Abstract
This study was carried out to assess the groundwater pollution in the northern Develi Closed Basin by using the heavy metal pollution index (HPI). Samples from 10 wells and 5 springs were collected in dry and wet seasons and concentrations of Pb, Zn, Cr, Mn, Fe, Cu, Cd, As and B were determined. In both seasons, for more than half of the samples, As, B and Fe concentrations exceeded the Turkish drinking water guideline values. Due to the occurrence of these metals in high concentrations in some samples HPI values are up to 1740. The source of these metals is geogenic and attributed to the interaction of these waters with highly altered volcanic and pyroclastic rocks. The overall HPI for wet and dry periods are reported as 360 and 440, respectively. Accordingly, the pollution level in the groundwater of this area is unacceptable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Groundwater is a significant source of drinking water, which is withdrawn not only as a drinking water supply, but also to supply water for irrigation, livestock, industry and domestic purposes. In fact, more than 2 billion people worldwide depend on groundwater for their daily supply (Kemper 2003). Increasing stresses on water supplies resulted in groundwater contamination issues in many parts of the world (Lugoli et al. 2011; Guler et al. 2012; Huang et al. 2013; Yolcubal et al. 2016 and many others). Until the recent past, water quality concerns have been neglected since adequate and good quality water supplies have been available and also the adverse effects of some heavy metals on human health were not fully understood. Today, heavy metal pollution of groundwater is considered as one of the most substantial environmental problems since heavy metals are highly toxic to humans even at low concentrations (Marcovecchio et al. 2007; Varghese and Jaya 2014; Abou Zakhem and Hafez 2015; Bhuiyan et al. 2016). It is difficult to evaluate the water contamination by only elemental concentrations (Nimick and Moore 1991), therefore water quality indices are used to assess the overall effects of pollution and to commentate on the influence of the individual pollution parameters (Prasad and Sangita 2008; Bhuiyan et al. 2016). There are several different methods developed to calculate water quality indices and these methods usually depend on the available data and the solicited results (Horton 1965; Nishida et al. 1982 and others). Heavy metal pollution index (HPI) is one of these methods which is proven to be a powerful way to evaluate the heavy metal pollution in surface water and groundwater (Mohan et al. 1996; Prasad and Jaiprakas 1999; Prasad and Sangita 2008; Milivojević et al. 2016). The HPI is a practical and easy to understand guiding tool that can be used by environmental managers, decision makers and users of a groundwater system (Prasanna et al. 2012). It represents the composite influence of the selected pollution parameters and the total quality of groundwater with respect to heavy metals (Prasad and Jaiprakas 1999). Not only groundwater quality, but also its suitability for drinking purpose can be evaluated by using the HPI.
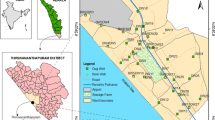
The Develi Closed Basin is located in Central Turkey within the borders of the Kayseri province (Fig. 1a, b). The basin is closed in character due to the mountainous parts surrounding the plain part (Fig. 1b) The study area is located in the northern part of the Develi Closed Basin, which hosts one of the most important bird paradises of Turkey, Sultansazligi wetland. The wetland area is protected according to the International Ramsar Agreement since 1994. The area attracted researchers and different studies were carried out around the wetland area in the last 10 years mainly to propose an approach to the hydrologic sustainability of the wetland (Dadaser-Celik et al. 2007), to put forward the spatial changes in the wetland by analyzing the satellite images (Dadaser-Celik et al. 2008), to calculate the water budget in the area (Yildiz and Gurer 2009), to determine the factors affecting the severe contraction that the wetland suffered over the years (Bayari and Yildiz 2012), to simulate the water levels in the wetland area by using an artificial neural network (Dadaser-Celik and Cengiz 2013) and to determine the evaporation from the wetland area (Yildiz and Gurer 2014). All of these studies concentrated on the wetland area and none of them focused on the heavy metal pollution issues, although the protection of the wetland from possible pollution sources depend on the identification of these sources. In the northern part of the basin, groundwater flow is towards the wetland area and the groundwater quality is more of an issue. Yildiz (2007) reported that although the groundwater of the study area does not seem to affect the wetland system in the short-term due to the occurrence of clay layers below the wetland area, in the long term there might be an interaction between the surface waters and groundwater. Hence, this study was carried out to exhibit and to investigate the heavy metal pollution in the groundwater of the northern Develi Basin. There are no remarkable anthropogenic point sources related to industrial and/or mining activities that can cause pollution in the northern Develi Basin and the pollution source is assumed to be geogenic. In order to assess the degree of this geogenic pollution in the basin, a geochemical survey was carried out and groundwater samples were collected in two different seasons.
