Abstract
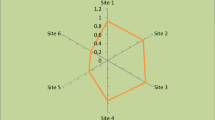
The trace metal pollution in the environment is a highly concerned issue in these days. One of the important causes of trace metal pollution is the exhaust gases released from the vehicles on the roads. These dangerous gases pose life-threatening effects on the forage plants grown along the roadside as these plants are at direct risk to these trace metals. The aims of the present study were to determine the cobalt (Co) concentrations in soil, forages, and blood plasma of the buffaloes and to evaluate the Co deficiencies and toxicities in these samples. All samples were collected from six sites (Faisalabad roadside, Bhalwal roadside, Shaheenabad roadside, Mateela roadside, 50 Chak roadside, and Dera Saudi-control) of Sargodha city. The Co concentrations in these samples were determined by atomic absorption spectrophotometer (AA-6300 Shimadzu Japan). In soil samples, Co level ranged from 1.958 to 3.457 mg/kg in the six sampling sites. The highest Co level was observed at site 6 and the lowest at site 2. In forage samples, Co level ranged from 0.770 to 2.309 mg/kg in the six sampling sites. The highest Co level was observed at site 3 and the lowest at site 2. In blood plasma samples, Co level ranged from 2.644 to 4.927 mg/kg in the six sampling sites. The highest Co level was observed at site 1 and the lowest at site 3. The results showed higher Co values in the samples collected from the site IV while the bioconcentration factor for forage-soil was found highest in the samples collected from Site III. On the other hand, a correlation was found positively significant when soil and forage were correlated, and it was found negatively significant when blood and forage were correlated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The livestock of a country is very important as it yields a list of healthy food (Devendra and Thomas 2002). A variety of food and other usable things for industrial use are also obtained from animals. This most important part of the ecosystem is under threat due to increased urbanization and industrialization (Ugulu 2015a, b). Such activities add toxic contaminants to the environment which are replete with dangerous chemicals like trace metals (Durkan et al. 2011; Ugulu et al. 2016). These trace metals then become part of various elements of ecosystems, accumulate there, and pose toxic effects on them (Dogan et al. 2010; Ahmad et al. 2018a; Khan et al. 2018a).
The soil and the plants which grow on soil get much contaminated by the trace metals (Ugulu and Baslar 2010; Nadeem et al. 2019). This condition of contamination is more adverse when the toxic smoke of the automobiles gets released and affects the roadside plants directly (Dogan et al. 2014a, b; Ugulu et al. 2012). The trace metals get accumulated in the plant bodies and soil and from the contaminated forages the trace metals get to enter into bodies of animals when they consume these forages (Khan et al. 2018b). As the fodder plants are the primary source of toxic trace metals for the animals (Ugulu et al. 2009; Yorek et al. 2016). Thus, forage plants analysis is the best indicator for mineral status as compared with the soil (McDowell 1983). Various types of metabolic disorders are observed in the animals which feed on such forages (Khan et al. 2018c). Tissue and blood of animals are reliable sources of upraising their metal status than forage due to soil contamination inconsistency in diet selection or availability of an ingested nutrient (Unver et al. 2015). Deficiency disease or symptoms occur secondary to illness and functional and structural abnormalities including impaired neuromuscular functions of skeletal, smooth, and cardiac muscle, muscular weakness, paralysis, and mental confusion (Murray et al. 2000).
There is no doubt naturally trace metals are found in the environment; they exceed their limits when continuously released into the environment as a result of anthropogenic activities (Ahmad et al. 2018b). In this direction, the present study was conducted to check the accumulation of cobalt in roadside soil, forages, and buffalo blood and to evaluate the Co deficiencies and toxicities in these samples.
