \(\hbox {Cr}^{6+}\), \(\hbox {Ni}^{2+}\). Positive values represent a greater RS, and negative values correspond to a lesser RS than L. fortunei SC1 (dotted line)
References
Abdel Gawad S (2006) Toxicity and bioaccumulation of Cadmium in the freshwater bivalve Corbicula Fluminalis Müller, 1774. EgyptJ Aquat Biol Fish 10(4):33–43
Abraham TJ, Salih KYM, Chacko J (1986) Effects of heavy metals on the filtration rate of bivalve Villorita cyprinoides (Hanley) var. cochinensis. Indian J Mar Sci 15(3):195–196
Belaich M, Oliver C, Pilloff M, Porta A (2006) Evaluation of a biomarker of cd (II) exposure on Limnoperna fortunei. Environ Pollut 144(1):280–288. https://doi.org/10.1016/j.envpol.2005.12.015
Bhamre PR, Thorat SP, Desai AE, Deoray BM (2010) Evaluation of acute toxicity of mercury, cadmium and zinc to a freshwater mussel Lamellidens consobrinus. Our Nature 8(1):180–184
Black MC (2003) Water quality standards for North Carolina’s endangered mussels. University of Georgia, Department of Environmental Health Science
Bonel N, Lorda J (2015) Growth and body weight variability of the invasive mussel Limnoperna fortunei (mytilidae) across habitat and season. Malacologia 58(1–2):129–146. https://doi.org/10.4002/040.058.0202
Cataldo D, Boltovskoy D, Pose M (2003) Toxicity of chlorine and three nonoxidizing molluscicides to the pest mussel Limnoperna fortunei. J Am Water Works Assoc 95(1):66–78. https://doi.org/10.1002/j.1551-8833.2003.tb10270.x
Chin HC, Chou FF (1978) Acute chromium toxicity of the freshwater mussel, Hyriopsis cumingii Lea. Nan-Ch-ing Ta Hsueh Hsueh PaoTsu Jan K’o Hsueh Pan 4:96–101
Chung KS (1980) Acute toxicity of selected heavy metals to mangrove oyster Crassostrea rhizophorae. Bull Jap Soc Sci Fish 46(6):777–780
do Amaral QDF, Da Rosa E, Wronski JG, Zuravski L, Querol MVM, dos Anjos B, de deAndrade CFF, Machado MM, de Oliveira LFS (2019) Golden mussel (Limnoperna fortunei) as a bioindicator in aquatic environments contaminated with mercury: cytotoxic and genotoxic aspects. Sci Total Environ 675:343–353. https://doi.org/10.1016/j.scitotenv.2019.04.108
Darrigran G, Damborenea C (2006) Capítulo 3 características de la especie. In: Darrigran G, Damborenea C (eds) Bio-invasón del mejillón dorado en el continente americano. Editorial de la Universidad Nacional de La Plata (EDULP), Argentina, pp 69–79
Darrigran G, Lagreca M (2005) Moluscos litorales del estuario del río de la plata, argentina. ProBiota 8:41
Ezcurra de Drago I, Montalto L, Oliveros O (2006) Desarrollo y ecología larval de Limnoperna fortunei. In: Darrigran G, Damborenea C (eds) Bioinvasilón del mejillón dorado en el continente americano. Editorial de la Universidad Nacional de La Plata (EDULP), Argentina, pp 85–93
Elder JF, Collins JJ (1991) Freshwater molluscs as indicators of bioavailability and toxicity of metals in surface-water systems. In: Ware G (ed) Reviews of environmental contamination and toxicology. Springer, New York, pp 37–79
Finney DJ (1971) Probit analysis, 3d edn. Cambridge University Press, Cambridge
Gibson KJ (2015) Acute toxicity testing on freshwater mussels (Bivalvia: Unionidae) and freshwater snails (Gastropoda: Caenogastropoda). Doctoral dissertation, Troy University
Gupta SK, Singh J et al (2011) Evaluation of mollusc as sensitive indicator of heavy metal pollution in aquatic system: a review. IIOAB J 2(1):49–57
Ituarte CF (1988) Características de la incubación branquial en Eupera platensis doello-jurado, 1921 y Byssanodonta paranensis d’orbigny, 1846 (pelecypoda: Sphaeriidae. Iheringia 68:41–47
Iummato MM, Sabatini SE, Cacciatore LC, Cochón AC, Cataldo D, de Molina MdCR, Juárez ÁB (2018) Biochemical responses of the golden mussel Limnoperna fortunei under dietary glyphosate exposure. Ecotoxicol Environ Saf 163:69–75. https://doi.org/10.1016/j.ecoenv.2018.07.046
Keller AE (2000) Memorandum to Rob Pepin. Subject: water quality and toxicity data for unpublished unionid mussels tests. USEPA, Chicago, IL, 14 p
Keller AE, Zam SG (1991) The acute toxicity of selected metals to the fresh water mussel, Anodonta imbecillis. Environ Toxicol Chem 10:539–546