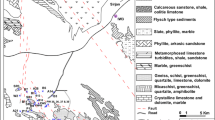
a Location map of the Kayseri province (Source Google Earth), b map showing the location of the study area in the Develi Closed Basin, c geological map of the study area (Modified from Donmez et al. 2005)
Materials and Methods
The Develi Closed Basin is located in central Turkey (Fig. 1a). The total drainage area of the basin is 3190 km2, however, the present study was carried out in the northern part of the basin in an area which is approximately 761 km2 (Fig. 1b). The study area has a terrestrial climate with hot, dry summers and cold winters. According to the long-term measurements carried out at the Kayseri meteorological station between 1929 and 2008, the average annual temperature and precipitation are around 10.6°C and 380.2 mm, respectively. The highest elevation is the summit of Mount Erciyes with 3917 m elevation above sea level, which is located in the northeastern part of the area (Fig. 1c). Actually, Mount Erciyes is the highest peak in Central Anatolia. The bottom of Çöl Lake is the lowest elevation in the area (1071 m) (Fig. 1c).
The study area is characterized by formations of volcanic origin formed during the latest stages of Mount Erciyes volcanism in between the Miocene and Quaternary (Donmez et al. 2005). The geologic units outcropping in the area are Miocene volcanics, Upper Miocene sedimentary rocks, Pliocene volcanic rocks and Quaternary volcanic and sedimentary units (Fig. 1c). Lower Miocene sedimentary rocks consist of gray, red colored sandstones, mudstones and pebble stones. The Upper Miocene andesites are the products of a viscous magma and are in porphyritic texture. Upper Miocene basalts are comprised of plagioclase, olivine and clinopyroxene phenocrystals. Upper Miocene pyroclastic rocks are comprised of grey–purple colored tuffs and ignimbrites (Donmez et al. 2003) whereas the sedimentary rocks are composed of brown to red colored pebble stones, sandstones, mudstones with gypsum, anhydrite and ignimbrite levels (Donmez et al. 2005). Pliocene aged rocks are the pyroclastic rocks composed of tuffs and agglomerates, being the products of an andesitic lava, the basaltic rocks which are dark colored and have a vesicular texture and the andesitic rocks which are dark colored and massif (Turkecan et al. 1998). Pliocene units are overlain by Quaternary aged volcanic glasses, pyroclastic rocks (pumices and volcanic ashes), massif and dark colored olivine basalts and grey–black colored andesites (Turkecan et al. 1998). Quaternary alluvium units cover the plain parts of the area and comprised of clay, sand and pebbles. Groundwater occurs under both unconfined and confined conditions in the altered and fractured parts of volcanic rocks (SHW 1995).
Samples from 10 groundwater wells whose depths vary between 101 and 215 m, and 5 springs were collected in both wet (October 2013) and dry (March 2014) seasons (Table 1). All of the wells are equipped with an electrical submersible pump. During sampling from the wells, extensive purging procedure has been completed to ensure that more than three times the well volume has been discharged while monitoring the indicator field parameters (pH, temperature and electrical conductivity) with a Hach HQ40d multi-analyzer. Sampling was carried out only after the field parameters were stabilized to guarantee that the sample represents the groundwater from the aquifer. Sampling from the springs was carried out directly from the outlet of the spring. The samples were collected in acid washed polyethylene bottles (250 ml), they were passed through 0.45 µm filters to remove sediment particles, acidified to pH <2 (65% HNO3) after filtering and kept refrigerated at 4°C prior to analyses. Duplicate samples were collected from each sampling point for quality assurance/quality control and the average of replicates are presented as the analysis results. The analyses were carried out in ISO-certified State Hydraulic Works (SHW) laboratories in Kayseri by using a Bruker Aurora M90 Inductively Coupled Plasma-Mass Spectrometer (ICP-MS) following standard methods given by United States Environmental Protection Agency (EPA Method 200.8). Before introducing the heavy metals in question to the MS, they were ionized in ICP by argon and then quantified by their mass/charge ratio. The analytical data quality was ensured by performing periodic measurements of the calibration standards, analysis of laboratory reagent blanks, replicates and internal standards. All of the standards used were traceable to the U.S. Department of Commerce, National Institute of Standards and Technology (NIST). Analyses were carried out in duplicates and the results are expressed as the mean. The detection limits were found to be 0.04 µg/l for Pb, 0.83 µg/l for Zn, 0.17 µg/l for Cr, 0.25 µg/l for Mn, 1.23 µg/l for Fe, 0.10 µg/l for Cu, 0.09 µg/l for Cd, 0.64 µg/l for As and 0.01 mg/l for B. Analytical precision was reported as ±5% for all metals.