Materials and methods
Study area
The area selected for study was Sargodha, Punjab, Pakistan (Fig. 1). The relative humidity in Sargodha is almost 30–48%; the temperature in summer season is almost 24–49 °C during day time unlike the temperature of winter which is a minimum of up to 8 °C. In Sargodha, the forages and the wastes of the agricultural products are the common food of the ruminants. The samples of four types of forages jowar (Sorghum bicolor (L.) Moench), wheat (Triticum aestivum L.), berseem (Trifolium alexandrinum L.), and bajra (Pennisetum typhoideum Rich.) were collected from six different sites of Sargodha which were Faisalabad roadside (site 1), Bhalwal roadside (site 2), Shaheenabad roadside (site 3), Mateela roadside (Site 4), 50 Chak roadside (site 5), and Dera Saudi (site 6), the site away from the road. The collection of the samples of forages, soil, and buffalo blood were collected separately in winter and summer seasons. Winter sampling was done in December–January 2016 while the summer sampling was done in May–June 2016.
Sample collection
Soil samples
Top soil samples (0–20 cm) were collected from the six sites of Sargodha. Rocky granules and large lumps were removed from the soil samples. A total of 120 samples of soil separately were collected as 60 replicates in winter and 60 in summer season. Soil samples were air dried followed by their drying in an oven at 70–75 °C for almost 7 days. Then, after the oven drying, the samples were crushed and saved for a further procedure that was acid digestion.
Forage samples
A total of 120 samples of four forages were collected, 60 samples of two forages (Gandum and Sorghum) from winter and 60 samples of two forages (Millet and Berseem) from summer seasons. This sampling was done as 5 replicates of each forage from each site. Forage samples were collected in polythene bags. Samples of sorghum, berseem, gandum, and millet samples were collected from the roadsides of Mateela, Bhalwal, Shaheenabad, 50 chak, and Faisalabad roadside. The samples from the site away from the road were collected from Dera Saudi. All samples of forages were subjected to air drying which was then followed by oven drying at 70–75 °C for 7 days. The samples were ground after they were removed from the oven; about 2 g of the weighed sample was saved for the further procedure.
Blood samples
The samples of the buffalo blood were collected from the jugular veins of buffaloes in sealed test tubes. Sixty samples of buffaloes’ blood were collected from six sites in winter and summer season, respectively. The serum of the blood samples was separated by centrifugation (3000 rpm for 15–30 min). The serum was then saved (at 20 °C) for further procedure.
Metal analysis
Soil
Kacar (1996) gave the method of acid digestion of the soil samples utilizing 2 g of soil sample and 30% H2O2 (10 mL). This mixture was then dried at 90 °C. This procedure was repeated twice, and the temperature was increased up to 200 °C after the addition of a 1-mL concentration of HClO4 and a 2-mL concentration of H2SO4 to the dried sample.
Forage
The procedure for the acid digestion of forage samples utilizes around 1 g of the crushed and ground forage sample in an Erlenmeyer where it was processed for 3 h at 85 °C with a mixture of HCL and HNO3 in the ratio 1:3. Along with this mixture, concentrated HCLO4 was also added in Erlenmeyer (Sabudak et al. 2007).
Blood plasma
Serum of all the blood samples was digested separately using a 10-mL concentration of nitric acid in around a 100-mL digestion flask with 0.5 g sample of blood serum. The temperature of this apparatus was about 80 °C. Five milliliters per chloric acid was also added to this mixture. Heating was continued until the solution became colorless (Richards 1968).
Dilution and filtration
Digested samples were filtered and diluted up to 50 mL with distilled water. These samples were now ready for analysis of cobalt.
Reagents and apparatus
The step dilution of the stock solution (1000 mg/L Merck AAS solutions) along with the distilled deionized water was used to prepare the standard working solution of cobalt. To avoid contamination, the containers were rinsed with distilled water after their dipping in concentrated HNO3 for one night. Atomic absorption spectrophotometer (AA-6300 Shimadzu Japan) equipped with a graphite furnace was used to analyze the cobalt in the digested samples of blood, soil, and forage.