Mackie GL (1989) Tolerances of five benthic invertebrates to hydrogen ions and metals (Cd, Pb, Al). Arch Environ Contam Toxicol 18(1–2):215–223
Oliveira MD, Campos MC, Paolucci EM, Mansur MC, Hamilton SK (2015) Colonization and spread of Limnoperna fortunei in south america. In: Boltovskoy D (ed) Limnoperna Fortunei. Springer, Cham, pp 333–355. https://doi.org/10.1007/978-3-319-13494-9_19
Olson KR, Harrel RC (1973) Effect of salinity on acute toxicity of mercury, copper, and chromium for Rangia cuneata (Pelecypoda, Mactridae). Contrib Mar Sci 17:9–13
Pastorino G, Darrigran G, Stella Maris M, Lunaschi L (1993) Limnoperna fortunei (dunker, 1857) (mytilidae), nuevo bivalvo invasor en aguas del río de la plata. Neotropica 39:34
Pereyra PJ, Bulus Rossini G, Darrigran G (2011) Toxicity of three commercial tannins to the nuisance invasive species Limnoperna fortunei(dunker, 1857): implications for control. Fresenius Environ Bull 20(6):1432–1437
Pereyra PJ, Bulus Rossini G, Darrigran G (2012) Toxicity of neem’s oil, a potential biocide against the invasive mussel Limnoperna fortunei (dunker 1857). Anais da Academia Brasileira de Ciências 84(4):1065–1071. https://doi.org/10.1590/S0001-37652012005000059
Raj AIM, Hameed PS (1990) Freshwater mussel, Lamellidens marginalis (Lamarck) (mollusca: bivalvia: unionidae) as an indicator of river pollution. Chem Ecol 4(2):57–64
Ricciardi A (1998) Global range expansion of the asian mussel Limnoperna fortunei (mytilidae): another fouling threat to freshwater systems. Biofouling 13(2):97–106. https://doi.org/10.1080/08927019809378374
Santos-Medrano GE, Martinez RR (2019) Acute sensitivity comparison among daphnia magna straus, 1820 daphnia pulex leydig, 1860 and simocephalus vetulus müller, 1776, exposed to nine toxicants. Turk J Fish Aquat Sci 19(7):615–623. https://doi.org/10.4194/1303-2712-v19_7_08
Silva J, Fuentealba C, Bay-Schmith E, Larrain A (2007) Estandarizacion del bioensayo de toxicidad aguad con Diplodon chilensis usando un toxico de referencia. Gayana (Concepción) 71(2):135–141
Soares M, Pereira D, Santos C, Mansur M, Pires M, Breintenbach J, Grespan C (2009) Toxicidade do sulfato de cobre ao mexilhão dourado, Limnoperna fortunei (dunker, 1857), em água bruta. J Braz Soc Ecotoxicol 4(1–3):37–48. https://doi.org/10.5132/jbse.2009.01.006
USEPA (1996) Water quality criteria documents for the protection of aquatic life in ambient water: 1995 updates
Vareda JP, Valente AJ, Durães L (2019) Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: a review. J Environ Manag 246:101–118. https://doi.org/10.1016/j.jenvman.2019.05.126
Villar C, Stripeikis J, D’huicque L, Tudino M, Troccoli O, Bonetto C (1999) Cd, cu and zn concentrations in sediments and the invasive bivalves Limnoperna fortunei and Corbicula fluminea at the rio de la plata basin, argentina. Hydrobiologia 416:41–49. https://doi.org/10.1023/A:1003811223880
Wang N, Ivey CD, Ingersoll CG, Brumbaugh WG, Alvarez D, Hammer EJ, Bauer CR, Augspurger T, Raimondo S, Christopher Barnhart M (2017) Acute sensitivity of a broad range of freshwater mussels to chemicals with different modes of toxic action. Environ Toxicol Chem 36(3):786–796
Zhou Q, Zhang J, Fu J, Shi J, Jiang G (2008) Biomonitoring: an appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal Chim Acta 606(2):135–150. https://doi.org/10.1016/j.aca.2007.11.018
Acknowledgements
The authors thank the Faculty of Exact Science of the National University of La Plata (FCEx-UNLP) for providing the space to conduct the experiments involved in this article, and to the reviewers whose valuable observations helped us improve and clarify our manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no confict of interest.
Ethical Approval
The authors declare that all studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bálsamo Crespo, E., Pereyra, P.J., Silvestro, A. et al. Acute Toxicity of \(\hbox {Cd}^{2+}\), \(\hbox {Cr}^{6+}\), and \(\hbox {Ni}^{2+}\) to the Golden Mussel Limnoperna fortunei (Dunker 1857). Bull Environ Contam Toxicol 104, 748–754 (2020). https://doi.org/10.1007/s00128-020-02854-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-020-02854-5