Statistical analysis hierarchical agglomerative clustering (HAC) was carried out by using Excel Statistical software. HCA was performed by using Ward’s method for linkage (Ward 1963) and squared Euclidean distance as a measure of similarity. Besides, HCA was conducted in R-mode where clusters of variables are searched (Bhuiyan et al. 2016). A dendrogram was then used for visual inspection of the results (Davis 1986; Guler 2002) (Fig. 2). Prior to analysis, data was log-transformed and standardized to assure that the influence of every each metal would be equal in the analysis (Guler et al. 2002).
The HPI was calculated using Eq. 1 by Mohan et al. (1996). It was developed by assigning a weighting for each selected parameter (Wi) between zero and one, depending on the relative importance of the parameter in quality considerations. Wi is usually defined as inversely proportional to the recommended standard (Si) for each parameter (Horton 1965; Mohan et al. 1996; Prasad and Sangita 2008).
where n is the number of the parameters considered, Wi and Qi are the unit weight and the sub-index of the ith parameter, respectively. The sub-index was calculated using Eq. 2:
where Mi, Ii and Si are monitored value of the metal, desirable maximum and standard values of the ith parameter, respectively. Prasad and Sangita (2008) reported that the critical pollution index value is 100.
Results and Discussion
Concentrations of nine metals in two different seasons (dry and wet) are presented in Table 1 together with pH, Electrical Conductivity (EC), WHO (2008) and ITASHY (2005) guideline values. According to Table 1, pH of the waters is near neutral ranging between 6.5 and 7.8 in dry season and between 6.8 and 7.4 in the wet season. Electrical Conductivity values vary from 63 to 1543 and from 61 to 1532 in dry and wet seasons, respectively. The lowest EC is observed in a spring (SP4) and the highest one is observed in a well (w4). Concentrations of some metals (As, Fe and B) in most of the samples exceed the desirable limits prescribed by WHO (2008). In fact, for As, 60% of the samples in both the dry and wet seasons exhibit concentrations above the guideline values (10 µg/l), the maximum concentrations reaching up to 593 µg/l in dry season and 774 µg/l in the wet season. The mean concentrations tend to increase from dry (164 µg/l) to wet (188 µg/l) seasons. Such high concentrations of As in drinking water represent an enormous threat to human health (Nriagu 1994). Moreover, Imamul Huq et al. (2006) stated that arsenic contaminated irrigation water may have a negative effect upon both food safety and crop production. Imamul Huq (2008) also put forward that irrigation water with high levels of As can cause contamination of agricultural soils due to the accumulation of As in soil. The Canadian Guidelines for dissolved arsenic in irrigation water is 100 µg/l (CCME 1993). Accordingly, 60% of the samples exceeds these guidelines and not suitable for irrigation, although most of these wells are already utilized to supply water for especially sugar beets (Yildiz 2007).
The mean concentrations of Zn, Fe and B also show an increasing trend from dry to wet seasons. B concentrations in 20% (same for both seasons) and Fe concentrations in 46.7% (33.3% for wet season) of the samples have concentrations above the limits recommended by ITASHY (2005). The increase in the mean Fe concentrations is especially attention striking since they have almost been doubled in between the two seasons (Table 1). In contrary to As, B, Fe and Zn, concentrations of Pb, Cr, Mn and Cu show a decreasing trend in between dry and wet seasons.