Statistical analysis
Data obtained from soil, forage, and blood samples were statistically analyzed and the mean concentration of cobalt was found in the samples. Variance and correlation were determined using SPSS Software and ANOVA. The mean significance values were at 0.05, 0.001, and 0.01 probability levels represented by Steel and Torrie (1980).
Bioconcentration factor
The metal content of the forage plant (without taking into consideration different parts of the plant) is determined by calculating the bioaccumulation factor. Bioconcentration factor (BCF) is defined as a measure of the ability of metal uptake by plant and the transport of the trace metal into different parts of plant (Sainger et al. 2011).
Health risk index
The estimated exposure ratio of trace metals via forage intake and oral reference dose is called health risk index (HRI) (Cui et al. 2004; USEPA 2002).
From the integrated risk information system, the RfD value for Co was 0.043 mg/kg/day (USEPA 2002).
Daily intake of metals
The formula to find the daily intake of metals (DIM) is:
Baverage weight means the average body weight of buffalo which is 550 kg while Dfood intake means the daily intake of forage by animals which is 12.5 kg and Cmetal represents the trace metal concentrations in forage (Briggs and Briggs 1980).
Pollution load index
Pollution load index has been evaluated as:
PLI = Metal concentration in investigated soil ∕ reference value of the metal in soil (Liu et al. 2005).
The reference value for Co was 9.1 mg/kg (Dutch Standards 2000).
Results and discussion
Soil
In soil samples, Co level ranged from 1.958 to 3.457 mg/kg in the six sampling sites. The highest Co level was observed at site 6 and the lowest at site 2 (Table 1). The concentration of Co in the six sites of sampling was of the order: site 6 > site 5 > site 3 > site 4 > site 1 > site 2, respectively. The results from analysis of variance of the soil data showed a non-significant effect (P > 0.05) of sites on concentration of soil Co for season, sites, and sites × seasons, respectively (Table 2).
The study performed by Marianna et al. (2016) showed a higher concentration of Co as compared with the concentrations of this metal found in this study. Considerably low industrialization and limited anthropogenic activities in the study area could be responsible for less accumulation of Co in the agricultural soils (Aksoy and Ozturk 1997). Adriano (1986) reported that the trace metal accumulation in soil is a dominant factor. Haktanir (1983) found that in organic matter, the percentage of clay and high pH does not allow the trace metals to get transferred to the next trophic level rather increase binding capacity of trace metals in the system in which they are present. In addition to that, trace metals get adsorbed by silt or clay fraction along with the organic matter.
Forage
In forage samples, Co level ranged from 0.770 to 2.309 mg/kg in the six sampling sites. The highest Co level was observed at site 3 and the lowest at site 2 (Table 3). The concentration of cobalt in the six sites of sampling was of the order: site 3 > site 5 > site 6 > site 4 > site 1 > site 2, respectively. Non-significant results of cobalt were found in the concentration of soil Co examined in the samples of forage for season and sites × seasons. Significant effects of Co were found on sites, respectively (Table 4).
Umar et al. (2015) studied gum Arabic tree (Acacia nilotica) and found higher Co concentrations unlike the Co concentration found in samples of forages collected and tested in the current study. The reason behind the exceeded concentration of Co in the samples tested in this study was due to enhanced automobile emissions in the region of Sargodha. The presence of hydrogen ions in the soil, mineral status of soil, and composition of the forages and soil samples greatly affect the concentration of Co in the examined samples (Huston et al. 2006) Trace metal accumulation and their distribution in the different parts of the environment depend on many factors. The factors may be organic matter content, pH, and clay contents of soil. In addition to them are the number of automobiles on the roads, the distance of forages, and soil from the road and duration of exposure of living content of environment to the dangerous gases (Kalavrouziotis et al. 2007)
Blood
In blood plasma samples, Co level ranged from 2.644 to 4.927 mg/kg in the six sampling sites. The highest Co level was observed at site 1 and the lowest at site 3 (Table 5). The concentration of cobalt in the six sites of sampling was of the order: site 3 > site 2 > site 4 > site 5 > site 6 > site 1, respectively. The results from analysis of variance of the soil data showed non-significant effect (P > 0.05) of sites on concentration of soil Co for season and sites × seasons and on the other hand, the variance of soil data showed significant effects of cobalt on sites, respectively (Table 6).
The Co existence in the samples of the blood of buffaloes was studied by Nwede et al. (2010) and these results were compared by the Co concentration determined in the current study. It was found that the Co concentration in the current study was higher than that found in the samples of buffalo studied by Nwede et al. (2010). It was alarming to note that along with blood, cobalt also accumulated and resisted in the sensitive organs of buffalos like kidneys (Minervino et al. 2009). The need of the hour is that the smoke of the traffic should be managed properly in order to avoid the damages caused by the various trace metals like cobalt in the toxic smoke. The plant contamination and the associated contaminations of the other trophic levels like animals and human beings have really become a concerning issue (Abbas et al. 2010).
Bioconcentration factor for forage-soil
The minimum BCF value of Co (0.32) was observed at site 6 while the maximum BCF value of Co (1.054) was observed at site 2 (Table 7). The order of BCF for forage-soil was in the following order at six sites of sampling: site 2 > site 4 > site 3 > site 6 > site 5 > site 1, respectively.
Bioconcentration factor calculated for Co showed higher value in the samples examined by Kamal et al. (2015) unlike the BCF results found in the current study. Loose bonding of Co with the soil was the reason behind lower BCF in the samples examined in the current study. The reason behind the lowest BCF was the tight bonding of Co with the soil and thus, it does not get transferred into the forages. Khan et al. (2018d) reported that the value of the BCF greater than 1 means that the forages can accumulate a large concentration of trace metals in them. The extent up to which the trace metals like Co get accumulated in the soil or forages or any part of the ecosystem depends upon the age, the composition, the climatic conditions of that area, and the edaphic factors (Alloway and Ayres 1997). In addition to this, the other reasons behind the elevated Co in the samples was the mining process in the area found in the vicinity of Sargodha, emissions from the automobiles, fertilizer application, burning of fossil fuels, smelting etc. (Smith and Carson 1981).
Bioconcentration factor for blood-forage
The minimum BCF value of Co (1.34) was observed at site 6 while the maximum BCF value of Co (5.55) was observed at site 2 (Table 8). The order of BCF for blood plasma-forage samples was in the following order at six sites of sampling: site 2 > site 4 > site 3 > site 5 > site 1 > site 6.
When the data of results found in the current study were compared with the data obtained in the study of Tshibangu et al. (2014), it was found that the lowest concentration of Co was found in the samples studied by Tshibangu et al. (2014). The concentration of the hydrogen ions in the soil interferes with the cobalt concentration in the soil (Zhang et al. 2007; Cui et al. 2004). The exceeded concentration of hydrogen ions in the soil does not allow the movement of trace metals like cobalt and on the other hand, lesser concentration of hydrogen ions (Ph) does not pose much influence on the concentration of the trace metals in the soil and their transfer to plants (Celechovska et al. 2008).
Daily intake of metals
The minimum DIM value of Co (0.001) was observed at site 1 while the maximum DIM value of Co (0.03) was observed at site 4 (Table 9). The order of DIM values for cobalt was as follows: site 4 > site 3 > site 2 > site 6 > site 5 > site 1.
The Co concentration found as the result the DIM found by Saskia et al. (2013) showed a value lower than 1 like that found in the current study. The daily intake of cobalt was found almost 0.1 which suggests the absence of any health risk after the consumption of such forages by animals (Radwan and Salama 2006). Cobalt concentration was found lowest as no considerable source of dust, burning coal, and fossil fuels and forest fires existed in the area of sampling.