According to the dendrogram obtained as a result of hierarchical agglomerative clustering of October 2013 data, there are three clusters formed. Cluster 1 contains As and B, Cluster 2 includes Pb, Cu, Mn and Zn; Cluster 3 includes Cr, Cd and Fe (Fig. 2). The dendrogram prepared for March 2014 is different from the aforementioned one since Cluster 1 is formed by Cu, Pb, Zn, Fe and Mn, Cluster 2 consists of Cd and Cluster 3 consists of As, B and Cr. As is highly correlated with B in both dry and wet seasons. Fe shows correlation with Cr and Cd in dry season, but in the wet season Fe is highly correlated with Mn, Zn, Pb and Cu. Mn, Zn, Pb and Cu are correlated in both seasons. The considerable increase/decrease in Fe, As, B and Mn concentrations between two seasons changes the correlation systematics. The controls on dissolved As, Fe and Mn concentrations in the groundwater are often complex. In order to find out the distribution of the aqueous species and the precipitation state of the solids (saturation index) in the samples, the computer program PHREEQC (Parkhust and Appelo 1999) utilizing database llnl was used. This program relies on an ion-association aqueous model and is able to carry out aqueous geochemical calculations (Somay et al. 2008). In this manner, As (V) is dominant in all of the samples and the major As species are HAsO4 −2 and H2AsO4 − without any seasonal variations except for the percent change in the species occurrences, Mn (II) is dominant in all of the samples and the major species is in the ionic form as Mn2+. In case of iron, Fe3+ components (Fe(OH)3) are more abundant than Fe2+ components (FeHCO3 + and Fe2+) for most of the samples (i.e. W3, W4, W5, W9, W10, SP1, SP2 and SP4). This may be attributed to the oxidation of dissolved Fe2+ ions due to biological activities resulting in the precipitation of Fe(OH)3, to temperature and pH or Eh-controlled reactions with aquifer material or to the seasonal changes in the potential hydraulic conditions between the wetland area and the groundwater system. Dissolved iron can precipitate rapidly with increasing pH or Eh, therefore considerable seasonal changes of dissolved iron in groundwater can be observed (van der Grift et al. 2014). It should be noted herein that wells W1, W2, W3, W4, W5, W8, W9 draws water from the unconfined aquifer, whereas W10 draws water from both the unconfined and the confined aquifer due to the screens installed in both aquifers. W6 and W7, on the other hand, draw water from the confined aquifer. Among the samples, the oversaturation of the mineral phases containing iron includes goethite, magnetite and hematite. Further evaluation is required to reveal out the exact mechanisms of the dissolution/precipitation of Fe together with As, B and Mn in such a complex groundwater system where the extend and the thickness of the aquifer is highly variable due to the characteristics of the volcanic terrain.
There are no industrial or mining activities that can cause anthropogenic contamination in the study area; however the outcrops of young and altered products of Erciyes volcanism (Fig. 1) are enriched in various heavy metals, therefore the heavy metal pollution source of the groundwater is considered to be geogenic. Arslan et al. (2016) mentioned that there is a change in the chemical character of the groundwater between wet and dry seasons due to the water–rock interaction especially during the wet season. This interaction occurs as a result of the dissolution in the altered zone formed due to the weathering of the volcanic and the pyroclastic rocks of Erciyes volcanism under the influence of the tectonic components (faults) (SHW 1995; Donmez et al. 2005). To be more specific, for sample W2, the water type in the dry season is Ca–Mg–HCO3 and in the wet season it is Ca–HCO3. For sample W5, the water type in the dry season is Na–Ca–Cl–HCO3 and in the wet season it is Na–Cl–HCO3. In the same manner, the spring SP3 sample is Na–Ca–Mg–HCO3 type in dry season and the type changes to Na–Ca–HCO3–SO4 in the wet season. For SP4, the water type is Na–Ca–HCO3 in dry season and Na–Ca–HCO3–SO4 in the wet season. Although there is a slight decrease in the mean electrical conductivity values of the samples between dry and wet seasons, some of the major ion concentrations between dry and wet periods increase, whereas some decrease.