Health risk index
The minimum HRI value of Co (0.240) was observed at site 1 while the maximum HRI value of Co (0.697) was observed in site 3 (Table 10). The order of HRI values for CO was: site 3 > site 4 > site 2 > site 5 > site 6 > site 1.
The HRI value found after the analysis of the results found in this study was found highest when was compared with the results of HRI found in the study of Zahara et al. (2014). The HRI value greater than 1 if found in the results suggests the greater health risk associated with the consumption of the contaminated diets, forages (USEPA 2002). But if the HRI value comes out to be less than 1 after the analysis of the results, then it means no considerable health risk is associated with the consumption of such diet (IRIS 2003). USEPA (2002) reported that the value of HRI due to the contaminated diet depends upon the soil chemical and physical characteristics, the kind of forage, and the rate up to which the contaminated forage is being consumed. The value of health risk index is quite usable and affective in providing a quantitative determination for future management of risk and the checking of the environment from every aspect.
Pollution load index
The minimum pollution load index (PLI) value of Co (0.216) was observed at site 1 while the maximum PLI value of Co (0.379) was observed at site 6 (Table 11). The PLI for cobalt was in the order: site 6 > site 3 > site 5 > site 4 > site 2 > site 1.
The PLI value found the lowest in the samples examined in the current study unlike the samples studied by Ahmad et al. (2014). The value of PLI lower than 1 means the area is less contaminated and polluted while the PLI greater than 1 suggest higher contamination. Tomlinson et al. (1980) gave the criteria for using PLI for determining the contamination status of the soil. According to them, the PLI value greater than 1 means the soil is very much destroyed, and the value less than 1 means perfection, while the value of PLI equal to 1 presents only baseline levels of pollutants present.
Correlation
The correlation for soil-forage for cobalt was found positively significant; on the other hand, the correlation for blood-forage was found negatively significant (Table 12).
The lowest correlation was found in the samples of soil, forages, and blood examined in the study done by Ahmad et al. (2014). A positively non-significant correlation for Co means a strong relationship which indicates the balance flow of trace metals between soil and forage. A positively non-significant correlation found in the samples means a weaker relationship existed between cobalt found in soil and forage. More easy transfer of trace metals from soil-forage also leads to positive correlation (Amlan et al. 2012). A negatively non-significant correlation means an imbalance of cobalt between soil and forage leading to a convoluted relationship.
Conclusion
The daily intake of cobalt was found almost 0.1 which suggests the absence of any health risk after the consumption of such forages by animals. Moreover, the pollution load index and health risk index values below 1 showed that the study area is less contaminated and no considerable health risk for an animal is associated with the consumption of such diet. The basic factors influencing the accumulation of metals in buffaloes depend upon the occurrence of metals, their concentration in fodder and soil, and the duration of exposure. The correlation for soil-forage for cobalt was found positively significant; on the other hand, the correlation for blood-forage was found negatively significant. The bioconcentration factor values of Co were higher for blood plasma of buffaloes. So, the higher uptake of Co by buffaloes might be toxic to exert dangerous effect on them in the future.
References
Abbas M, Parveen Z, Iqbal Z, Riazuddin M, Iqbal S, Ahmed M, Bhutto R (2010) Monitoring of toxic metals (cadmium, lead, arsenic and mercury) in vegetables of Sindh, Pakistan, Kathmandu University. J Sci Engin Technol 6(2):60–65
Adriano DC (1986) Trace elements in the terrestrial environment. Springer-Verlag Inc, New York, pp 1–45
Ahmad K, Khan ZI, Ashfaq A, Ashraf M, Yasmin S (2014) Assessment of heavy metal and metalloid levels in spinach (Spinacia oleracea L.) grown in wastewater irrigated agricultural soil of Sargodha, Pakistan. Pak J Bot 46(5):1805–1810
Ahmad K, Nawaz K, Khan ZI et al (2018a) Effect of diverse regimes of irrigation on metals accumulation in wheat crop: an assessment-dire need of the day. Fresen Environ Bull 27(2):846–855
Ahmad K, Kokab R, Khan ZI, Ashfaq A, Bashir H, Mudasra M et al (2018b) Assessment of heavy metals in wheat variety “Chagi-2” under short-term wastewater irrigation. Biologia (Pakistan) 64(1):15–25
Aksoy A, Ozturk MA (1997) Nerium oleander L. as a biomonitor of lead and other heavy metal pollution in Mediterranean environments. Sci Total Environ 205:145–150
Alloway BJ, Ayres DC (1997) Chemical principles for environmental pollution. Blackie Academic Profes, pp 190–220
Amlan K, Ghosh MA, Bhatt HP, Agrawal A (2012) Effect of long-term application of treated sewage water on heavy metal accumulation in vegetables grown in Northern India. Environ Monit Assess 184:1025–1036
Briggs H, Briggs HM (1980) Modern breeds of livestock, Fourth edn. McMillion Publishing Co, London
Celechovska O, Malota L, Zima Z (2008) Entry of heavy metals into food chains: a 20-years comparison study in the Northern Moravia (Czech Republic). Act Vet Brnao 77:645–652
Cui YL, Zhu RH, Zhi RH et al (2004) Transfer of metals from soils to vegetables in an area near a smelter in Nanning, China. Environ Int 30:785–791
Devendra C, Thomas D (2002) Crop–animal systems in Asia: importance of livestock and characterization of agro-ecological zones. Agric Sys 71(1–2):5–15
Dogan Y, Ugulu I, Baslar S (2010) Turkish red pine as a biomonitor: a comparative study of the accumulation of trace elements in needles and barks. Ekoloji 19(75):88–96. https://doi.org/10.5053/ekoloji.2010.7512
Dogan Y, Baslar S, Ugulu I (2014a) A study on detecting heavy metal accumulation through biomonitoring: content of trace elements in plants at Mount Kazdagi in Turkey. Appl Ecol Environ Res 12(3):627–636. https://doi.org/10.15666/aeer/1203_627636
Dogan Y, Unver MC, Ugulu I, Calis M, Durkan N (2014b) Heavy metal accumulation in the bark and leaves of Juglans regia planted in Artvin City, Turkey. Biotech Biotechnol Equip 28(4):643–649. https://doi.org/10.1080/13102818.2014.947076
Durkan N, Ugulu I, Unver MC, Dogan Y, Baslar S (2011) Concentrations of trace elements aluminum, boron, cobalt and tin in various wild edible mushroom species from Buyuk Menderes River Basin of Turkey by ICP-OES. Trace Elements and Electrolytes 28(4):242–248. https://doi.org/10.5414/TEX01198
Haktanir K (1983) Cevre Kirliligi, Ankara Un. Ziraat Fak. Yay., Ankara, Turkey (in Turkish).
Huston JE, White RG, Bequette BJ et al (2006) Nutrient requirements of small ruminants: sheep, goats, cervids, and New World camelids. The National Academies Press, Washington, D.C.
IRIS (2003) Integrated Risk Information System database. US Environmental Protection Agency
Kacar B (1996) Toprak Analizleri, Ankara Universitesi Ziraat Fak Egitim Arastırma Gelistirme Vakfi Yay, Ankara, Turkey (in Turkish).