As mentioned previously, HPI is considered as an effective tool used in the characterization of the composite influence of metals on the overall water quality (Sheyki and Moore 2012). In Tables 2 and 3, the HPI calculations for groundwater of the study area and HPI of the individual sampling points are presented. In this study, Ii values are assumed to be zero for all of the metals that are considered and Si values are the highest permissible value for drinking water as specified by the drinking water quality guidelines for Turkey (ITASHY 2005). The HPI values calculated for both dry and wet seasons are more than three times higher than the critical value of the pollution index (100) suggested by Prasad and Sangita 2008. Therefore, the level of pollution in the area is unacceptable in both seasons. The HPI in dry season (360) is slightly lower than the one in wet season (~440). Kumar et al. (2012) classified HPI into three categories as low (<19), medium (19–38) and high (>38). According to this classification, in both dry and wet seasons, most of the samples (46.7% and 60%, respectively) fall in the high category. Some of the samples exhibit very high HPI values (as high as 1740) due to high concentrations of As, B and Fe in these samples (e.g. W4, W5, W7, W8, W9, SP-2 and SP-5). These high HPI values are due to the geogenic pollution source. For the rest of the samples, in dry season, 26.7% of the samples fall in both low and medium categories and in the wet season, 20% of the samples fall in both low and medium categories respectively.
In conclusion, in this study heavy metal pollution is examined and pollution index calculations are carried out for groundwater resources located in the northern part of the Develi Closed Basin. This is the first study focusing on the heavy metal pollution of groundwater resources around the Sultansazlığı wetland. The results indicate that for almost half of the samples As, B and Fe concentrations are much higher than the maximum permissible level for drinking water. For As, the highest concentration exceeds the drinking water standard about 77 times and is detected in the wet season in one of the samples (W4). The source of the heavy metals (As, B, Fe, Pb, Zn, Fe and Cu) occurring at substantially high concentrations in some of the groundwater samples is attributed to geogenic sources. To be specific about the geogenic sources, the volcanic rocks formed during the latest stages of Erciyes volcanism are products of a fast cooling magma and they are highly heterogeneous in nature. Some heavy metals present naturally in these rocks dissolve especially from the altered parts and incorporate into groundwater. The alteration in these volcanic rocks occurs due to various reasons including the multi-phase volcanism in effect during the formation of these rocks and the existence of a pressure ridge in the area. The overall HPI for wet and dry periods are calculated as 360 and 440, respectively. For some of the samples (about 47%) HPI values are extremely high (up to 1740) due to presence of especially As, B and Fe in high concentrations. To summarize, it is concluded that geogenic sources contribute to the high scores of HPI in the study area and the pollution level in the groundwater is unacceptable. Therefore, the treatment of groundwater in this area is highly recommended before it is to be consumed and/or used for agricultural purposes.
References
Abou Zakhem B, Hafez R (2015) Heavy metal pollution index for groundwater quality assessment in Damascus Oasis, Syria. Environ Earth Sci 73:6591–6600. doi:10.1007/s12665-014-3882-5
Arslan S, Yucel C, Celik M (2016) Geogenic arsenic and other trace elements in the groundwater system of Develi Closed Basin, Kayseri, Turkey. 43rd International Association of Hydrogeologists Congress, Montpellier, France, Abstract number 1418
Bayari S, Yildiz FE (2012) Effects of the North Atlantic oscillation and groundwater use on the contraction of Sultansazligi Wetland. Hydrogeol J 20:369–383
Bhuiyan MAH, Bodrud-Doza M, Islam A, Rakib MA, Rahman MS, Ramanathan AL (2016). Assessment of groundwater quality of Lakshimpur district of Bangladesh using water quality indices, geostatistical methods, and multivariate analysis. Environ Earth Sci 75(12)
CCME (Canadian Council of Ministers of the Environment) (1993) Appendix XV—Protocols for deriving water quality guidelines for the protection of agricultural water uses (October 1993). In: Canadian water quality guidelines, Canadian Council of Resource and Environment Ministers. 1987. Prepared by the Task Force on Water Quality Guidelines.