Kalavrouziotis IK, Carter J, Varnavas SP, Mehra AA, Drakatos PA (2007) Towards an understanding of the effect of road pollution on adjacent food crops: zea mays as an example. Int J Environ Pollut 30(3/4):576–592
Kamal KT, Lofti K, Omer KD, Mohammad RE, Obuleiz KB (2015) Heavy metals concentrations in fish from Red sea and Arabian gulf. Health benefits and risk assessments due to their consumptions. Asian J Chem 27:4411–4416
Khan ZI, Ahmad K, Safdar H, Ugulu I, Wajid K, Bashir H, Dogan Y (2018d) Manganese bioaccumulation and translocation of in forages grown in soil irrigated with city effluent: an evaluation on health risk. Res J Pharmaceut Biol Chem Sci 9(5):759–770
Khan ZI, Ugulu I, Sahira S, Ahmad K, Ashfaq A, Mehmood N, Dogan Y (2018a) determination of toxic metals in fruits of Abelmoschus esculentus grown in contaminated soils with different irrigation sources by spectroscopic method. Int J Environ Res 12:503–511. https://doi.org/10.1007/s41742-018-0110-2
Khan ZI, Ugulu I, Umar S, Ahmad K, Mehmood N, Ashfaq A, Bashir H, Sohail M (2018b) Potential toxic metal accumulation in soil, forage and blood plasma of buffaloes sampled from Jhang, Pakistan. Bull Environ Contam Toxicol 101:235–242. https://doi.org/10.1007/s00128-018-2353-1
Khan ZI, Ugulu I, Ahmad K, Yasmeen S, Noorka IR, Mehmood N, Sher M (2018c) Assessment of trace metal and metalloid accumulation and human health risk from vegetables consumption through spinach and coriander specimens irrigated with wastewater. Bull Environ Contam Toxicol 101:787–795. https://doi.org/10.1007/s00128-018-2448-8
Liu W, Zhao JZ, Ouyang ZY, Soderlund L, Liu GH (2005) Impacts of sewage irrigation on heavy metals distribution and contamination. Environ Int 31:805–812
Marianna B, Cortivo C, Barion G, Mosca G, Vamerali T (2016) Phytoremediation opportunities with alimurgic species in metal-contaminated environments. Pak J Bot:213–218
McDowell LR (1983) Minerals in animal and human nutrition. Academic Press, New York
Minervino A, Junior H, Ferreira RA (2009) Clinical observations of cattle and buffalos with experimentally induced chronic copper poisoning. Res Vet Sci 87:473–478
Murray RK, Granner DK, Mayes PA, Rodwell VW (2000) Harper’s Biochemistry, 25th edn. McGraw-Hill, Health Profession Division, USA
Nadeem M, Qureshi TM, Ugulu I, Riaz MN, An QU, Khan ZI, Ahmad K, Ashfaq A, Bashir H, Dogan Y (2019) Mineral, vitamin and phenolic contents and sugar profiles of some prominent date palm (Phoenix dactylifera) varieties of Pakistan. Pak J Bot 51(1):171–178. https://doi.org/10.30848/PJB2019-1(14)
Nwede D, Okoye O, Babayemi P (2010) Heavy metal levels in animal muscle tissue: a case study of Nigerian raises cattles. Res J Appl Sci 5(2):146–150
Radwan MA, Salama AK (2006) Market basket survey for some heavy metals in Egyptian fruits and vegetables. Food Chem Toxicol 44:1273–1278
Richards LA (1968) Diagnosis and improvement of saline and alkaline soils., Agri. Handbook No. 60, 1st edn. IBH Pub. Co., New Delhi
Sabudak T, Seren G, Kaykıoglu G, Dincer AR (2007) Determination of trace elements in soil and sunflower (Helianthus annuus L.) plant parts. Fresen Environ Bull 16:1274–1278
Sainger P, Dhankhar A, Sainger R, Kawushik M, Singh RP (2011) Assessment of heavy metal tolerance in native plant species from soils contaminated with electroplating effluent. Ecotoxicol Environ Saf 74:2284–2291
Saskia A, Yamaji N, Yokosho K, Ma JF (2013) Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Environ Int 24(5):2155–2167
Smith I, Carson BL (1981) Trace metals in the environment. Ann. Arbor, M.I. Ann Arbor Science Publishers, New York, p 32
Dutch standards (2000) Circular on target values and intervention values for soil remediation.
Steel R, Torrie JH (1980) Principle and procedures of statistics, a biometrical approach, 2nd edn. McGraw Hill Book Co. Inc, New York, pp 336–354
Tomlinson DL, Wilson JG, Harris CR, Jeffrey DW (1980) Problems in the assessment of heavy metal levels in estuaries and the formation of a pollution index. Helgolaender Meeresunter 33:566–575
Tshibangu T, Nsahlai VI, Kiatok MH, Hornick JL (2014) Heavy metals concentration in Adenodolichos rhomboideus (O. Hoffm.) Harms. forage growing on mining tailings in south east of Democratic Republic of Congo: Influence of washing, Ph and soil concentrations. Int J Curr Res Bio Sci Plant Biol 1(5):16–27
Ugulu I (2015a) A quantitative investigation on recycling attitudes of gifted/talented students. Biotech Biotechnol Equip 29:20–26. https://doi.org/10.1080/13102818.2015.1047168
Ugulu I (2015b) Determination of heavy metal accumulation in plant samples by spectrometric techniques in Turkey. Appl Spectros Rev 50(2):113–151. https://doi.org/10.1080/05704928.2014.935981
Ugulu I, Baslar S (2010) The determination and fidelity level of medicinal plants used to make traditional Turkish salves. J Alternative Compl Med 16(3):313–322. https://doi.org/10.1080/13102818.2009.10818451
Ugulu I, Baslar S, Dogan Y, Aydin H (2009) The determination of colour intensity of Rubia tinctorum and Chrozophora tinctoria distributed in Western Anatolia. Biotech Biotechnol Equip 23(SE):410–413
Ugulu I, Dogan Y, Baslar S, Varol O (2012) Biomonitoring of trace element accumulation in plants growing at Murat Mountain. Int J Environ Sci Tech 9:527–534. https://doi.org/10.1007/s13762-012-0056-4
Ugulu I, Unver MC, Dogan Y (2016) Determination and comparison of heavy metal accumulation level of Ficus carica bark and leaf samples in Artvin, Turkey. Oxid Commun 39(1):765–775
Umar A, Birnin AU, Muhammad C (2015) Acacia Nilotica as bio indicator of copper and cobalt production due to vehicular emission along the main entrance road of Usmanu Danfodio University Skoto, Nigeria. Int J Adv Res Chem Sci 2:1–8
Unver MC, Ugulu I, Durkan N, Baslar S, Dogan Y (2015) Heavy metal contents of Malva sylvestris sold as edible greens in the local markets of Izmir. Ekoloji 24(96):13–25. https://doi.org/10.5053/ekoloji.2015.01
USEPA (US Environmental Protection Agency) (2002) Region 9. In: Preliminary Remediation Goals
Yorek N, Ugulu I, Aydin H (2016) Using self-organizing neural network map combined with ward’s clustering algorithm for visualization of students’ cognitive structural models about aliveness concept. Computational Intelligence and Neuroscience, Article ID 2476256:1–14. https://doi.org/10.1155/2016/2476256
Zahara B, Khan ZI, Ahmad K et al (2014) Vegetables as a potential source of metals and metalloids for human nutrition: a case study of Momordica charantia Grown in Soil Irrigated with Domestic Sewage Water in Sargodha, Pakistan. Pak J Zool 46(3):633–641
Zhang H, Lou Y, Song J et al (2007) Predicting As, Cd and Pb uptake by rice and vegetables using fields data from China. J Environ Sci 23:70–78
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, Z.I., Arshad, N., Ahmad, K. et al. Toxicological potential of cobalt in forage for ruminants grown in polluted soil: a health risk assessment from trace metal pollution for livestock. Environ Sci Pollut Res 26, 15381–15389 (2019). https://doi.org/10.1007/s11356-019-04959-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04959-9