Dadaser-Celik F, Cengiz E (2013) A neural network model for simulation of water levels at the Sultan Marshes wetland in Turkey. Wetl Ecol Manag 21:297–306
Dadaser-Celik F, Brezonik PL, Stefan HG (2007) Hydrologic sustainability of the Sultan Marshes in Turkey. Water Int 32 (5):856–876
Dadaser-Celik F, Bauer ME, Brezonik PL, Stefan HG (2008) Changes in the Sultan Marshes ecosystem (Turkey) in satellite images 1980–2003. Wetlands 28:852–865
Davis JC (1986) Statistics and data analysis in geology. 2nd edn, Wiley, New York
Donmez M, Turkecan A, Akçay AE (2003) Tertiary volcanics of Kayseri Niğde Nevşehir region. General Directorate of Mineral Research and Exploration Institute, Ankara, Turkey, Report No: 10575
Donmez M, Akçay AE, Turkecan A (2005) Turkish Geological Maps Report of Kayseri K34 section. General Directorate of Mineral Research and Exploration Institute, Ankara, pp 2–19
Guler C, Thyne GD, McCray JE, Turner KA (2002) Evaluation of graphical and multivariate statistical methods for classification of water chemistry data. Hydrogeol J 10:455–474
Guler C, Kurt MA, Alpaslan M, Akbulut C (2012) Assessment of the impact of anthropogenic activities on the groundwater hydrology and chemistry in Tarsus coastal plain (Mersin, SE Turkey) using fuzzy clustering, multivariate statistics and GIS techniques. J Hydrol 414:435–451
Horton RK (1965) An index number system for rating water quality. J Water Pollut Control Fed 37(3):300–306
Huang GX, Sun JC, Zhang Y, Chen ZY, Liu F (2013) Impact of anthropogenic and natural processes on the evolution of groundwater chemistry in a rapidly urbanized coastal area, South China. Sci Total Environ 463:209–221. doi:10.1016/j.scitotenv.2013.05.078
Imamul Huq SM (2008) Fate of arsenic in irrigation water and its potential impact on the food chain. In: Ahuja S (ed) Arsenic contamination of groundwater: mechanism, analysis and remediation, Ch 2. Wiley, Hoboken, pp 23–49
Imamul Huq SM, Joardar JC, Parvin S, Correll R, Naidu R (2006) Arsenic contamination in food chain: transfer of arsenic into food materials through groundwater irrigation. J Health Popul Nutr 24:305–306
ITASHY (2005) Regulation on waters for human consumption. Official Gazette dated 17/02/2005, No. 25730, Ankara (in Turkish)
Kemper KE (2003) Groundwater from development to management. Hydrogeol J 12(1):3–5. doi:10.1007/s10040-003-0305-1
Kumar PJS, Delson PD, Babu PT (2012) Appraisal of heavy metals in groundwater in Chennai city using a HPI model. Bull Environ Contam Toxicol 89:793–798. doi:10.1007/s00128-012-0794-5
Lugoli F, Leopizzi MI, Bagordo F, Grassi T, Guido M, De Donno A (2011) Widespread microbiological groundwater contamination in the South-eastern Salento (Puglia-Italy). J Environ Monit 13(1):192–200. doi:10.1039/c0em00193g
Marcovecchio JE, Botte SE, Freije RH (2007) Heavy metals, major metals, trace elements. In: Nollet LML, De Gelder LSP (eds) Handbook of water analysis. 2nd edn, CRC Press, London, pp 273–309
Milivojević J, Krstić D, Šmit B, Djekić V (2016) Assessment of heavy metal contamination and calculation of its pollution index for Uglješnica River, Serbia. Bull Environ Contam Toxicol 97:737–742. doi:10.1007/s00128-016-1918-0
Mohan SV, Nithila P, Reddy SJ (1996) Estimation of heavy metals in drinking water and development of heavy metal pollution index. J Environ Sci Health Part a-Environ Sci Eng Tox Hazard Subst Control 31(2):283–289
Nimick DA, Moore JN (1991) Prediction of water-soluble metal concentrations in fluvially deposited tailings sediments, Upper Clark Fork Valley, Montana, USA. Appl Geochem 6:635–646. doi:10.1016/0883-2927(91)90074-Y
Nishida H, Miyai M, Tada F, Suzuki S (1982) Computation of the index of pollution caused by heavy-metals in river sediment. Environ Pollut Ser B-Chem Phys 4(4):241–248. doi:10.1016/0143-148x(82)90010-6
Nriagu JO (1994) Arsenic in the environment: Part II, human health and ecosystem effects. Wiley, New York
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (version 2)—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations: U.S. Geological Survey Water-Resources Investigations Report 99-4259, p 312
Prasad B, Jaiprakas KC (1999) Evaluation of heavy metals in ground water near mining area and development of heavy metal pollution index. J Environ Sci Health Part a-Tox/Hazard Subst Environ Eng 34(1):91–102
Prasad B, Sangita K (2008) Heavy metal pollution index of groundwater of an abandoned open cast mine filled with fly ash: a case study. Mine Water Environ 27(4):265–267. doi:10.1007/s10230-008-0050-8
Prasanna MV, Praveena SM, Chidambaram S, Nagarajan R, Elayaraja A (2012) Evaluation of water quality pollution indices for heavy metal contamination monitoring: a case study from Curtin Lake, Miri City, East Malaysia. Environ Earth Sci 67:1987–2001
Sheyki V, Moore F (2012) Geochemical characterization of Kor River water quality, Fars Province, southwest Iran. Water Qual Expo Health (Lond) 4:25–38
SHW (1995) Revised hydrogeological study report of Kayseri Develi-Yeşilhisar plains. State Hydraulic Works 12nd district, Kayseri 1–19 (in Turkish)
Somay AM, Gemici U, Filiz S (2008) Hydrogeochemical investigation of Kucuk Menderes River coastal wetland, Selcuk-Izmir, Turkey. Eviron Geol 55: 149–164
Turkecan A, Acarlar M, Donmez M, Hepsen N, Bilgin R (1998) Petrology of volcanic rocks and geology of Kayseri area (Bünyan-Develi-Tomarza). Miner Res and Explor Inst, Turkey, Report No:10186 (in Turkish)
van der Grift B, Rozemeijer JC, Griffioen J, van der Velde Y (2014) Iron oxidation kinetics and phosphate immobilization along the flow-path from groundwater into surface water. Hydrol Earth Syst Sci 18:4687–4702
Varghese J, Jaya DS (2014) Metal pollution of groundwater in the vicinity of Valiathura Sewage Farm in Kerala, South India. Bull Environ Contam Toxicol 93:694–698. doi:10.1007/s00128-014-1410-7
Ward JH (1963) Hierarchical grouping to optimize an objective function. J Am Stat Assoc 58:236–244
WHO (2008) World Health Organization guidelines for drinking-water quality (3rd edn). WHO Press, Geneva
Yildiz FE (2007) Determination of relationship between surface water and groundwater in Sultansazligi wetland-Kayseri, PhD Thesis, Gazi University Department of Civil Engineering, Ankara
Yildiz FE, Gurer I (2009) An integrated water resources management of Sultansazligi Closed Basin in Kayseri Turkey (in Turkish). GU J Sci 22(3):203–213
Yildiz FE, Gurer I (2014) Comparison of the evaporation methods for Sultansazligi Wetland (in Turkish). GU J Sci Part C 2(3):247–254
Yolcubal I, Gunduz OC, Sonmez F (2016) Assessment of impact of environmental pollution on groundwater and surface water qualities in a heavily industrialized district of Kocaeli (Dilovasi), Turkey. Environ Earth Sci 75(2):170. doi:10.1007/s12665-015-4986-2
Acknowledgements
The authors wish to express their grateful appreciation to Nurhan Öztürk from the 12th District Office of the State Hydraulic Works for her help throughout the study. Part of the data published in this paper will also be included in the master’s thesis of the second author. The authors are grateful for the constructive comments and suggestions of Editor-in-Chief Erin Bennett, editorial board editor and two anonymous reviewers for improving the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arslan, Ş., Yücel, Ç., Çallı, S.S. et al. Assessment of Heavy Metal Pollution in the Groundwater of the Northern Develi Closed Basin, Kayseri, Turkey. Bull Environ Contam Toxicol 99, 244–252 (2017). https://doi.org/10.1007/s00128-017-2119-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2119-1